The Neurobiological Basis of Love: A Meta-Analysis of Human Functional Neuroimaging Studies of Maternal and Passionate Love
Abstract
1. Introduction
2. Methods
2.1. Systematic Review and Selection of Studies
2.2. Meta-Analysis: ALE
3. Results
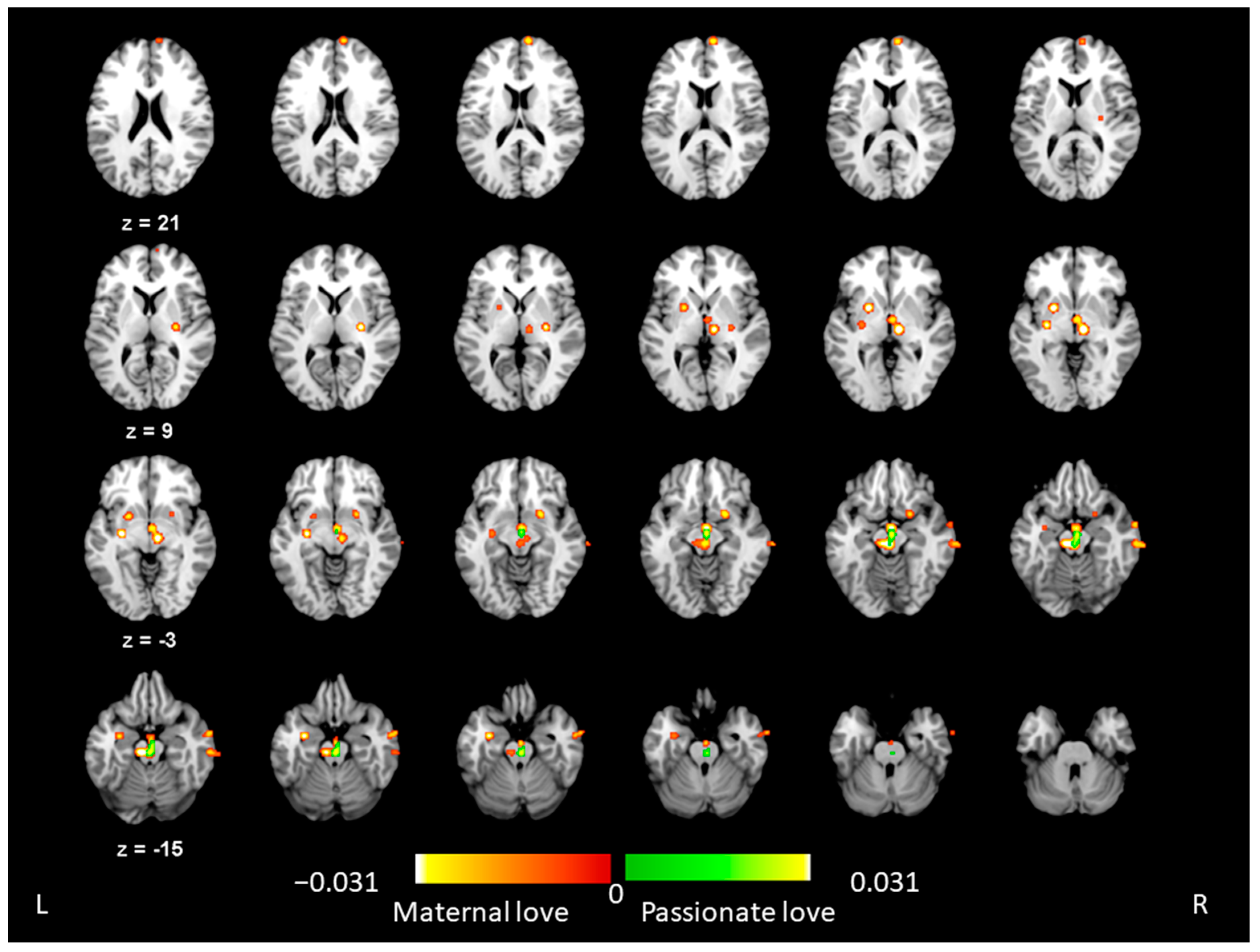
3.1. ALE Results for Maternal Love
3.2. ALE Results for Passionate Love
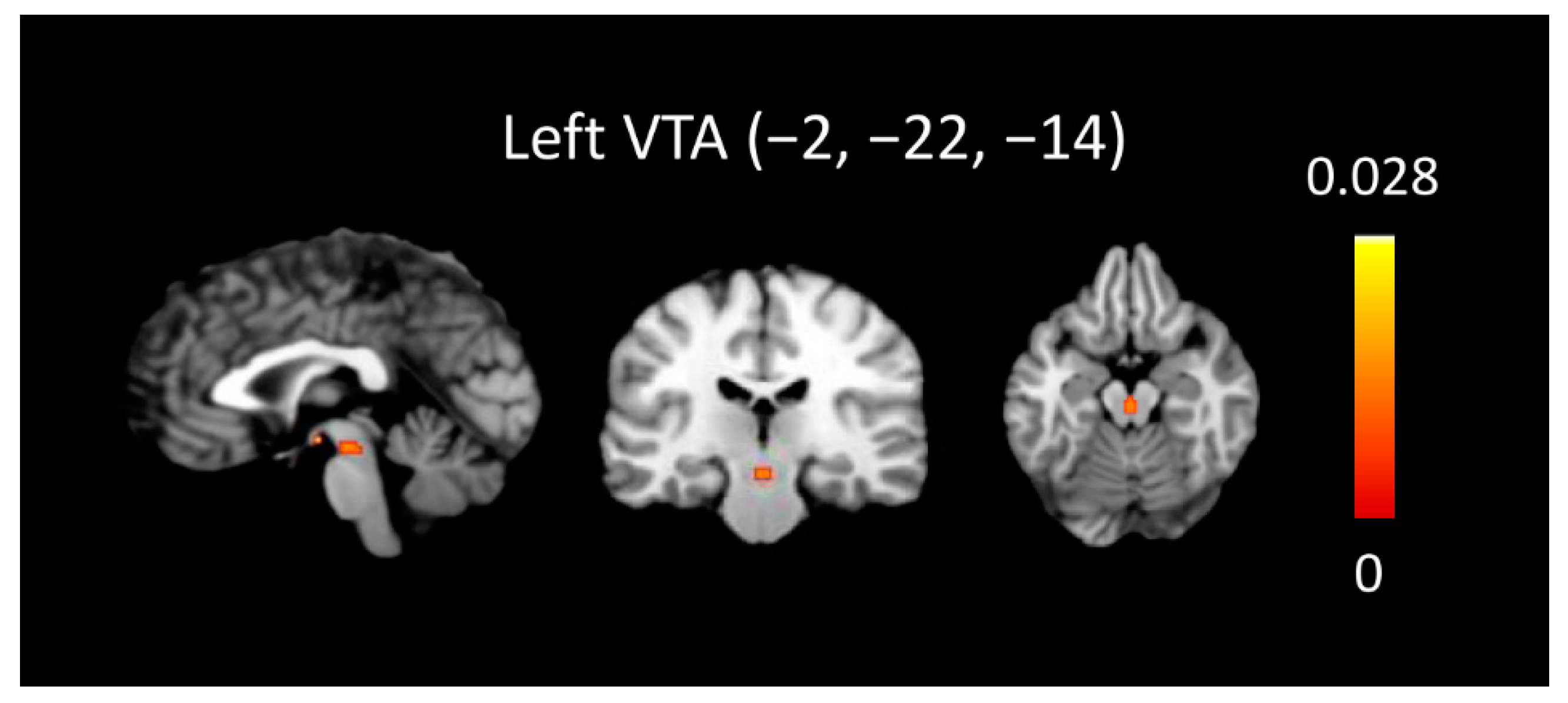
3.3. ALE Conjunction Results across Maternal and Passionate Love
3.4. ALE Results Indicating Differences between Maternal and Passionate Love
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fearon, R.M.P.; Roisman, G.I. Attachment theory: Progress and future directions. Curr. Opin. Psychol. 2017, 15, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S. The Role of Oxytocin and Vasopressin in Attachment. Psychodyn Psychiatry 2017, 45, 499–517. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R. The Neurobiology of Human Attachments. Trends. Cogn. Sci. 2017, 21, 80–99. [Google Scholar] [CrossRef] [PubMed]
- Gobrogge, K.L.; Jia, X.X.; Liu, Y.; Wang, Z.X. Neurochemical Mediation of Affiliation and Aggression Associated with Pair-Bonding. Biol. Psychiat. 2017, 81, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Stoop, R.; Hegoburu, C.; van den Burg, E. New Opportunities in Vasopressin and Oxytocin Research: A Perspective from the Amygdala. Annu. Rev. Neurosci. 2015, 38, 369–388. [Google Scholar] [CrossRef]
- He, Z.; Zhang, L.; Hou, W.; Zhang, X.; Young, L.J.; Li, L.; Liu, L.; Ma, H.; Xun, Y.; Lv, Z.; et al. Paraventricular Nucleus Oxytocin Subsystems Promote Active Paternal Behaviors in Mandarin Voles. J. Neurosci. 2021, 41, 6699–6713. [Google Scholar] [CrossRef]
- Song, Z.M.; Borland, J.M.; Larkin, T.E.; O’Malley, M.; Albers, H.E. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrino 2016, 74, 164–172. [Google Scholar] [CrossRef]
- Song, Z.M.; McCann, K.E.; McNeill, J.K.; Larkin, T.E.; Huhman, K.L.; Albers, H.E. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrino 2014, 50, 14–19. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Nemeroff, C.B.; Prange, A.J. Increase in Spontaneous Motor-Activity Following Infusion of Neurotensin into the Ventral Tegmental Area. Brain Res. 1981, 229, 525–529. [Google Scholar] [CrossRef]
- Kauer, J.A. Learning mechanisms in addiction: Synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev. Physiol. 2004, 66, 447–475. [Google Scholar] [CrossRef]
- Schultz, W.; Dayan, P.; Montague, P.R. A neural substrate of prediction and reward. Science 1997, 275, 1593–1599. [Google Scholar] [CrossRef]
