Evidence of Neuroplastic Changes after Transcranial Magnetic, Electric, and Deep Brain Stimulation
Abstract
:1. Introduction
2. Transcranial Magnetic Stimulation (TMS)
2.1. Overview of TMS Methods
2.2. Neuroplasticity
2.2.1. After-Effects of TMS: Changes in MEPs
2.2.2. Combining TMS with EEG
2.2.3. After-Effects Revealed by PET and MR Imaging
2.2.4. Pharmacological Evidence
2.2.5. Behavioral and Therapeutic Evidence
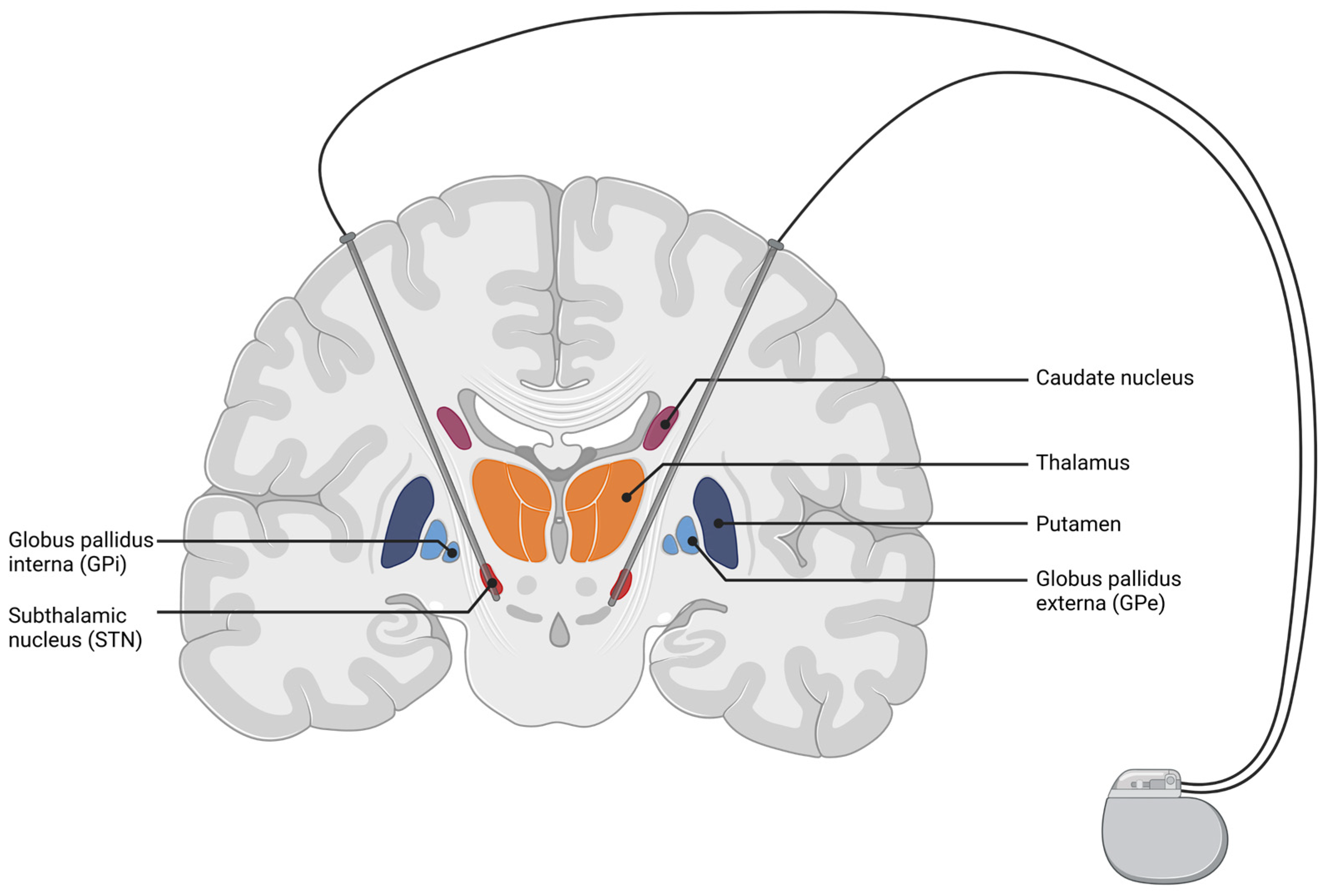
3. Deep Brain Stimulation (DBS)
3.1. Overview of DBS Methods
3.2. Studies Demonstrating Effects of Neuroplasticity
3.2.1. Evidence for Neuroplasticity in Essential Tremor
3.2.2. Evidence for Neuroplasticity in Parkinson’s Disease
3.2.3. Evidence for Neuroplasticity in Dystonia
4. Transcranial Electric Stimulation (tES)
4.1. Overview of tES Methods
4.2. Assumed Neuronal Mechanisms
4.2.1. tDCS
4.2.2. tACS
4.2.3. tRNS
5. Comparison of Methods Regarding Neuronal Plasticity
Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Griesbach, G.S.; Hovda, D.A. Cellular and Molecular Neuronal Plasticity. Handb. Clin. Neurol. 2015, 128, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Berlucchi, G.; Buchtel, H.A. Neuronal Plasticity: Historical Roots and Evolution of Meaning. Exp. Brain Res. 2009, 192, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Draganski, B.; Gaser, C.; Kempermann, G.; Kuhn, H.G.; Winkler, J.; Büchel, C.; May, A. Temporal and Spatial Dynamics of Brain Structure Changes during Extensive Learning. J. Neurosci. 2006, 26, 6314–6317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holtmaat, A.J.G.D.; Trachtenberg, J.T.; Wilbrecht, L.; Shepherd, G.M.; Zhang, X.; Knott, G.W.; Svoboda, K. Transient and Persistent Dendritic Spines in the Neocortex in Vivo. Neuron 2005, 45, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Lanza, G.; DelRosso, L.M.; Ferri, R. Sleep and Homeostatic Control of Plasticity. Handb. Clin. Neurol. 2022, 184, 53–72. [Google Scholar] [CrossRef]
- Arcos-Burgos, M.; Lopera, F.; Sepulveda-Falla, D.; Mastronardi, C. Neural Plasticity during Aging. Neural Plast. 2019, 2019, 6042132. [Google Scholar] [CrossRef]
- Timmermans, W.; Xiong, H.; Hoogenraad, C.C.; Krugers, H.J. Stress and Excitatory Synapses: From Health to Disease. Neuroscience 2013, 248, 626–636. [Google Scholar] [CrossRef]
- Mendez Colmenares, A.; Voss, M.W.; Fanning, J.; Salerno, E.A.; Gothe, N.P.; Thomas, M.L.; McAuley, E.; Kramer, A.F.; Burzynska, A.Z. White Matter Plasticity in Healthy Older Adults: The Effects of Aerobic Exercise. Neuroimage 2021, 239, 118305. [Google Scholar] [CrossRef]
- Mitterová, K.; Klobušiaková, P.; Šejnoha Minsterová, A.; Kropáčová, S.; Balážová, Z.; Točík, J.; Vaculíková, P.; Skotáková, A.; Grmela, R.; Rektorová, I. Impact of Cognitive Reserve on Dance Intervention-Induced Changes in Brain Plasticity. Sci. Rep. 2021, 11, 18527. [Google Scholar] [CrossRef]
- Shigihara, Y.; Hoshi, H.; Shinada, K.; Okada, T.; Kamada, H. Non-Pharmacological Treatment Changes Brain Activity in Patients with Dementia. Sci. Rep. 2020, 10, 6744. [Google Scholar] [CrossRef] [Green Version]
- Minzenberg, M.J.; Leuchter, A.F. The Effect of Psychotropic Drugs on Cortical Excitability and Plasticity Measured with Transcranial Magnetic Stimulation: Implications for Psychiatric Treatment. J. Affect. Disord. 2019, 253, 126–140. [Google Scholar] [CrossRef]
- Zhuang, X.; Mazzoni, P.; Kang, U.J. The Role of Neuroplasticity in Dopaminergic Therapy for Parkinson Disease. Nat. Rev. Neurol. 2013, 9, 248–256. [Google Scholar] [CrossRef]
- Barker, A.T.; Jalinous, R.; Freeston, I.L. Non-Invasive Magnetic Stimulation of Human Motor Cortex. Lancet 1985, 1, 1106–1107. [Google Scholar] [CrossRef]
- Hallett, M. Transcranial Magnetic Stimulation: A Primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Tsodyks, M.; Wu, S. Short-Term Synaptic Plasticity. Scholarpedia 2013, 8, 3153. [Google Scholar] [CrossRef]
- Brown, J.C.; Higgins, E.S.; George, M.S. Synaptic Plasticity 101: The Story of the AMPA Receptor for the Brain Stimulation Practitioner. Neuromodulation Technol. Neural Interface 2022, in press. [Google Scholar] [CrossRef]
- Wagner, T.; Valero-Cabre, A.; Pascual-Leone, A. Noninvasive Human Brain Stimulation. Annu. Rev. Biomed. Eng. 2007, 9, 527–565. [Google Scholar] [CrossRef] [Green Version]
- Rothwell, J.C.; Hallett, M.; Berardelli, A.; Eisen, A.; Rossini, P.; Paulus, W. Magnetic Stimulation: Motor Evoked Potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 97–103. [Google Scholar]
- Cohen, S.L.; Bikson, M.; Badran, B.W.; George, M.S. A Visual and Narrative Timeline of US FDA Milestones for Transcranial Magnetic Stimulation (TMS) Devices. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2022, 15, 73–75. [Google Scholar] [CrossRef]
- Snow, N.J.; Wadden, K.P.; Chaves, A.R.; Ploughman, M. Transcranial Magnetic Stimulation as a Potential Biomarker in Multiple Sclerosis: A Systematic Review with Recommendations for Future Research. Neural Plast. 2019, 2019, e6430596. [Google Scholar] [CrossRef] [Green Version]
- Amassian, V.E.; Cracco, R.Q.; Maccabee, P.J.; Cracco, J.B.; Rudell, A.P.; Eberle, L. Transcranial Magnetic Stimulation in Study of the Visual Pathway. J. Clin. Neurophysiol. 1998, 15, 288–304. [Google Scholar] [CrossRef] [Green Version]
- Ilmoniemi, R.J.; Virtanen, J.; Ruohonen, J.; Karhu, J.; Aronen, H.J.; Näätänen, R.; Katila, T. Neuronal Responses to Magnetic Stimulation Reveal Cortical Reactivity and Connectivity. Neuroreport 1997, 8, 3537–3540. [Google Scholar] [CrossRef] [Green Version]
- Modak, A.; Fitzgerald, P.B. Personalising Transcranial Magnetic Stimulation for Depression Using Neuroimaging: A Systematic Review. World J. Biol. Psychiatry 2021, 22, 647–669. [Google Scholar] [CrossRef]
- Aydogan, D.B.; Souza, V.H.; Lioumis, P.; Ilmoniemi, R.J. Towards Real-Time Tractography-Based TMS Neuronavigation. Brain Stimul. 2021, 14, 1609. [Google Scholar] [CrossRef]
- Ziemann, U. TMS Induced Plasticity in Human Cortex. Rev. Neurosci. 2004, 15, 253–266. [Google Scholar] [CrossRef]
- Ziemann, U.; Paulus, W.; Nitsche, M.A.; Pascual-Leone, A.; Byblow, W.D.; Berardelli, A.; Siebner, H.R.; Classen, J.; Cohen, L.G.; Rothwell, J.C. Consensus: Motor Cortex Plasticity Protocols. Brain Stimul. 2008, 1, 164–182. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Valls-Solé, J.; Wassermann, E.M.; Hallett, M. Responses to Rapid-Rate Transcranial Magnetic Stimulation of the Human Motor Cortex. Brain 1994, 117 Pt 4, 847–858. [Google Scholar] [CrossRef]
- Eldaief, M.C.; Halko, M.A.; Buckner, R.L.; Pascual-Leone, A. Transcranial Magnetic Stimulation Modulates the Brain’s Intrinsic Activity in a Frequency-Dependent Manner. Proc. Natl. Acad. Sci. USA 2011, 108, 21229–21234. [Google Scholar] [CrossRef] [Green Version]
- Raij, T.; Nummenmaa, A.; Marin, M.-F.; Porter, D.; Furtak, S.; Setsompop, K.; Milad, M.R. Prefrontal Cortex Stimulation Enhances Fear Extinction Memory in Humans. Biol. Psychiatry 2018, 84, 129–137. [Google Scholar] [CrossRef]
- Siebner, H.R.; Bergmann, T.O.; Bestmann, S.; Massimini, M.; Johansen-Berg, H.; Mochizuki, H.; Bohning, D.E.; Boorman, E.D.; Groppa, S.; Miniussi, C.; et al. Consensus Paper: Combining Transcranial Stimulation with Neuroimaging. Brain Stimul. 2009, 2, 58–80. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.X.; Rogers, L.M.; Gross, E.Z.; Ryals, A.J.; Dokucu, M.E.; Brandstatt, K.L.; Hermiller, M.S.; Voss, J.L. Targeted Enhancement of Cortical-Hippocampal Brain Networks and Associative Memory. Science 2014, 345, 1054–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefaucheur, J.-P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-Based Guidelines on the Therapeutic Use of Repetitive Transcranial Magnetic Stimulation (RTMS): An Update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef] [PubMed]
- Kiebs, M.; Hurlemann, R.; Mutz, J. Repetitive Transcranial Magnetic Stimulation in Non-Treatment-Resistant Depression. Br. J. Psychiatry 2019, 215, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Mutz, J.; Vipulananthan, V.; Carter, B.; Hurlemann, R.; Fu, C.H.Y.; Young, A.H. Comparative Efficacy and Acceptability of Non-Surgical Brain Stimulation for the Acute Treatment of Major Depressive Episodes in Adults: Systematic Review and Network Meta-Analysis. BMJ 2019, 364, l1079. [Google Scholar] [CrossRef] [Green Version]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef]
