The Influence of Neurotrophic Factors BDNF and GDNF Overexpression on the Functional State of Mice and Their Adaptation to Audiogenic Seizures
Abstract
:1. Introduction
2. Materials and Methods
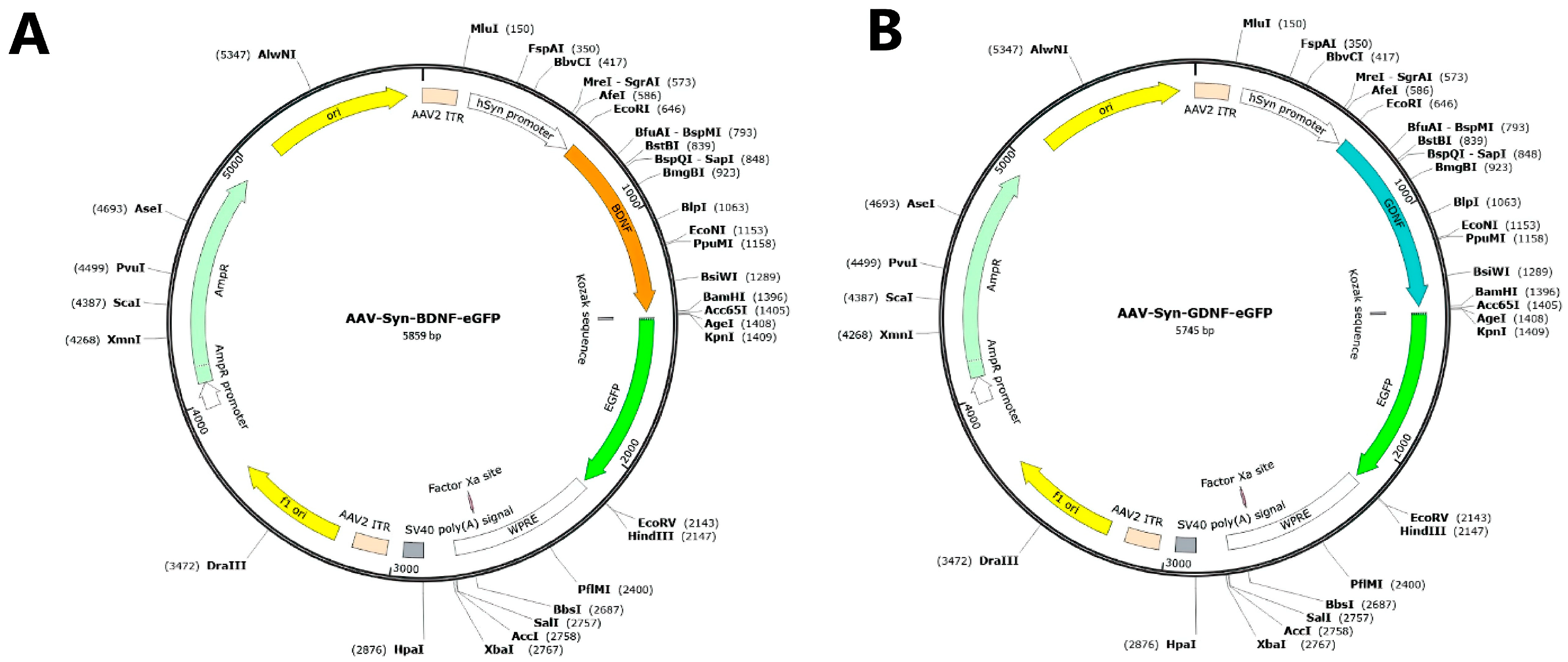
2.1. Research Object
2.2. Scheme of the Experiment
2.3. Audiogenic Seizures Model
2.4. Neurological Status Assessment
2.5. Open Field Test
2.6. Test of Conditioned Passive Avoidance Reflex (CPAR)
2.7. Real-Time PCR
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarmast, S.T.; Abdullahi, A.M.; Jahan, N. Current Classification of Seizures and Epilepsies: Scope, Limitations and Recommendations for Future Action. Cureus 2020, 12, 10549. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Fonseca, E.; Ettcheto, M.; Sánchez-López, E.; de Rojas, I.; Alonso-Lana, S.; Morató, X.; Souto, E.B.; Toledo, M.; Boada, M.; et al. Epilepsy in Neurodegenerative Diseases: Related Drugs and Molecular Pathways. Pharmaceuticals 2021, 14, 1057. [Google Scholar] [CrossRef]
- Perucca, P.; Bahlo, M.; Berkovic, S.F. The Genetics of Epilepsy. Annu. Rev. Genom. Hum. Genet. 2020, 21, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Giri, M.; Xia, Z.; Subedi, Y.N.; Li, Y. Genetic and epigenetic mechanisms of epilepsy: A review. Neuropsychiatr Dis. Treat 2017, 13, 1841–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitroshina, E.V.; Mishchenko, T.A.; Shirokova, O.M.; Astrakhanova, T.A.; Loginova, M.M.; Epifanova, E.A.; Babaev, A.A.; Tarabykin, V.S.; Vedunova, M.V. Intracellular Neuroprotective Mechanisms in Neuron-Glial Networks Mediated by Glial Cell Line-Derived Neurotrophic Factor. Oxidative Med. Cell. Longev. 2019, 18. [Google Scholar] [CrossRef]
- Mitroshina, E.V.; Mishchenko, T.A.; Shishkina, T.V.; Vedunova, M.V. Role of Neurotrophic Factors BDNF and GDNF in Nervous System Adaptation to the Influence of Ischemic Factors. Bull. Exp. Biol. Med. 2019, 167, 574–579. [Google Scholar] [CrossRef]