- Watabe-Uchida, M.; Eshel, N.; Uchida, N. Neural Circuitry of Reward Prediction Error. Annu Rev. Neurosci 2017, 40, 373–394. [Google Scholar] [CrossRef]
- Carter, C.S. Oxytocin Pathways and the Evolution of Human Behavior. Annu Rev. Psychol. 2014, 65, 17–39. [Google Scholar] [CrossRef]
- Neumann, I.D.; Slattery, D.A. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol. Psychiat. 2016, 79, 213–221. [Google Scholar] [CrossRef]
- Floresco, S.B. The Nucleus Accumbens: An Interface between Cognition, Emotion, and Action. Annu. Rev. Psychol. 2015, 66, 25–52. [Google Scholar] [CrossRef]
- Numan, M.; Young, L.J. Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm. Behav. 2016, 77, 98–112. [Google Scholar] [CrossRef]
- Barrett, C.E.; Arambula, S.E.; Young, L.J. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl. Psychiat. 2015, 5, e606. [Google Scholar] [CrossRef]
- Bosch, O.J.; Dabrowska, J.; Modi, M.E.; Johnson, Z.V.; Keebaugh, A.C.; Barrett, C.E.; Ahern, T.H.; Guo, J.D.; Grinevich, V.; Rainnie, D.G.; et al. Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrino 2016, 64, 66–78. [Google Scholar] [CrossRef]
- Baez-Mendoza, R.; Schultz, W. The role of the striatum in social behavior. Front. Neurosci. Switz. 2013, 7, 233. [Google Scholar] [CrossRef]
- Schore, A.N. Relational Trauma and the Developing Right Brain An Interface of Psychoanalytic Self Psychology and Neuroscience. Ann. N. Y. Acad. Sci. 2009, 1159, 189–203. [Google Scholar] [CrossRef]
- Bartels, A.; Zeki, S. The neural basis of romantic love. Neuroreport 2000, 11, 3829–3834. [Google Scholar] [CrossRef]
- Bartels, A.; Zeki, S. The neural correlates of maternal and romantic love. Neuroimage 2004, 21, 1155–1166. [Google Scholar] [CrossRef]
- Ortigue, S.; Bianchi-Demicheli, F.; Patel, N.; Frum, C.; Lewis, J.W. Neuroimaging of Love: fMRI Meta-Analysis Evidence toward New Perspectives in Sexual Medicine. J. Sex Med. 2010, 7, 3541–3552. [Google Scholar] [CrossRef]
- Watanuki, S.; Akama, H. Neural Substrates of Brand Love: An Activation Likelihood Estimation Meta-Analysis of Functional Neuroimaging Studies. Front. Neurosci. Switz. 2020, 14, 973. [Google Scholar] [CrossRef]
- Hatfield, E.; Sprecher, S. Measuring passionate love in intimate relationships. J. Adolesc. 1986, 9, 383–410. [Google Scholar] [CrossRef]
- Acevedo, B.P.; Aron, A.; Fisher, H.E.; Brown, L.L. Neural correlates of long-term intense romantic love. Soc. Cogn. Affect. Neurosci. 2012, 7, 145–159. [Google Scholar] [CrossRef]