- Freitas, C.; Farzan, F.; Pascual-Leone, A. Assessing Brain Plasticity across the Lifespan with Transcranial Magnetic Stimulation: Why, How, and What Is the Ultimate Goal? Front. Neurosci. 2013, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Kujirai, T.; Caramia, M.D.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Ferbert, A.; Wroe, S.; Asselman, P.; Marsden, C.D. Corticocortical Inhibition in Human Motor Cortex. J. Physiol. 1993, 471, 501–519. [Google Scholar] [CrossRef]
- Turco, C.V.; Nelson, A.J. Transcranial Magnetic Stimulation to Assess Exercise-Induced Neuroplasticity. Front. Neuroergonomics 2021, 2, 17. [Google Scholar] [CrossRef]
- Chen, R.; Classen, J.; Gerloff, C.; Celnik, P.; Wassermann, E.M.; Hallett, M.; Cohen, L.G. Depression of Motor Cortex Excitability by Low-Frequency Transcranial Magnetic Stimulation. Neurology 1997, 48, 1398–1403. [Google Scholar] [CrossRef]
- Berardelli, A.; Inghilleri, M.; Rothwell, J.C.; Romeo, S.; Currà, A.; Gilio, F.; Modugno, N.; Manfredi, M. Facilitation of Muscle Evoked Responses after Repetitive Cortical Stimulation in Man. Exp. Brain Res. 1998, 122, 79–84. [Google Scholar] [CrossRef]
- Maeda, F.; Keenan, J.P.; Tormos, J.M.; Topka, H.; Pascual-Leone, A. Modulation of Corticospinal Excitability by Repetitive Transcranial Magnetic Stimulation. Clin. Neurophysiol. 2000, 111, 800–805. [Google Scholar] [CrossRef]
- Peinemann, A.; Reimer, B.; Löer, C.; Quartarone, A.; Münchau, A.; Conrad, B.; Siebner, H.R. Long-Lasting Increase in Corticospinal Excitability after 1800 Pulses of Subthreshold 5 Hz Repetitive TMS to the Primary Motor Cortex. Clin. Neurophysiol. 2004, 115, 1519–1526. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Fountain, S.; Daskalakis, Z.J. A Comprehensive Review of the Effects of RTMS on Motor Cortical Excitability and Inhibition. Clin. Neurophysiol. 2006, 117, 2584–2596. [Google Scholar] [CrossRef]
- Rothkegel, H.; Sommer, M.; Paulus, W. Breaks during 5Hz RTMS Are Essential for Facilitatory after Effects. Clin. Neurophysiol. 2010, 121, 426–430. [Google Scholar] [CrossRef]
- Touge, T.; Gerschlager, W.; Brown, P.; Rothwell, J.C. Are the After-Effects of Low-Frequency RTMS on Motor Cortex Excitability Due to Changes in the Efficacy of Cortical Synapses? Clin. Neurophysiol. 2001, 112, 2138–2145. [Google Scholar] [CrossRef]
- Tang, Z.; Xuan, C.; Li, X.; Dou, Z.; Lan, Y.; Wen, H. Effect of Different Pulse Numbers of Transcranial Magnetic Stimulation on Motor Cortex Excitability: Single-blind, Randomized Cross-over Design. CNS Neurosci. Ther. 2019, 25, 1277–1281. [Google Scholar] [CrossRef] [Green Version]
- McCalley, D.M.; Lench, D.H.; Doolittle, J.D.; Imperatore, J.P.; Hoffman, M.; Hanlon, C.A. Determining the Optimal Pulse Number for Theta Burst Induced Change in Cortical Excitability. Sci. Rep. 2021, 11, 8726. [Google Scholar] [CrossRef]
- Gamboa, O.L.; Antal, A.; Moliadze, V.; Paulus, W. Simply Longer Is Not Better: Reversal of Theta Burst after-Effect with Prolonged Stimulation. Exp. Brain Res. 2010, 204, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.H.; Shin, J.E.; Jeong, Y.-S.; Shin, H.-I. Changes in Motor Cortical Excitability Induced by High-Frequency Repetitive Transcranial Magnetic Stimulation of Different Stimulation Durations. Clin. Neurophysiol. 2008, 119, 71–79. [Google Scholar] [CrossRef]
- Rossini, P.M.; Barker, A.T.; Berardelli, A.; Caramia, M.D.; Caruso, G.; Cracco, R.Q.; Dimitrijević, M.R.; Hallett, M.; Katayama, Y.; Lücking, C.H.; et al. Non-Invasive Electrical and Magnetic Stimulation of the Brain, Spinal Cord and Roots: Basic Principles and Procedures for Routine Clinical Application. Report of an IFCN Committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 79–92. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Brown, T.L.; Daskalakis, Z.J.; Chen, R.; Kulkarni, J. Intensity-Dependent Effects of 1 Hz RTMS on Human Corticospinal Excitability. Clin. Neurophysiol. 2002, 113, 1136–1141. [Google Scholar] [CrossRef]
- Modugno, N.; Nakamura, Y.; MacKinnon, C.; Filipovic, S.; Bestmann, S.; Berardelli, A.; Rothwell, J. Motor Cortex Excitability Following Short Trains of Repetitive Magnetic Stimuli. Exp. Brain Res. 2001, 140, 453–459. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta Burst Stimulation of the Human Motor Cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Larson, J.; Wong, D.; Lynch, G. Patterned Stimulation at the Theta Frequency Is Optimal for the Induction of Hippocampal Long-Term Potentiation. Brain Res. 1986, 368, 347–350. [Google Scholar] [CrossRef]
- Cárdenas-Morales, L.; Nowak, D.A.; Kammer, T.; Wolf, R.C.; Schönfeldt-Lecuona, C. Mechanisms and Applications of Theta-Burst RTMS on the Human Motor Cortex. Brain Topogr. 2010, 22, 294–306. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.E.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of Theta Burst versus High-Frequency Repetitive Transcranial Magnetic Stimulation in Patients with Depression (THREE-D): A Randomised Non-Inferiority Trial. Lancet 2018, 391, 1683–1692. [Google Scholar] [CrossRef]
- Stefan, K.; Kunesch, E.; Cohen, L.G.; Benecke, R.; Classen, J. Induction of Plasticity in the Human Motor Cortex by Paired Associative Stimulation. Brain 2000, 123 Pt 3, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Stefan, K.; Kunesch, E.; Benecke, R.; Cohen, L.G.; Classen, J. Mechanisms of Enhancement of Human Motor Cortex Excitability Induced by Interventional Paired Associative Stimulation. J. Physiol. 2002, 543, 699–708. [Google Scholar] [CrossRef]
- Wolters, A.; Sandbrink, F.; Schlottmann, A.; Kunesch, E.; Stefan, K.; Cohen, L.G.; Benecke, R.; Classen, J. A Temporally Asymmetric Hebbian Rule Governing Plasticity in the Human Motor Cortex. J. Neurophysiol. 2003, 89, 2339–2345. [Google Scholar] [CrossRef]
- Feldman, D.E. The Spike-Timing Dependence of Plasticity. Neuron 2012, 75, 556–571. [Google Scholar] [CrossRef] [Green Version]
- Arai, N.; Müller-Dahlhaus, F.; Murakami, T.; Bliem, B.; Lu, M.-K.; Ugawa, Y.; Ziemann, U. State-Dependent and Timing-Dependent Bidirectional Associative Plasticity in the Human SMA-M1 Network. J. Neurosci. 2011, 31, 15376–15383. [Google Scholar] [CrossRef] [PubMed]
- Buch, E.R.; Johnen, V.M.; Nelissen, N.; O’Shea, J.; Rushworth, M.F.S. Noninvasive Associative Plasticity Induction in a Corticocortical Pathway of the Human Brain. J. Neurosci. 2011, 31, 17669–17679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udupa, K.; Bahl, N.; Ni, Z.; Gunraj, C.; Mazzella, F.; Moro, E.; Hodaie, M.; Lozano, A.M.; Lang, A.E.; Chen, R. Cortical Plasticity Induction by Pairing Subthalamic Nucleus Deep-Brain Stimulation and Primary Motor Cortical Transcranial Magnetic Stimulation in Parkinson’s Disease. J. Neurosci. 2016, 36, 396–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shulga, A.; Lioumis, P.; Kirveskari, E.; Savolainen, S.; Mäkelä, J.P. A Novel Paired Associative Stimulation Protocol with a High-Frequency Peripheral Component: A Review on Results in Spinal Cord Injury Rehabilitation. Eur. J. Neurosci. 2021, 53, 3242–3257. [Google Scholar] [CrossRef]
- Esser, S.K.; Huber, R.; Massimini, M.; Peterson, M.J.; Ferrarelli, F.; Tononi, G. A Direct Demonstration of Cortical LTP in Humans: A Combined TMS/EEG Study. Brain Res. Bull. 2006, 69, 86–94. [Google Scholar] [CrossRef]
- Casarotto, S.; Lauro, L.J.R.; Bellina, V.; Casali, A.G.; Rosanova, M.; Pigorini, A.; Defendi, S.; Mariotti, M.; Massimini, M. EEG Responses to TMS Are Sensitive to Changes in the Perturbation Parameters and Repeatable over Time. PLoS ONE 2010, 5, e10281. [Google Scholar] [CrossRef] [Green Version]
- Lioumis, P.; Kicić, D.; Savolainen, P.; Mäkelä, J.P.; Kähkönen, S. Reproducibility of TMS-Evoked EEG Responses. Hum. Brain Mapp. 2009, 30, 1387–1396. [Google Scholar] [CrossRef]