- Lorberbaum, J.P.; Newman, J.D.; Horwitz, A.R.; Dubno, J.R.; Lydiard, R.B.; Hamner, M.B.; Bohning, D.E.; George, M.S. A potential role for thalamocingulate circuitry in human maternal behavior. Biol. Psychiat. 2002, 51, 431–445. [Google Scholar] [CrossRef]
- Nitschke, J.B.; Nelson, E.E.; Rusch, B.D.; Fox, A.S.; Oakes, T.R.; Davidson, R.J. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage 2004, 21, 583–592. [Google Scholar] [CrossRef]
- Leibenluft, E.; Gobbini, M.I.; Harrison, T.; Haxby, J.V. Mothers’ neural activation in response to pictures of their children and other children. Biol. Psychiat. 2004, 56, 225–232. [Google Scholar] [CrossRef]
- Ranote, S.; Elliott, R.; Abel, K.M.; Mitchell, R.; Deaking, J.F.W.; Appleby, L. The neural basis of maternal responsiveness to infants: An fMRI study. Neuroreport 2004, 15, 1825–1829. [Google Scholar]
- Noriuchi, M.; Kikuchi, Y.; Senoo, A. The functional neuroanatomy of maternal love: Mother’s response to infant’s attachment behaviors. Biol. Psychiat. 2008, 63, 415–423. [Google Scholar] [CrossRef]
- Lenzi, D.; Trentini, C.; Pantano, P.; Macaluso, E.; Iacoboni, M.; Lenzi, G.L.; Ammaniti, M. Neural Basis of Maternal Communication and Emotional Expression Processing during Infant Preverbal Stage. Cereb. Cortex. 2009, 19, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Strathearn, L.; Li, J.; Fonagy, P.; Montague, P.R. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics 2008, 122, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Strathearn, L.; Fonagy, P.; Amico, J.; Montague, P.R. Adult Attachment Predicts Maternal Brain and Oxytocin Response to Infant Cues. Neuropsychopharmacol 2009, 34, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Atzil, S.; Hendler, T.; Feldman, R. Specifying the Neurobiological Basis of Human Attachment: Brain, Hormones, and Behavior in Synchronous and Intrusive Mothers. Neuropsychopharmacol 2011, 36, 2603–2615. [Google Scholar] [CrossRef]
- Barrett, J.; Wonch, K.E.; Gonzalez, A.; Ali, N.; Steiner, M.; Hall, G.B.; Fleming, A.S. Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Soc. Neurosci. UK 2012, 7, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.W.; Downey, D.; Strachan, H.; Elliott, R.; Williams, S.R.; Abel, K.M. The neural basis of maternal bonding. PLoS ONE 2014, 9, e88436. [Google Scholar] [CrossRef]
- Aron, A.; Fisher, H.; Mashek, D.J.; Strong, G.; Li, H.F.; Brown, L.L. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J. Neurophysiol. 2005, 94, 327–337. [Google Scholar] [CrossRef]
- Ortigue, S.; Bianchi-Demicheli, F.; Hamilton, A.F.D.C.; Grafton, S.T. The neural basis of love as a subliminal prime: An event-related functional magnetic resonance imaging study. J. Cognitive. Neurosci. 2007, 19, 1218–1230. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.; Jeong, J.; Lee, K.U.; Ahn, K.J.; Chung, Y.A.; Hong, K.Y.; Chae, J.H. Temporal Changes in Functional Magnetic Resonance Imaging Activation of Heterosexual Couples for Visual Stimuli of Loved Partners. Psychiat. Invest 2009, 6, 19–25. [Google Scholar] [CrossRef]
- Zeki, S.; Romaya, J.P. The brain reaction to viewing faces of opposite- and same-sex romantic partners. PLoS ONE 2010, 5, e15802. [Google Scholar] [CrossRef][Green Version]
- Stoessel, C.; Stiller, J.; Bleich, S.; Boensch, D.; Doerfler, A.; Garcia, M.; Richter-Schmidinger, T.; Kornhuber, J.; Forster, C. Differences and Similarities on Neuronal Activities of People Being Happily and Unhappily in Love: A Functional Magnetic Resonance Imaging Study. Neuropsychobiology 2011, 64, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Aron, A.; Brown, L.; Cao, G.; Feng, T.; Weng, X. Reward and motivation systems: A brain mapping study of early-stage intense romantic love in Chinese participants. Hum. Brain Mapp. 2011, 32, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, J.; Aron, A.; Lei, W.; Westmaas, J.L.; Weng, X. Intense passionate love attenuates cigarette cue-reactivity in nicotine-deprived smokers: An FMRI study. PLoS ONE 2012, 7, e42235. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P.T. Activation likelihood estimation meta-analysis revisited. Neuroimage 2012, 59, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Laird, A.R.; Grefkes, C.; Wang, L.E.; Zilles, K.; Fox, P.T. Coordinate-Based Activation Likelihood Estimation Meta-Analysis of Neuroimaging Data: A Random-Effects Approach Based on Empirical Estimates of Spatial Uncertainty. Hum. Brain Mapp. 2009, 30, 2907–2926. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eickhoff, S.B.; Laird, A.R.; Fox, M.; Wiener, M.; Fox, P. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum. Brain Mapp. 2012, 33, 1–13. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eden, G.F.; Jones, K.M.; Zeffiro, T.A. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. Neuroimage 2002, 16, 765–780. [Google Scholar] [CrossRef]
- Laird, A.R.; Fox, P.M.; Price, C.J.; Glahn, D.C.; Uecker, A.M.; Lancaster, J.L.; Turkeltaub, P.E.; Kochunov, P.; Fox, P.T. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Hum. Brain Mapp. 2005, 25, 155–164. [Google Scholar] [CrossRef]
- Adolphs, R.; Damasio, H.; Tranel, D.; Cooper, G.; Damasio, A.R. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J. Neuroscience 2000, 20, 2683–2690. [Google Scholar] [CrossRef]