- Kerwin, L.J.; Keller, C.J.; Wu, W.; Narayan, M.; Etkin, A. Test-Retest Reliability of Transcranial Magnetic Stimulation EEG Evoked Potentials. Brain Stimul. 2018, 11, 536–544. [Google Scholar] [CrossRef]
- Casula, E.P.; Tarantino, V.; Basso, D.; Arcara, G.; Marino, G.; Toffolo, G.M.; Rothwell, J.C.; Bisiacchi, P.S. Low-Frequency RTMS Inhibitory Effects in the Primary Motor Cortex: Insights from TMS-Evoked Potentials. NeuroImage 2014, 98, 225–232. [Google Scholar] [CrossRef]
- Van Der Werf, Y.D.; Paus, T. The Neural Response to Transcranial Magnetic Stimulation of the Human Motor Cortex. I. Intracortical and Cortico-Cortical Contributions. Exp. Brain Res. 2006, 175, 231–245. [Google Scholar] [CrossRef]
- Vernet, M.; Bashir, S.; Yoo, W.-K.; Perez, J.M.; Najib, U.; Pascual-Leone, A. Insights on the Neural Basis of Motor Plasticity Induced by Theta Burst Stimulation from TMS–EEG. Eur. J. Neurosci. 2013, 37, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Veniero, D.; Maioli, C.; Miniussi, C. Potentiation of Short-Latency Cortical Responses by High-Frequency Repetitive Transcranial Magnetic Stimulation. J. Neurophysiol. 2010, 104, 1578–1588. [Google Scholar] [CrossRef] [Green Version]
- Komssi, S.; Kähkönen, S.; Ilmoniemi, R.J. The Effect of Stimulus Intensity on Brain Responses Evoked by Transcranial Magnetic Stimulation. Hum. Brain Mapp. 2004, 21, 154–164. [Google Scholar] [CrossRef]
- Komssi, S.; Kähkönen, S. The Novelty Value of the Combined Use of Electroencephalography and Transcranial Magnetic Stimulation for Neuroscience Research. Brain Res. Rev. 2006, 52, 183–192. [Google Scholar] [CrossRef]
- Komssi, S.; Aronen, H.J.; Huttunen, J.; Kesäniemi, M.; Soinne, L.; Nikouline, V.V.; Ollikainen, M.; Roine, R.O.; Karhu, J.; Savolainen, S.; et al. Ipsi- and Contralateral EEG Reactions to Transcranial Magnetic Stimulation. Clin. Neurophysiol. 2002, 113, 175–184. [Google Scholar] [CrossRef]
- Bonato, C.; Miniussi, C.; Rossini, P.M. Transcranial Magnetic Stimulation and Cortical Evoked Potentials: A TMS/EEG Co-Registration Study. Clin. Neurophysiol. 2006, 117, 1699–1707. [Google Scholar] [CrossRef]
- Chung, S.W.; Lewis, B.P.; Rogasch, N.C.; Saeki, T.; Thomson, R.H.; Hoy, K.E.; Bailey, N.W.; Fitzgerald, P.B. Demonstration of Short-Term Plasticity in the Dorsolateral Prefrontal Cortex with Theta Burst Stimulation: A TMS-EEG Study. Clin. Neurophysiol. 2017, 128, 1117–1126. [Google Scholar] [CrossRef]
- Lioumis, P.; Zomorrodi, R.; Hadas, I.; Daskalakis, Z.J.; Blumberger, D.M. Combined Transcranial Magnetic Stimulation and Electroencephalography of the Dorsolateral Prefrontal Cortex. J. Vis. Exp. 2018, 138, e57983. [Google Scholar] [CrossRef]
- Tremblay, S.; Rogasch, N.C.; Premoli, I.; Blumberger, D.M.; Casarotto, S.; Chen, R.; Di Lazzaro, V.; Farzan, F.; Ferrarelli, F.; Fitzgerald, P.B.; et al. Clinical Utility and Prospective of TMS–EEG. Clin. Neurophysiol. 2019, 130, 802–844. [Google Scholar] [CrossRef]
- Chung, S.W.; Rogasch, N.C.; Hoy, K.E.; Fitzgerald, P.B. Measuring Brain Stimulation Induced Changes in Cortical Properties Using TMS-EEG. Brain Stimul. 2015, 8, 1010–1020. [Google Scholar] [CrossRef]
- Qiu, S.; Yi, W.; Wang, S.; Zhang, C.; He, H. The Lasting Effects of Low-Frequency Repetitive Transcranial Magnetic Stimulation on Resting State EEG in Healthy Subjects. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Casula, E.P.; Pellicciari, M.C.; Ponzo, V.; Stampanoni Bassi, M.; Veniero, D.; Caltagirone, C.; Koch, G. Cerebellar Theta Burst Stimulation Modulates the Neural Activity of Interconnected Parietal and Motor Areas. Sci. Rep. 2016, 6, 36191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tervo, A.E.; Nieminen, J.O.; Lioumis, P.; Metsomaa, J.; Souza, V.H.; Sinisalo, H.; Stenroos, M.; Sarvas, J.; Ilmoniemi, R.J. Closed-Loop Optimization of Transcranial Magnetic Stimulation with Electroencephalography Feedback. Brain Stimul. 2022, 15, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, C.; Belardinelli, P.; Müller-Dahlhaus, F.; Ziemann, U. Closed-Loop Neuroscience and Non-Invasive Brain Stimulation: A Tale of Two Loops. Front. Cell. Neurosci. 2016, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Strafella, A.P.; Paus, T.; Barrett, J.; Dagher, A. Repetitive Transcranial Magnetic Stimulation of the Human Prefrontal Cortex Induces Dopamine Release in the Caudate Nucleus. J. Neurosci. 2001, 21, RC157. [Google Scholar] [CrossRef]
- Pogarell, O.; Koch, W.; Pöpperl, G.; Tatsch, K.; Jakob, F.; Mulert, C.; Grossheinrich, N.; Rupprecht, R.; Möller, H.-J.; Hegerl, U.; et al. Acute Prefrontal RTMS Increases Striatal Dopamine to a Similar Degree as D-Amphetamine. Psychiatry Res. 2007, 156, 251–255. [Google Scholar] [CrossRef]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological After-Effects of Non-Invasive Brain Stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef]
- Lee, L.; Siebner, H.R.; Rowe, J.B.; Rizzo, V.; Rothwell, J.C.; Frackowiak, R.S.J.; Friston, K.J. Acute Remapping within the Motor System Induced by Low-Frequency Repetitive Transcranial Magnetic Stimulation. J. Neurosci. 2003, 23, 5308–5318. [Google Scholar] [CrossRef]
- Rounis, E.; Lee, L.; Siebner, H.R.; Rowe, J.B.; Friston, K.J.; Rothwell, J.C.; Frackowiak, R.S.J. Frequency Specific Changes in Regional Cerebral Blood Flow and Motor System Connectivity Following RTMS to the Primary Motor Cortex. NeuroImage 2005, 26, 164–176. [Google Scholar] [CrossRef]
- Ridding, M.C.; Rothwell, J.C. Is There a Future for Therapeutic Use of Transcranial Magnetic Stimulation? Nat. Rev. Neurosci. 2007, 8, 559–567. [Google Scholar] [CrossRef]
- O’Shea, J.; Johansen-Berg, H.; Trief, D.; Göbel, S.; Rushworth, M.F.S. Functionally Specific Reorganization in Human Premotor Cortex. Neuron 2007, 54, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Bestmann, S.; Baudewig, J.; Siebner, H.R.; Rothwell, J.C.; Frahm, J. Subthreshold High-Frequency TMS of Human Primary Motor Cortex Modulates Interconnected Frontal Motor Areas as Detected by Interleaved FMRI-TMS. Neuroimage 2003, 20, 1685–1696. [Google Scholar] [CrossRef]
- Beynel, L.; Powers, J.P.; Appelbaum, L.G. Effects of Repetitive Transcranial Magnetic Stimulation on Resting-State Connectivity: A Systematic Review. NeuroImage 2020, 211, 116596. [Google Scholar] [CrossRef]
- Mielacher, C.; Schultz, J.; Kiebs, M.; Dellert, T.; Metzner, A.; Graute, L.; Högenauer, H.; Maier, W.; Lamm, C.; Hurlemann, R. Individualized Theta-Burst Stimulation Modulates Hippocampal Activity and Connectivity in Patients with Major Depressive Disorder. Pers. Med. Psychiatry 2020, 23–24, 100066. [Google Scholar] [CrossRef]
- May, A.; Hajak, G.; Gänßbauer, S.; Steffens, T.; Langguth, B.; Kleinjung, T.; Eichhammer, P. Structural Brain Alterations following 5 Days of Intervention: Dynamic Aspects of Neuroplasticity. Cereb. Cortex 2007, 17, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Lan, M.J.; Chhetry, B.T.; Liston, C.; Mann, J.J.; Dubin, M. Transcranial Magnetic Stimulation of Left Dorsolateral Prefrontal Cortex Induces Brain Morphological Changes in Regions Associated with a Treatment Resistant Major Depressive Episode: An Exploratory Analysis. Brain Stimul. 2016, 9, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Boes, A.D.; Uitermarkt, B.D.; Albazron, F.M.; Lan, M.J.; Liston, C.; Pascual-Leone, A.; Dubin, M.J.; Fox, M.D. Rostral Anterior Cingulate Cortex Is a Structural Correlate of Repetitive TMS Treatment Response in Depression. Brain Stimul. 2018, 11, 575–581. [Google Scholar] [CrossRef]
- Hayasaka, S.; Nakamura, M.; Noda, Y.; Izuno, T.; Saeki, T.; Iwanari, H.; Hirayasu, Y. Lateralized Hippocampal Volume Increase Following High-Frequency Left Prefrontal Repetitive Transcranial Magnetic Stimulation in Patients with Major Depression. Psychiatry Clin. Neurosci. 2017, 71, 747–758. [Google Scholar] [CrossRef] [Green Version]
- Noda, Y.; Zomorrodi, R.; Daskalakis, Z.J.; Blumberger, D.M.; Nakamura, M. Enhanced Theta-Gamma Coupling Associated with Hippocampal Volume Increase Following High-Frequency Left Prefrontal Repetitive Transcranial Magnetic Stimulation in Patients with Major Depression. Int. J. Psychophysiol. 2018, 133, 169–174. [Google Scholar] [CrossRef]
- Asan, L.; Falfán-Melgoza, C.; Beretta, C.A.; Sack, M.; Zheng, L.; Weber-Fahr, W.; Kuner, T.; Knabbe, J. Cellular Correlates of Gray Matter Volume Changes in Magnetic Resonance Morphometry Identified by Two-Photon Microscopy. Sci. Rep. 2021, 11, 4234. [Google Scholar] [CrossRef]
- Vlachos, A.; Müller-Dahlhaus, F.; Rosskopp, J.; Lenz, M.; Ziemann, U.; Deller, T. Repetitive Magnetic Stimulation Induces Functional and Structural Plasticity of Excitatory Postsynapses in Mouse Organotypic Hippocampal Slice Cultures. J. Neurosci. 2012, 32, 17514–17523. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Chen, R.-S.; Rothwell, J.C.; Wen, H.-Y. The After-Effect of Human Theta Burst Stimulation Is NMDA Receptor Dependent. Clin. Neurophysiol. 2007, 118, 1028–1032. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Benitez, J.; Oxley, T.; Daskalakis, J.Z.; de Castella, A.R.; Kulkarni, J. A Study of the Effects of Lorazepam and Dextromethorphan on the Response to Cortical 1 Hz Repetitive Transcranial Magnetic Stimulation. Neuroreport 2005, 16, 1525–1528. [Google Scholar] [CrossRef]
- Brown, J.C.; DeVries, W.H.; Korte, J.E.; Sahlem, G.L.; Bonilha, L.; Short, E.B.; George, M.S. NMDA Receptor Partial Agonist, d-Cycloserine, Enhances 10 Hz RTMS-Induced Motor Plasticity, Suggesting Long-Term Potentiation (LTP) as Underlying Mechanism. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2020, 13, 530–532. [Google Scholar] [CrossRef] [Green Version]
- Selby, B.; MacMaster, F.P.; Kirton, A.; McGirr, A. D-Cycloserine Blunts Motor Cortex Facilitation after Intermittent Theta Burst Transcranial Magnetic Stimulation: A Double-Blind Randomized Placebo-Controlled Crossover Study. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2019, 12, 1063–1065. [Google Scholar] [CrossRef]
- Teo, J.T.H.; Swayne, O.B.; Rothwell, J.C. Further Evidence for NMDA-Dependence of the after-Effects of Human Theta Burst Stimulation. Clin. Neurophysiol. 2007, 118, 1649–1651. [Google Scholar] [CrossRef]
- Wankerl, K.; Weise, D.; Gentner, R.; Rumpf, J.-J.; Classen, J. L-Type Voltage-Gated Ca2+ Channels: A Single Molecular Switch for Long-Term Potentiation/Long-Term Depression-like Plasticity and Activity-Dependent Metaplasticity in Humans. J. Neurosci. 2010, 30, 6197–6204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weise, D.; Mann, J.; Rumpf, J.-J.; Hallermann, S.; Classen, J. Differential Regulation of Human Paired Associative Stimulation-Induced and Theta-Burst Stimulation-Induced Plasticity by L-Type and T-Type Ca2+ Channels. Cereb. Cortex 2017, 27, 4010–4021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziemann, U.; Hallett, M.; Cohen, L.G. Mechanisms of Deafferentation-Induced Plasticity in Human Motor Cortex. J. Neurosci. 1998, 18, 7000–7007. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Chan, S.; Pringle, E.; Schallert, K.; Procaccio, V.; Jimenez, R.; Cramer, S.C. BDNF Val66met Polymorphism Is Associated with Modified Experience-Dependent Plasticity in Human Motor Cortex. Nat. Neurosci. 2006, 9, 735–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheeran, B.; Talelli, P.; Mori, F.; Koch, G.; Suppa, A.; Edwards, M.; Houlden, H.; Bhatia, K.; Greenwood, R.; Rothwell, J.C. A Common Polymorphism in the Brain-Derived Neurotrophic Factor Gene (BDNF) Modulates Human Cortical Plasticity and the Response to RTMS. J. Physiol. 2008, 586, 5717–5725. [Google Scholar] [CrossRef]
- Abraham, W.C. Metaplasticity: Tuning Synapses and Networks for Plasticity. Nat. Rev. Neurosci. 2008, 9, 387. [Google Scholar] [CrossRef]
- Karabanov, A.; Ziemann, U.; Hamada, M.; George, M.S.; Quartarone, A.; Classen, J.; Massimini, M.; Rothwell, J.; Siebner, H.R. Consensus Paper: Probing Homeostatic Plasticity of Human Cortex with Non-Invasive Transcranial Brain Stimulation. Brain Stimul. 2015, 8, 993–1006. [Google Scholar] [CrossRef] [Green Version]
- Bienenstock, E.L.; Cooper, L.N.; Munro, P.W. Theory for the Development of Neuron Selectivity: Orientation Specificity and Binocular Interaction in Visual Cortex. J. Neurosci. 1982, 2, 32–48. [Google Scholar] [CrossRef] [Green Version]
- Müller, J.F.M.; Orekhov, Y.; Liu, Y.; Ziemann, U. Homeostatic Plasticity in Human Motor Cortex Demonstrated by Two Consecutive Sessions of Paired Associative Stimulation. Eur. J. Neurosci. 2007, 25, 3461–3468. [Google Scholar] [CrossRef]
- Murakami, T.; Müller-Dahlhaus, F.; Lu, M.-K.; Ziemann, U. Homeostatic Metaplasticity of Corticospinal Excitatory and Intracortical Inhibitory Neural Circuits in Human Motor Cortex. J. Physiol. 2012, 590, 5765–5781. [Google Scholar] [CrossRef]
- Ni, Z.; Gunraj, C.; Kailey, P.; Cash, R.F.H.; Chen, R. Heterosynaptic Modulation of Motor Cortical Plasticity in Human. J. Neurosci. 2014, 34, 7314–7321. [Google Scholar] [CrossRef] [Green Version]
- Todd, G.; Flavel, S.C.; Ridding, M.C. Priming Theta-Burst Repetitive Transcranial Magnetic Stimulation with Low- and High-Frequency Stimulation. Exp. Brain Res. 2009, 195, 307–315. [Google Scholar] [CrossRef]
- Hamada, M.; Terao, Y.; Hanajima, R.; Shirota, Y.; Nakatani-Enomoto, S.; Furubayashi, T.; Matsumoto, H.; Ugawa, Y. Bidirectional Long-Term Motor Cortical Plasticity and Metaplasticity Induced by Quadripulse Transcranial Magnetic Stimulation. J. Physiol. 2008, 586, 3927–3947. [Google Scholar] [CrossRef]
- Stefan, K.; Wycislo, M.; Gentner, R.; Schramm, A.; Naumann, M.; Reiners, K.; Classen, J. Temporary Occlusion of Associative Motor Cortical Plasticity by Prior Dynamic Motor Training. Cereb. Cortex 2006, 16, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.; Silla, F.; Pierce, S.; Theule, J.; Girard, T.A. Cognitive Functioning before and after Repetitive Transcranial Magnetic Stimulation (RTMS): A Quantitative Meta-Analysis in Healthy Adults. Neuropsychologia 2020, 141, 107395. [Google Scholar] [CrossRef] [PubMed]
- Guse, B.; Falkai, P.; Wobrock, T. Cognitive Effects of High-Frequency Repetitive Transcranial Magnetic Stimulation: A Systematic Review. J. Neural Transm. 2010, 117, 105–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefaucheur, J.-P.; André-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-Based Guidelines on the Therapeutic Use of Repetitive Transcranial Magnetic Stimulation (RTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.J.; Phillips, A.L.; Bentzley, B.S.; Stimpson, K.H.; Nejad, R.; Barmak, F.; Veerapal, C.; Khan, N.; Cherian, K.; Felber, E.; et al. Stanford Neuromodulation Therapy (SNT): A Double-Blind Randomized Controlled Trial. Am. J. Psychiatry 2022, 179, 132–141. [Google Scholar] [CrossRef]
- Benabid, A.L. Lasker Award Winner Alim Louis Benabid. Nat. Med. 2014, 20, 1121–1123. [Google Scholar] [CrossRef]
- Benabid, A.L.; Pollak, P.; Louveau, A.; Henry, S.; de Rougemont, J. Combined (Thalamotomy and Stimulation) Stereotactic Surgery of the VIM Thalamic Nucleus for Bilateral Parkinson Disease. Appl. Neurophysiol. 1987, 50, 344–346. [Google Scholar] [CrossRef]
- Benabid, A.L.; Benazzouz, A.; Hoffmann, D.; Limousin, P.; Krack, P.; Pollak, P. Long-Term Electrical Inhibition of Deep Brain Targets in Movement Disorders. Mov. Disord. 1998, 13 (Suppl. 3), 119–125. [Google Scholar] [CrossRef]
- Eldridge, R.; Rocca, W.A. The Clinical Syndrome of Striatal Dopamine Deficiency: Parkinsonism Induced by MPTP. N. Engl. J. Med. 1985, 313, 1159–1160. [Google Scholar] [CrossRef]
- Kaakkola, S.; Teräväinen, H. Animal Models of Parkinsonism. Pharmacol. Toxicol. 1990, 67, 95–100. [Google Scholar] [CrossRef]
- Deffains, M.; Holland, P.; Moshel, S.; Ramirez de Noriega, F.; Bergman, H.; Israel, Z. Higher Neuronal Discharge Rate in the Motor Area of the Subthalamic Nucleus of Parkinsonian Patients. J. Neurophysiol. 2014, 112, 1409–1420. [Google Scholar] [CrossRef] [Green Version]
- Kühn, A.A.; Trottenberg, T.; Kivi, A.; Kupsch, A.; Schneider, G.-H.; Brown, P. The Relationship between Local Field Potential and Neuronal Discharge in the Subthalamic Nucleus of Patients with Parkinson’s Disease. Exp. Neurol. 2005, 194, 212–220. [Google Scholar] [CrossRef]
- Bergman, H.; Wichmann, T.; DeLong, M.R. Reversal of Experimental Parkinsonism by Lesions of the Subthalamic Nucleus. Science 1990, 249, 1436–1438. [Google Scholar] [CrossRef]