- Gallagher, H.L.; Frith, C.D. Functional imaging of ‘theory of mind’. Trends Cogn. Sci. 2003, 7, 77–83. [Google Scholar] [CrossRef]
- Schurz, M.; Radua, J.; Aichhorn, M.; Richlan, F.; Perner, J. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci. Biobehav. R 2014, 42, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Austin, J.; Elliott, R.; Ellison-Wright, I.; Wan, M.W.; Drake, R.; Downey, D.; Elmadih, A.; Mukherjee, I.; Heaney, L.; et al. Neural pathways of maternal responding: Systematic review and meta-analysis. Arch. Women Ment. Hlth. 2019, 22, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Arnow, B.A.; Desmond, J.E.; Banner, L.L.; Glover, G.H.; Solomon, A.; Polan, M.L.; Lue, T.F.; Atlas, S.W. Brain activation and sexual arousal in healthy, heterosexual males. Brain 2002, 125, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.; Newman, J.L.; Longe, O.A.; Deakin, J.F.W. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: A parametric functional magnetic resonance imaging study. J. Neuroscience. 2003, 23, 303–307. [Google Scholar] [CrossRef]
- Harsanyi, A.; Csigo, K.; Demeter, G.; Nemeth, A. New approach to obsessive-compulsive disorder: Dopaminergic theories. Psychiatr. Hung. 2007, 22, 248–258. [Google Scholar]
- Arias-Carrion, O.; Stamelou, M.; Murillo-Rodriguez, E.; Menendez-Gonzalez, M.; Poppel, E. Dopaminergic reward system: A short integrative review. Int. Arch. Med. 2010, 3, 24. [Google Scholar] [CrossRef]
- Donaldson, Z.R.; Young, L.J. Oxytocin, vasopressin, and the neurogenetics of sociality. Science 2008, 322, 900–904. [Google Scholar] [CrossRef]
- Caldwell, H.K.; Aulino, E.A.; Freeman, A.R.; Miller, T.V.; Witchey, S.K. Oxytocin and behavior: Lessons from knockout mice. Dev. Neurobiol. 2017, 77, 190–201. [Google Scholar] [CrossRef]
- Shahrokh, D.K.; Zhang, T.Y.; Diorio, J.; Gratton, A.; Meaney, M.J. Oxytocin-Dopamine Interactions Mediate Variations in Maternal Behavior in the Rat. Endocrinology 2010, 151, 2276–2286. [Google Scholar] [CrossRef]
- Douglas, A.J. Baby love? Oxytocin-dopamine interactions in mother-infant bonding. Endocrinology 2010, 151, 1978–1980. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.X. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 2003, 121, 537–544. [Google Scholar] [CrossRef]

| (A) | ||||||||
| First Author | Year | Categories of Love | Numbers of Participants | Mean Age of Participants’ Own Child | Mean Age of Participants | Experimental Stimuli | Contrasts | Numbers of Foci |