- Chiken, S.; Nambu, A. High-Frequency Pallidal Stimulation Disrupts Information Flow through the Pallidum by GABAergic Inhibition. J. Neurosci. 2013, 33, 2268–2280. [Google Scholar] [CrossRef] [Green Version]
- Chiken, S.; Nambu, A. Mechanism of Deep Brain Stimulation: Inhibition, Excitation, or Disruption? Neuroscientist 2016, 22, 313–322. [Google Scholar] [CrossRef]
- Brown, P. Oscillatory Nature of Human Basal Ganglia Activity: Relationship to the Pathophysiology of Parkinson’s Disease. Mov. Disord. 2003, 18, 357–363. [Google Scholar] [CrossRef]
- Hammond, C.; Bergman, H.; Brown, P. Pathological Synchronization in Parkinson’s Disease: Networks, Models and Treatments. Trends Neurosci. 2007, 30, 357–364. [Google Scholar] [CrossRef]
- Tinkhauser, G.; Pogosyan, A.; Tan, H.; Herz, D.M.; Kühn, A.A.; Brown, P. Beta Burst Dynamics in Parkinson’s Disease OFF and ON Dopaminergic Medication. Brain 2017, 140, 2968–2981. [Google Scholar] [CrossRef] [Green Version]
- Moran, A.; Stein, E.; Tischler, H.; Belelovsky, K.; Bar-Gad, I. Dynamic Stereotypic Responses of Basal Ganglia Neurons to Subthalamic Nucleus High-Frequency Stimulation in the Parkinsonian Primate. Front. Syst. Neurosci. 2011, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Gradinaru, V.; Mogri, M.; Thompson, K.R.; Henderson, J.M.; Deisseroth, K. Optical Deconstruction of Parkinsonian Neural Circuitry. Science 2009, 324, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Nambu, A.; Tokuno, H.; Hamada, I.; Kita, H.; Imanishi, M.; Akazawa, T.; Ikeuchi, Y.; Hasegawa, N. Excitatory Cortical Inputs to Pallidal Neurons via the Subthalamic Nucleus in the Monkey. J. Neurophysiol. 2000, 84, 289–300. [Google Scholar] [CrossRef]
- Flora, E.D.; Perera, C.L.; Cameron, A.L.; Maddern, G.J. Deep Brain Stimulation for Essential Tremor: A Systematic Review. Mov. Disord. 2010, 25, 1550–1559. [Google Scholar] [CrossRef]
- Groppa, S.; Herzog, J.; Falk, D.; Riedel, C.; Deuschl, G.; Volkmann, J. Physiological and Anatomical Decomposition of Subthalamic Neurostimulation Effects in Essential Tremor. Brain 2014, 137, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Barbe, M.T.; Liebhart, L.; Runge, M.; Pauls, K.A.M.; Wojtecki, L.; Schnitzler, A.; Allert, N.; Fink, G.R.; Sturm, V.; Maarouf, M.; et al. Deep Brain Stimulation in the Nucleus Ventralis Intermedius in Patients with Essential Tremor: Habituation of Tremor Suppression. J. Neurol. 2011, 258, 434–439. [Google Scholar] [CrossRef]
- Favilla, C.G.; Ullman, D.; Wagle Shukla, A.; Foote, K.D.; Jacobson, C.E.; Okun, M.S. Worsening Essential Tremor Following Deep Brain Stimulation: Disease Progression versus Tolerance. Brain 2012, 135, 1455–1462. [Google Scholar] [CrossRef]
- Putzke, J.D.; Whaley, N.R.; Baba, Y.; Wszolek, Z.K.; Uitti, R.J. Essential Tremor: Predictors of Disease Progression in a Clinical Cohort. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1235–1237. [Google Scholar] [CrossRef]
- Paschen, S.; Forstenpointner, J.; Becktepe, J.; Heinzel, S.; Hellriegel, H.; Witt, K.; Helmers, A.-K.; Deuschl, G. Long-Term Efficacy of Deep Brain Stimulation for Essential Tremor: An Observer-Blinded Study. Neurology 2019, 92, e1378–e1386. [Google Scholar] [CrossRef]
- Patel, N.; Ondo, W.; Jimenez-Shahed, J. Habituation and Rebound to Thalamic Deep Brain Stimulation in Long-Term Management of Tremor Associated with Demyelinating Neuropathy. Int. J. Neurosci. 2014, 124, 919–925. [Google Scholar] [CrossRef]
- Witt, K.; Kopper, F.; Deuschl, G.; Krack, P. Subthalamic Nucleus Influences Spatial Orientation in Extra-Personal Space. Mov. Disord. 2006, 21, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Schmalbach, B.; Günther, V.; Raethjen, J.; Wailke, S.; Falk, D.; Deuschl, G.; Witt, K. The Subthalamic Nucleus Influences Visuospatial Attention in Humans. J. Cogn. Neurosci. 2014, 26, 543–550. [Google Scholar] [CrossRef]
- Benabid, A.L.; Koudsié, A.; Benazzouz, A.; Fraix, V.; Ashraf, A.; Le Bas, J.F.; Chabardes, S.; Pollak, P. Subthalamic Stimulation for Parkinson’s Disease. Arch. Med. Res. 2000, 31, 282–289. [Google Scholar] [CrossRef]
- Kumar, R.; Lozano, A.M.; Kim, Y.J.; Hutchison, W.D.; Sime, E.; Halket, E.; Lang, A.E. Double-Blind Evaluation of Subthalamic Nucleus Deep Brain Stimulation in Advanced Parkinson’s Disease. Neurology 1998, 51, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Sestini, S.; Scotto di Luzio, A.; Ammannati, F.; De Cristofaro, M.T.R.; Passeri, A.; Martini, S.; Pupi, A. Changes in Regional Cerebral Blood Flow Caused by Deep-Brain Stimulation of the Subthalamic Nucleus in Parkinson’s Disease. J. Nucl. Med. 2002, 43, 725–732. [Google Scholar] [PubMed]
- Østergaard, K.; Sunde, N.A. Evolution of Parkinson’s Disease during 4 Years of Bilateral Deep Brain Stimulation of the Subthalamic Nucleus. Mov. Disord. 2006, 21, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Oroz, M.C.; Gorospe, A.; Guridi, J.; Ramos, E.; Linazasoro, G.; Rodriguez-Palmero, M.; Obeso, J.A. Bilateral Deep Brain Stimulation of the Subthalamic Nucleus in Parkinson’s Disease. Neurology 2000, 55, S45–S51. [Google Scholar]
- Simuni, T.; Jaggi, J.L.; Mulholland, H.; Hurtig, H.I.; Colcher, A.; Siderowf, A.D.; Ravina, B.; Skolnick, B.E.; Goldstein, R.; Stern, M.B.; et al. Bilateral Stimulation of the Subthalamic Nucleus in Patients with Parkinson Disease: A Study of Efficacy and Safety. J. Neurosurg. 2002, 96, 666–672. [Google Scholar] [CrossRef]
- Sestini, S.; Ramat, S.; Formiconi, A.R.; Ammannati, F.; Sorbi, S.; Pupi, A. Brain Networks Underlying the Clinical Effects of Long-Term Subthalamic Stimulation for Parkinson’s Disease: A 4-Year Follow-up Study with RCBF SPECT. J. Nucl. Med. 2005, 46, 1444–1454. [Google Scholar]
- Sestini, S.; Pupi, A.; Ammannati, F.; Silvia, R.; Sorbi, S.; Castagnoli, A. Are There Adaptive Changes in the Human Brain of Patients with Parkinson’s Disease Treated with Long-Term Deep Brain Stimulation of the Subthalamic Nucleus? A 4-Year Follow-up Study with Regional Cerebral Blood Flow SPECT. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1646–1657. [Google Scholar] [CrossRef]
- Haberler, C.; Alesch, F.; Mazal, P.R.; Pilz, P.; Jellinger, K.; Pinter, M.M.; Hainfellner, J.A.; Budka, H. No Tissue Damage by Chronic Deep Brain Stimulation in Parkinson’s Disease. Ann. Neurol. 2000, 48, 372–376. [Google Scholar] [CrossRef]
- Temperli, P.; Ghika, J.; Villemure, J.-G.; Burkhard, P.R.; Bogousslavsky, J.; Vingerhoets, F.J.G. How Do Parkinsonian Signs Return after Discontinuation of Subthalamic DBS? Neurology 2003, 60, 78–81. [Google Scholar] [CrossRef]
- Lokkegaard, A.; Werdelin, L.M.; Regeur, L.; Karlsborg, M.; Jensen, S.R.; Brødsgaard, E.; Madsen, F.F.; Lonsdale, M.N.; Friberg, L. Dopamine Transporter Imaging and the Effects of Deep Brain Stimulation in Patients with Parkinson’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 508–516. [Google Scholar] [CrossRef]
- Álvarez-Bueno, C.; Deeks, J.J.; Cavero-Redondo, I.; Jolly, K.; Torres-Costoso, A.I.; Price, M.; Fernandez-Rodriguez, R.; Martínez-Vizcaíno, V. Effect of Exercise on Motor Symptoms in Patients with Parkinson’s Disease: A Network Meta-Analysis. J. Geriatr. Phys. Ther. 2021. [Google Scholar] [CrossRef]
- Trager, M.H.; Koop, M.M.; Velisar, A.; Blumenfeld, Z.; Nikolau, J.S.; Quinn, E.J.; Martin, T.; Bronte-Stewart, H. Subthalamic Beta Oscillations Are Attenuated after Withdrawal of Chronic High Frequency Neurostimulation in Parkinson’s Disease. Neurobiol. Dis. 2016, 96, 22–30. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, C.; Tian, Y.; Orlov, N.; Zhang, J.; Guo, Y.; Xu, S.; Jiang, C.; Hao, H.; Neumann, W.-J.; et al. Neuromodulation Effects of Deep Brain Stimulation on Beta Rhythm: A Longitudinal Local Field Potential Study. Brain Stimul. 2020, 13, 1784–1792. [Google Scholar] [CrossRef]
- Turco, C.V.; El-Sayes, J.; Savoie, M.J.; Fassett, H.J.; Locke, M.B.; Nelson, A.J. Short- and Long-Latency Afferent Inhibition; Uses, Mechanisms and Influencing Factors. Brain Stimul. 2018, 11, 59–74. [Google Scholar] [CrossRef]
- Sailer, A.; Molnar, G.F.; Paradiso, G.; Gunraj, C.A.; Lang, A.E.; Chen, R. Short and Long Latency Afferent Inhibition in Parkinson’s Disease. Brain 2003, 126, 1883–1894. [Google Scholar] [CrossRef] [Green Version]
- Shukla, A.W.; Moro, E.; Gunraj, C.; Lozano, A.; Hodaie, M.; Lang, A.; Chen, R. Long-Term Subthalamic Nucleus Stimulation Improves Sensorimotor Integration and Proprioception. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1020–1028. [Google Scholar] [CrossRef]
- Sankar, T.; Li, S.X.; Obuchi, T.; Fasano, A.; Cohn, M.; Hodaie, M.; Chakravarty, M.M.; Lozano, A.M. Structural Brain Changes Following Subthalamic Nucleus Deep Brain Stimulation in Parkinson’s Disease. Mov. Disord. 2016, 31, 1423–1425. [Google Scholar] [CrossRef]
- Kern, D.S.; Uy, D.; Rhoades, R.; Ojemann, S.; Abosch, A.; Thompson, J.A. Discrete Changes in Brain Volume after Deep Brain Stimulation in Patients with Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2020, 91, 928–937. [Google Scholar] [CrossRef]
- Ge, J.; Wang, M.; Lin, W.; Wu, P.; Guan, Y.; Zhang, H.; Huang, Z.; Yang, L.; Zuo, C.; Jiang, J.; et al. Metabolic Network as an Objective Biomarker in Monitoring Deep Brain Stimulation for Parkinson’s Disease: A Longitudinal Study. EJNMMI Res. 2020, 10, 131. [Google Scholar] [CrossRef]
- Spieles-Engemann, A.L.; Steece-Collier, K.; Behbehani, M.M.; Collier, T.J.; Wohlgenant, S.L.; Kemp, C.J.; Cole-Strauss, A.; Levine, N.D.; Gombash, S.E.; Thompson, V.B.; et al. Subthalamic Nucleus Stimulation Increases Brain Derived Neurotrophic Factor in the Nigrostriatal System and Primary Motor Cortex. J. Parkinsons Dis. 2011, 1, 123–136. [Google Scholar] [CrossRef]
- Sortwell, C.E.; Hacker, M.L.; Fischer, D.L.; Konrad, P.E.; Davis, T.L.; Neimat, J.S.; Wang, L.; Song, Y.; Mattingly, Z.R.; Cole-Strauss, A.; et al. BDNF Rs6265 Genotype Influences Outcomes of Pharmacotherapy and Subthalamic Nucleus Deep Brain Stimulation in Early-Stage Parkinson’s Disease. Neuromodulation 2021, in press. [Google Scholar] [CrossRef]
- Vidailhet, M.; Jutras, M.-F.; Roze, E.; Grabli, D. Deep Brain Stimulation for Dystonia. Handb. Clin. Neurol. 2013, 116, 167–187. [Google Scholar] [CrossRef]
- Volkmann, J.; Mueller, J.; Deuschl, G.; Kühn, A.A.; Krauss, J.K.; Poewe, W.; Timmermann, L.; Falk, D.; Kupsch, A.; Kivi, A.; et al. Pallidal Neurostimulation in Patients with Medication-Refractory Cervical Dystonia: A Randomised, Sham-Controlled Trial. Lancet Neurol. 2014, 13, 875–884. [Google Scholar] [CrossRef]
- Grips, E.; Blahak, C.; Capelle, H.H.; Bäzner, H.; Weigel, R.; Sedlaczek, O.; Krauss, J.K.; Wöhrle, J.C. Patterns of Reoccurrence of Segmental Dystonia after Discontinuation of Deep Brain Stimulation. J. Neurol. Neurosurg. Psychiatry 2007, 78, 318–320. [Google Scholar] [CrossRef] [Green Version]
- Cheung, T.; Zhang, C.; Rudolph, J.; Alterman, R.L.; Tagliati, M. Sustained Relief of Generalized Dystonia despite Prolonged Interruption of Deep Brain Stimulation. Mov. Disord. 2013, 28, 1431–1434. [Google Scholar] [CrossRef]
- Goto, S.; Yamada, K. Long Term Continuous Bilateral Pallidal Stimulation Produces Stimulation Independent Relief of Cervical Dystonia. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1506–1507. [Google Scholar] [CrossRef] [Green Version]
- Hebb, M.O.; Chiasson, P.; Lang, A.E.; Brownstone, R.M.; Mendez, I. Sustained Relief of Dystonia Following Cessation of Deep Brain Stimulation. Mov. Disord. 2007, 22, 1958–1962. [Google Scholar] [CrossRef]
- Udupa, K.; Chen, R. Motor Cortical Plasticity in Parkinson’s Disease. Front. Neurol. 2013, 4, 128. [Google Scholar] [CrossRef] [Green Version]
- Quartarone, A.; Hallett, M. Emerging Concepts in the Physiological Basis of Dystonia. Mov. Disord. 2013, 28, 958–967. [Google Scholar] [CrossRef]
- Ruge, D.; Tisch, S.; Hariz, M.I.; Zrinzo, L.; Bhatia, K.P.; Quinn, N.P.; Jahanshahi, M.; Limousin, P.; Rothwell, J.C. Deep Brain Stimulation Effects in Dystonia: Time Course of Electrophysiological Changes in Early Treatment. Mov. Disord. 2011, 26, 1913–1921. [Google Scholar] [CrossRef] [Green Version]
- Kroneberg, D.; Plettig, P.; Schneider, G.-H.; Kühn, A.A. Motor Cortical Plasticity Relates to Symptom Severity and Clinical Benefit From Deep Brain Stimulation in Cervical Dystonia. Neuromodulation Technol. Neural Interface 2018, 21, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Kim, S.J.; Phielipp, N.; Ghosh, S.; Udupa, K.; Gunraj, C.A.; Saha, U.; Hodaie, M.; Kalia, S.K.; Lozano, A.M.; et al. Pallidal Deep Brain Stimulation Modulates Cortical Excitability and Plasticity. Ann. Neurol. 2018, 83, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Qiao, L.; Fan, D.; Zhang, S.; Turel, O.; Li, Y.; Li, J.; Xue, G.; Chen, A.; He, Q. Modulation of Brain Activity with Noninvasive Transcranial Direct Current Stimulation (TDCS): Clinical Applications and Safety Concerns. Front. Psychol. 2017, 8, 685. [Google Scholar] [CrossRef] [PubMed]
- Elyamany, O.; Leicht, G.; Herrmann, C.S.; Mulert, C. Transcranial Alternating Current Stimulation (TACS): From Basic Mechanisms towards First Applications in Psychiatry. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 135–156. [Google Scholar] [CrossRef]
- Kasten, F.H.; Duecker, K.; Maack, M.C.; Meiser, A.; Herrmann, C.S. Integrating Electric Field Modeling and Neuroimaging to Explain Inter-Individual Variability of TACS Effects. Nat. Commun. 2019, 10, 5427. [Google Scholar] [CrossRef] [Green Version]
- Bindman, L.J.; Lippold, O.C.; Redfearn, J.W. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J. Physiol. 1964, 172, 369–382. [Google Scholar] [CrossRef]
- Bikson, M.; Inoue, M.; Akiyama, H.; Deans, J.K.; Fox, J.E.; Miyakawa, H.; Jefferys, J.G.R. Effects of Uniform Extracellular DC Electric Fields on Excitability in Rat Hippocampal Slices in Vitro. J. Physiol. 2004, 557, 175–190. [Google Scholar] [CrossRef]
- Creutzfeldt, O.D.; Fromm, G.H.; Kapp, H. Influence of Transcortical D-c Currents on Cortical Neuronal Activity. Exp. Neurol. 1962, 5, 436–452. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained Excitability Elevations Induced by Transcranial DC Motor Cortex Stimulation in Humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Rahman, A.; Reato, D.; Arlotti, M.; Gasca, F.; Datta, A.; Parra, L.C.; Bikson, M. Cellular Effects of Acute Direct Current Stimulation: Somatic and Synaptic Terminal Effects. J. Physiol. 2013, 591, 2563–2578. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Kuo, M.-F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of Late LTP-like Plasticity in the Human Motor Cortex by Repeated Non-Invasive Brain Stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef]
- Batsikadze, G.; Moliadze, V.; Paulus, W.; Kuo, M.-F.; Nitsche, M.A. Partially Non-Linear Stimulation Intensity-Dependent Effects of Direct Current Stimulation on Motor Cortex Excitability in Humans. J. Physiol. 2013, 591, 1987–2000. [Google Scholar] [CrossRef]
- Jamil, A.; Batsikadze, G.; Kuo, H.-I.; Labruna, L.; Hasan, A.; Paulus, W.; Nitsche, M.A. Systematic Evaluation of the Impact of Stimulation Intensity on Neuroplastic After-Effects Induced by Transcranial Direct Current Stimulation. J. Physiol. 2017, 595, 1273–1288. [Google Scholar] [CrossRef]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive Cortical Stimulation Enhances Motor Skill Acquisition over Multiple Days through an Effect on Consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef] [Green Version]
- Boggio, P.S.; Nunes, A.; Rigonatti, S.P.; Nitsche, M.A.; Pascual-Leone, A.; Fregni, F. Repeated Sessions of Noninvasive Brain DC Stimulation Is Associated with Motor Function Improvement in Stroke Patients. Restor. Neurol. Neurosci. 2007, 25, 123–129. [Google Scholar]
- Miller, J.; Berger, B.; Sauseng, P. Anodal Transcranial Direct Current Stimulation (TDCS) Increases Frontal-Midline Theta Activity in the Human EEG: A Preliminary Investigation of Non-Invasive Stimulation. Neurosci. Lett. 2015, 588, 114–119. [Google Scholar] [CrossRef]