| Lorberbaum [27] | 2002 | Maternal | 10 | 6.18 weeks | 30.55 | Cry sound and white noise (auditory) | Infant cry > Rest | 80 |
| Bartles [22] | 2004 | Maternal | 20 | 24.4 months | 34 | Pictures (visual) | Own child > Acquainted child | 28 |
| Nitschke [28] | 2004 | Maternal | 6 | 3–5 months | No report | Pictures of faces (visual) | Own infant > Unfamiliar infant | 6 |
| Leibenluft [29] | 2004 | Maternal | 7 | 5–12 years | 32.9 | Pictures of faces (visual) | Own child > Familiar child | 36 |
| Ranote [30] | 2004 | Maternal | 10 | 25.6 months | 26 | Video Clip (visual-auditory) | Own infant > Unknown infant | 3 |
| Noriuchi [31] | 2008 | Maternal | 13 | 16.5 months | 31.1 | Video Clip (visual-auditory) | Own infant > Other infant | 81 |
| Lenzi [32] | 2009 | Maternal | 16 | 9.5 months | 33.7 | Pictures of faces (visual) | Own child > Acquainted child | 7 |
| Strathearn [33] | 2008 | Maternal | 28 | 6.7 months | 30.2 | Pictures of faces (visual) | Own infant > Unknown infant | 67 |
| Strathearn [34] | 2009 | Maternal | 30 | 7 months | No report | Pictures of faces (visual) | Own infant > Unknown infant | 46 |
| Atzil [35] | 2011 | Maternal | 23 | 4–6 months | 22–37 | Pictures of faces (visual) | Own infant > Unfamiliar infant | 21 |
| Barret [36] | 2012 | Maternal | 22 | 3 months | 25–35 | Pictures of faces (visual) | Own infant > Unfamiliar infant | 32 |
| Wan [37] | 2014 | Maternal | 20 | 6.2 months | 32 | Video Clip (visual-auditory) | Own infant > Unknown infant | 40 |
| (B) | ||||||||
| First Author | Year | Categories of Love | Numbers of Participants (Female) | Mean Age of Participants | Experimental Stimuli | Contrasts | Numbers of Foci | |
| Bartels & Zeki [21] | 2000 | Passionate | 17 (11) | 24.5 | Pictures of faces (visual) | Lover > Familiar friend | 13 | |
| Aron [38] | 2005 | Passionate | 17 (10) | 20.6 | Picture (visual) | Lover > Familiar friend | 8 | |
| Ortigue [39] | 2007 | Passionate | 36 (36) | 20.1 | Words (visual) | Lover > Familiar friend Lover > Noun | 14 4 | |
| Kim [40] | 2009 | Passionate | 10 (5) | 21.1 | Pictures of faces (visual) | Lover > Familiar friend | 29 | |
| Zeki [41] | 2010 | Passionate (opposite and same sex) | 24 (12) | 26.3 | Pictures of faces (visual) | Lover > Familiar friend | 11 | |
| Stoessel [42] | 2011 | Passionate | 12 (6) | 24.08 | Picture (visual) | Lover > Erotic pictures | 16 | |
| Xu [43] | 2011 | Passionate (Chinese participants) | 18 (10) | 21.61 | Pictures of faces (visual) | Lover > Familiar friend | 8 | |
| Xu [44] | 2012 | Passionate | 18 (0) | 25.11 | Picture (visual) | Lover > Familiar friend | 10 | |