- Zaehle, T.; Sandmann, P.; Thorne, J.D.; Jäncke, L.; Herrmann, C.S. Transcranial Direct Current Stimulation of the Prefrontal Cortex Modulates Working Memory Performance: Combined Behavioural and Electrophysiological Evidence. BMC Neurosci. 2011, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Ardolino, G.; Bossi, B.; Barbieri, S.; Priori, A. Non-Synaptic Mechanisms Underlie the after-Effects of Cathodal Transcutaneous Direct Current Stimulation of the Human Brain. J. Physiol. 2005, 568, 653–663. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, J.; Fujiwara, T.; Takahashi, O.; Liu, M.; Kimura, A.; Ushiba, J. Modulation of Mu Rhythm Desynchronization during Motor Imagery by Transcranial Direct Current Stimulation. J. Neuroeng. Rehabil. 2010, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Spitoni, G.F.; Cimmino, R.L.; Bozzacchi, C.; Pizzamiglio, L.; Di Russo, F. Modulation of Spontaneous Alpha Brain Rhythms Using Low-Intensity Transcranial Direct-Current Stimulation. Front. Hum. Neurosci. 2013, 7, 529. [Google Scholar] [CrossRef] [Green Version]
- Mangia, A.L.; Pirini, M.; Cappello, A. Transcranial Direct Current Stimulation and Power Spectral Parameters: A TDCS/EEG Co-Registration Study. Front. Hum. Neurosci. 2014, 8, 601. [Google Scholar] [CrossRef] [Green Version]
- Reed, T.; Cohen Kadosh, R. Transcranial Electrical Stimulation (TES) Mechanisms and Its Effects on Cortical Excitability and Connectivity. J. Inherit. Metab. Dis. 2018, 41, 1123–1130. [Google Scholar] [CrossRef] [Green Version]
- Keeser, D.; Meindl, T.; Bor, J.; Palm, U.; Pogarell, O.; Mulert, C.; Brunelin, J.; Möller, H.-J.; Reiser, M.; Padberg, F. Prefrontal Transcranial Direct Current Stimulation Changes Connectivity of Resting-State Networks during FMRI. J. Neurosci. 2011, 31, 15284–15293. [Google Scholar] [CrossRef]
- Palm, U.; Hasan, A.; Strube, W.; Padberg, F. TDCS for the Treatment of Depression: A Comprehensive Review. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 681–694. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-Based Guidelines on the Therapeutic Use of Transcranial Direct Current Stimulation (TDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Stagg, C.J.; Nitsche, M.A. Physiological Basis of Transcranial Direct Current Stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological Modulation of Cortical Excitability Shifts Induced by Transcranial Direct Current Stimulation in Humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct Current Stimulation Promotes BDNF-Dependent Synaptic Plasticity: Potential Implications for Motor Learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Podda, M.V.; Cocco, S.; Mastrodonato, A.; Fusco, S.; Leone, L.; Barbati, S.A.; Colussi, C.; Ripoli, C.; Grassi, C. Anodal Transcranial Direct Current Stimulation Boosts Synaptic Plasticity and Memory in Mice via Epigenetic Regulation of Bdnf Expression. Sci. Rep. 2016, 6, 22180. [Google Scholar] [CrossRef]
- Figurov, A.; Pozzo-Miller, L.D.; Olafsson, P.; Wang, T.; Lu, B. Regulation of Synaptic Responses to High-Frequency Stimulation and LTP by Neurotrophins in the Hippocampus. Nature 1996, 381, 706–709. [Google Scholar] [CrossRef]
- Woo, N.H.; Teng, H.K.; Siao, C.-J.; Chiaruttini, C.; Pang, P.T.; Milner, T.A.; Hempstead, B.L.; Lu, B. Activation of P75NTR by ProBDNF Facilitates Hippocampal Long-Term Depression. Nat. Neurosci. 2005, 8, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Chaieb, L.; Moliadze, V.; Monte-Silva, K.; Poreisz, C.; Thirugnanasambandam, N.; Nitsche, M.A.; Shoukier, M.; Ludwig, H.; Paulus, W. Brain-Derived Neurotrophic Factor (BDNF) Gene Polymorphisms Shape Cortical Plasticity in Humans. Brain Stimul. 2010, 3, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Riddle, J.; McPherson, T.; Atkins, A.K.; Walker, C.P.; Ahn, S.; Frohlich, F. Brain-Derived Neurotrophic Factor (BDNF) Polymorphism May Influence the Efficacy of TACS to Modulate Neural Oscillations. Brain Stimul. 2020, 13, 998–999. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Jaussi, W.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Consolidation of Human Motor Cortical Neuroplasticity by D-Cycloserine. Neuropsychopharmacology 2004, 29, 1573–1578. [Google Scholar] [CrossRef]
- Olma, M.C.; Dargie, R.A.; Behrens, J.R.; Kraft, A.; Irlbacher, K.; Fahle, M.; Brandt, S.A. Long-Term Effects of Serial Anodal TDCS on Motion Perception in Subjects with Occipital Stroke Measured in the Unaffected Visual Hemifield. Front. Hum. Neurosci. 2013, 7, 314. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, C.S.; Rach, S.; Neuling, T.; Strüber, D. Transcranial Alternating Current Stimulation: A Review of the Underlying Mechanisms and Modulation of Cognitive Processes. Front. Hum. Neurosci. 2013, 7, 279. [Google Scholar] [CrossRef] [Green Version]
- Hanslmayr, S.; Matuschek, J.; Fellner, M.-C. Entrainment of Prefrontal Beta Oscillations Induces an Endogenous Echo and Impairs Memory Formation. Curr. Biol. 2014, 24, 904–909. [Google Scholar] [CrossRef] [Green Version]
- Marshall, L.; Helgadóttir, H.; Mölle, M.; Born, J. Boosting Slow Oscillations during Sleep Potentiates Memory. Nature 2006, 444, 610–613. [Google Scholar] [CrossRef]
- Zaehle, T.; Rach, S.; Herrmann, C.S. Transcranial Alternating Current Stimulation Enhances Individual Alpha Activity in Human EEG. PLoS ONE 2010, 5, e13766. [Google Scholar] [CrossRef] [Green Version]
- Neuling, T.; Rach, S.; Herrmann, C.S. Orchestrating Neuronal Networks: Sustained after-Effects of Transcranial Alternating Current Stimulation Depend upon Brain States. Front. Hum. Neurosci. 2013, 7, 161. [Google Scholar] [CrossRef] [Green Version]
- Kasten, F.H.; Dowsett, J.; Herrmann, C.S. Sustained Aftereffect of α-TACS Lasts Up to 70 min after Stimulation. Front. Hum. Neurosci. 2016, 10, 245. [Google Scholar] [CrossRef] [Green Version]
- Kasten, F.H.; Herrmann, C.S. Transcranial Alternating Current Stimulation (TACS) Enhances Mental Rotation Performance during and after Stimulation. Front. Hum. Neurosci. 2017, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Harada, T.; Hara, M.; Matsushita, K.; Kawakami, K.; Kawakami, K.; Anan, M.; Sugata, H. Off-Line Effects of Alpha-Frequency Transcranial Alternating Current Stimulation on a Visuomotor Learning Task. Brain Behav. 2020, 10, e01754. [Google Scholar] [CrossRef]
- Nakazono, H.; Ogata, K.; Takeda, A.; Yamada, E.; Kimura, T.; Tobimatsu, S. Transcranial Alternating Current Stimulation of α but Not β Frequency Sharpens Multiple Visual Functions. Brain Stimul. 2020, 13, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Moliadze, V.; Sierau, L.; Lyzhko, E.; Stenner, T.; Werchowski, M.; Siniatchkin, M.; Hartwigsen, G. After-Effects of 10 Hz TACS over the Prefrontal Cortex on Phonological Word Decisions. Brain Stimul. 2019, 12, 1464–1474. [Google Scholar] [CrossRef] [Green Version]
- Berger, A.; Pixa, N.H.; Steinberg, F.; Doppelmayr, M. Brain Oscillatory and Hemodynamic Activity in a Bimanual Coordination Task Following Transcranial Alternating Current Stimulation (TACS): A Combined EEG-FNIRS Study. Front. Behav. Neurosci. 2018, 12, 67. [Google Scholar] [CrossRef]
- Vossen, A.; Gross, J.; Thut, G. Alpha Power Increase after Transcranial Alternating Current Stimulation at Alpha Frequency (α-TACS) Reflects Plastic Changes Rather Than Entrainment. Brain Stimul. 2015, 8, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Strüber, D.; Rach, S.; Neuling, T.; Herrmann, C.S. On the Possible Role of Stimulation Duration for After-Effects of Transcranial Alternating Current Stimulation. Front. Cell. Neurosci. 2015, 9, 311. [Google Scholar] [CrossRef] [Green Version]
- Zanto, T.P.; Jones, K.T.; Ostrand, A.E.; Hsu, W.-Y.; Campusano, R.; Gazzaley, A. Individual Differences in Neuroanatomy and Neurophysiology Predict Effects of Transcranial Alternating Current Stimulation. Brain Stimul. 2021, 14, 1317–1329. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Knepper, H.; Nolte, G.; Strüber, D.; Rach, S.; Herrmann, C.S.; Schneider, T.R.; Engel, A.K. Selective Modulation of Interhemispheric Functional Connectivity by HD-TACS Shapes Perception. PLoS Biol. 2014, 12, e1002031. [Google Scholar] [CrossRef] [Green Version]
- Strüber, D.; Rach, S.; Trautmann-Lengsfeld, S.A.; Engel, A.K.; Herrmann, C.S. Antiphasic 40 Hz Oscillatory Current Stimulation Affects Bistable Motion Perception. Brain Topogr. 2014, 27, 158–171. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Schneider, T.R.; Rach, S.; Trautmann-Lengsfeld, S.A.; Engel, A.K.; Herrmann, C.S. Entrainment of Brain Oscillations by Transcranial Alternating Current Stimulation. Curr. Biol. 2014, 24, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Benussi, A.; Cantoni, V.; Cotelli, M.S.; Cotelli, M.; Brattini, C.; Datta, A.; Thomas, C.; Santarnecchi, E.; Pascual-Leone, A.; Borroni, B. Exposure to Gamma TACS in Alzheimer’s Disease: A Randomized, Double-Blind, Sham-Controlled, Crossover, Pilot Study. Brain Stimul. 2021, 14, 531–540. [Google Scholar] [CrossRef]
- Mellin, J.M.; Alagapan, S.; Lustenberger, C.; Lugo, C.E.; Alexander, M.L.; Gilmore, J.H.; Jarskog, L.F.; Fröhlich, F. Randomized Trial of Transcranial Alternating Current Stimulation for Treatment of Auditory Hallucinations in Schizophrenia. Eur. Psychiatry 2018, 51, 25–33. [Google Scholar] [CrossRef]
- Ahn, S.; Mellin, J.M.; Alagapan, S.; Alexander, M.L.; Gilmore, J.H.; Jarskog, L.F.; Fröhlich, F. Targeting Reduced Neural Oscillations in Patients with Schizophrenia by Transcranial Alternating Current Stimulation. Neuroimage 2019, 186, 126–136. [Google Scholar] [CrossRef]
- Strüber, D.; Herrmann, C.S. Modulation of Gamma Oscillations as a Possible Therapeutic Tool for Neuropsychiatric Diseases: A Review and Perspective. Int. J. Psychophysiol. 2020, 152, 15–25. [Google Scholar] [CrossRef]
- Wischnewski, M.; Engelhardt, M.; Salehinejad, M.A.; Schutter, D.J.L.G.; Kuo, M.-F.; Nitsche, M.A. NMDA Receptor-Mediated Motor Cortex Plasticity after 20 Hz Transcranial Alternating Current Stimulation. Cereb. Cortex 2019, 29, 2924–2931. [Google Scholar] [CrossRef]
- Antal, A.; Herrmann, C.S. Transcranial Alternating Current and Random Noise Stimulation: Possible Mechanisms. Neural Plast. 2016, 2016, 3616807. [Google Scholar] [CrossRef] [Green Version]
- Terney, D.; Chaieb, L.; Moliadze, V.; Antal, A.; Paulus, W. Increasing Human Brain Excitability by Transcranial High-Frequency Random Noise Stimulation. J. Neurosci. 2008, 28, 14147–14155. [Google Scholar] [CrossRef]
- Chaieb, L.; Paulus, W.; Antal, A. Evaluating Aftereffects of Short-Duration Transcranial Random Noise Stimulation on Cortical Excitability. Neural Plast. 2011, 2011, 105927. [Google Scholar] [CrossRef]
- Chaieb, L.; Antal, A.; Paulus, W. Transcranial Random Noise Stimulation-Induced Plasticity Is NMDA-Receptor Independent but Sodium-Channel Blocker and Benzodiazepines Sensitive. Front. Neurosci. 2015, 9, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghin, F.; O’Hare, L.; Pavan, A. Electrophysiological Aftereffects of High-Frequency Transcranial Random Noise Stimulation (Hf-TRNS): An EEG Investigation. Exp. Brain Res. 2021, 239, 2399–2418. [Google Scholar] [CrossRef] [PubMed]
- Pilar-Cuellar, F.; Vidal, R.; Díaz, A.; Castro, E.; Anjos, S.; Vargas, V.; Romero, B.; Valdizan, E. Signaling Pathways Involved in Antidepressant-Induced Cell Proliferation and Synaptic Plasticity. Curr. Pharm. Des. 2014, 20, 3776–3794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossman, N.; Bono, D.; Dedic, N.; Kodandaramaiah, S.B.; Rudenko, A.; Suk, H.-J.; Cassara, A.M.; Neufeld, E.; Kuster, N.; Tsai, L.-H.; et al. Noninvasive Deep Brain Stimulation via Temporally Interfering Electric Fields. Cell 2017, 169, 1029–1041. [Google Scholar] [CrossRef] [Green Version]
- Von Conta, J.; Kasten, F.H.; Ćurčić-Blake, B.; Aleman, A.; Thielscher, A.; Herrmann, C.S. Interindividual Variability of Electric Fields during Transcranial Temporal Interference Stimulation (TTIS). Sci. Rep. 2021, 11, 20357. [Google Scholar] [CrossRef]
- Negahbani, E.; Kasten, F.H.; Herrmann, C.S.; Fröhlich, F. Targeting Alpha-Band Oscillations in a Cortical Model with Amplitude-Modulated High-Frequency Transcranial Electric Stimulation. Neuroimage 2018, 173, 3–12. [Google Scholar] [CrossRef]
- Esmaeilpour, Z.; Kronberg, G.; Reato, D.; Parra, L.C.; Bikson, M. Temporal Interference Stimulation Targets Deep Brain Regions by Modulating Neural Oscillations. Brain Stimul. 2021, 14, 55–65. [Google Scholar] [CrossRef]
- Ma, R.; Xia, X.; Zhang, W.; Lu, Z.; Wu, Q.; Cui, J.; Song, H.; Fan, C.; Chen, X.; Zha, R.; et al. High Gamma and Beta Temporal Interference Stimulation in the Human Motor Cortex Improves Motor Functions. Front. Neurosci. 2021, 15, 800436. [Google Scholar] [CrossRef]
- Reuter, S.; Deuschl, G.; Falk, D.; Mehdorn, M.; Witt, K. Uncoupling of Dopaminergic and Subthalamic Stimulation: Life-Threatening DBS Withdrawal Syndrome. Mov. Disord. 2015, 30, 1407–1413. [Google Scholar] [CrossRef]
- Reuter, S.; Deuschl, G.; Berg, D.; Helmers, A.; Falk, D.; Witt, K. Life-Threatening DBS Withdrawal Syndrome in Parkinson’s Disease Can Be Treated with Early Reimplantation. Parkinsonism Relat. Disord. 2018, 56, 88–92. [Google Scholar] [CrossRef]
- Orendáčová, M.; Kvašňák, E. Effects of Transcranial Alternating Current Stimulation and Neurofeedback on Alpha (EEG) Dynamics: A Review. Front. Hum. Neurosci. 2021, 15, 628229. [Google Scholar] [CrossRef]
- Nagaoka, T.; Katayama, Y.; Kano, T.; Kobayashi, K.; Oshima, H.; Fukaya, C.; Yamamoto, T. Changes in Glucose Metabolism in Cerebral Cortex and Cerebellum Correlate with Tremor and Rigidity Control by Subthalamic Nucleus Stimulation in Parkinson’s Disease: A Positron Emission Tomography Study. Neuromodulation 2007, 10, 206–215. [Google Scholar] [CrossRef]
- Chen, H.-M.; Sha, Z.-Q.; Ma, H.-Z.; He, Y.; Feng, T. Effective Network of Deep Brain Stimulation of Subthalamic Nucleus with Bimodal Positron Emission Tomography/Functional Magnetic Resonance Imaging in Parkinson’s Disease. CNS Neurosci. Ther. 2018, 24, 135–143. [Google Scholar] [CrossRef]
- Workman, C.D.; Fietsam, A.C.; Ponto, L.L.B.; Kamholz, J.; Rudroff, T. Individual Cerebral Blood Flow Responses to Transcranial Direct Current Stimulation at Various Intensities. Brain Sci. 2020, 10, 855. [Google Scholar] [CrossRef]
- Dhaynaut, M.; Sprugnoli, G.; Cappon, D.; Macone, J.; Sanchez, J.S.; Normandin, M.D.; Guehl, N.J.; Koch, G.; Paciorek, R.; Connor, A.; et al. Impact of 40Hz Transcranial Alternating Current Stimulation on Cerebral Tau Burden in Patients with Alzheimer’s Disease: A Case Series. J. Alzheimers Dis. 2022, 85, 1667–1676. [Google Scholar] [CrossRef]
- Sidtis, J.J.; Sidtis, D.V.L.; Dhawan, V.; Tagliati, M.; Eidelberg, D. Stimulation of the Subthalamic Nucleus Changes Cortical-Subcortical Blood Flow Patterns during Speech: A Positron Emission Tomography Study. Front. Neurol. 2021, 12, 684596. [Google Scholar] [CrossRef]
- Tremblay, S.; Tuominen, L.; Zayed, V.; Pascual-Leone, A.; Joutsa, J. The Study of Noninvasive Brain Stimulation Using Molecular Brain Imaging: A Systematic Review. Neuroimage 2020, 219, 117023. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nakajima, W.; Hatano, M.; Shibata, Y.; Kuroki, Y.; Arisawa, T.; Serizawa, A.; Sano, A.; Kogami, S.; Yamanoue, T.; et al. Visualization of AMPA Receptors in Living Human Brain with Positron Emission Tomography. Nat. Med. 2020, 26, 281–288. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kricheldorff, J.; Göke, K.; Kiebs, M.; Kasten, F.H.; Herrmann, C.S.; Witt, K.; Hurlemann, R. Evidence of Neuroplastic Changes after Transcranial Magnetic, Electric, and Deep Brain Stimulation. Brain Sci. 2022, 12, 929. https://doi.org/10.3390/brainsci12070929
Kricheldorff J, Göke K, Kiebs M, Kasten FH, Herrmann CS, Witt K, Hurlemann R. Evidence of Neuroplastic Changes after Transcranial Magnetic, Electric, and Deep Brain Stimulation. Brain Sciences. 2022; 12(7):929. https://doi.org/10.3390/brainsci12070929
Chicago/Turabian StyleKricheldorff, Julius, Katharina Göke, Maximilian Kiebs, Florian H. Kasten, Christoph S. Herrmann, Karsten Witt, and Rene Hurlemann. 2022. "Evidence of Neuroplastic Changes after Transcranial Magnetic, Electric, and Deep Brain Stimulation" Brain Sciences 12, no. 7: 929. https://doi.org/10.3390/brainsci12070929
APA StyleKricheldorff, J., Göke, K., Kiebs, M., Kasten, F. H., Herrmann, C. S., Witt, K., & Hurlemann, R. (2022). Evidence of Neuroplastic Changes after Transcranial Magnetic, Electric, and Deep Brain Stimulation. Brain Sciences, 12(7), 929. https://doi.org/10.3390/brainsci12070929