| Acevedo [26] | 2012 | Passionate (long-term) | 17 (10) | 52.85 | Pictures of faces (visual) | Lover > Familiar acquaintance Lover > Close friend | 30 26 | |
| Brain Regions | BA | MNI Coordinates | Voxels | Extrema Value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Left ventral tegmental area | - | −2 0 | −14 −24 | −20 −12 | 360 488 | 0.02679 # 0.03247 * |
| Right thalamus | - | 10 4 | −18 −8 | 0 −2 | 440 80 | 0.04450 * 0.03028 * |
| Left substantia nigra | - | −8 | −24 | −14 | 264 | 0.03697 * |
| Left putamen | - | −22 −28 | 4 −14 | 0 −4 | 240 184 | 0.04104 * 0.03775 * |
| Right medial prefrontal gyrus | 10 | 8 | 64 | 14 | 144 | 0.03046 # |
| Right superior temporal gyrus | 21 | 58 | −6 | −18 | 80 | 0.02490 # |
| Right middle temporal gyrus | 21 | 62 | −4 | −14 | 312 | 0.02606 # |
| Right putamen | - | 28 | −16 | 6 | 120 | 0.03611 # |
| Left amygdala | - | −32 | −6 | −18 | 312 | 0.03551 # |
| Brain Regions | MNI Coordinates | Voxels | Extrema Value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Left ventral tegmental area | −2 | −14 | −10 | 184 | 0.03104 * |
| Right ventral tegmental area | 2 | −22 | −16 | 480 | −0.02994 * |
| Brain Regions | MNI Coordinates | Voxels | Extrema Value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Maternal > Passionate love | |||||
| Left putamen | −22 | 4 | −4 | 184 | 0.02739 |
| Passionate > Maternal love | |||||
| No significance | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, H.-C.; Kuo, M.-E.; Wu, C.W.; Chao, Y.-P.; Huang, H.-W.; Huang, C.-M. The Neurobiological Basis of Love: A Meta-Analysis of Human Functional Neuroimaging Studies of Maternal and Passionate Love. Brain Sci. 2022, 12, 830. https://doi.org/10.3390/brainsci12070830
Shih H-C, Kuo M-E, Wu CW, Chao Y-P, Huang H-W, Huang C-M. The Neurobiological Basis of Love: A Meta-Analysis of Human Functional Neuroimaging Studies of Maternal and Passionate Love. Brain Sciences. 2022; 12(7):830. https://doi.org/10.3390/brainsci12070830
Chicago/Turabian StyleShih, Hsuan-Chu, Mu-En Kuo, Changwei W. Wu, Yi-Ping Chao, Hsu-Wen Huang, and Chih-Mao Huang. 2022. "The Neurobiological Basis of Love: A Meta-Analysis of Human Functional Neuroimaging Studies of Maternal and Passionate Love" Brain Sciences 12, no. 7: 830. https://doi.org/10.3390/brainsci12070830
APA StyleShih, H.-C., Kuo, M.-E., Wu, C. W., Chao, Y.-P., Huang, H.-W., & Huang, C.-M. (2022). The Neurobiological Basis of Love: A Meta-Analysis of Human Functional Neuroimaging Studies of Maternal and Passionate Love. Brain Sciences, 12(7), 830. https://doi.org/10.3390/brainsci12070830