- Mishchenko, T.A.; Mitroshina, E.V.; Usenko, A.V.; Voronova, N.V.; Astrakhanova, T.A.; Shirokova, O.M.; Kastalskiy, I.A.; Vedunova, M.V. Features of Neural Network Formation and Their Functions in Primary Hippocampal Cultures in the Context of Chronic TrkB Receptor System Influence. Front. Physiol. 2019, 9, 1925. [Google Scholar] [CrossRef] [Green Version]
- Skaper, S.D. Neurotrophic Factors: An Overview. Methods Mol. Biol. 2018, 1727, 1–17. [Google Scholar]
- Vilar, M.; Mira, H. Regulation of Neurogenesis by Neurotrophins during Adulthood: Expected and Unexpected Roles. Front. Neurosci. 2016, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Falcicchia, C.; Paolone, G.; Emerich, D.F.; Lovisari, F.; Bell, W.J.; Fradet, T.; Wahlberg, L.U.; Simonato, M. Seizure-Suppressant and Neuroprotective Effects of Encapsulated BDNF-Producing Cells in a Rat Model of Temporal Lobe Epilepsy. Mol. Ther. Methods Clin. Dev. 2018, 9, 211–224. [Google Scholar] [CrossRef] [Green Version]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of Brain-Derived Neurotrophin Factor in the Neurogenesis and Neuronal Function, and Its Involvement in the Pathophysiology of Brain Diseases. Int. J. Mol. Sci. 2018, 19, 3650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanter-Schlifke, I.; Fjord-Larsen, L.; Kusk, P.; Angehagen, M.; Wahlberg, L.; Kokaia, M. GDNF released from encapsulated cells suppresses seizure activity in the epileptic hippocampus. Exp. Neurol. 2009, 216, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Mahato, A.K.; Sidorova, Y.A. Glial cell line-derived neurotrophic factors (GFLs) and small molecules targeting RET receptor for the treatment of pain and Parkinson’s disease. Cell Tissue Res. 2020, 382, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, G.Y.; Ding, S. Glial Cell Line-Derived Neurotrophic Factor and Focal Ischemic Stroke. Neurochem. Res. 2021, 46, 2638–2650. [Google Scholar] [CrossRef]
- Cacialli, P. Neurotrophins Time Point Intervention after Traumatic Brain Injury: From Zebrafish to Human. Int. J. Mol. Sci. 2021, 22, 1585. [Google Scholar] [CrossRef]
- Castrén, E.; Antila, H. Neuronal plasticity and neurotrophic factors in drug responses. Mol. Psychiatry 2017, 22, 1085–1095. [Google Scholar] [CrossRef]
- Paolone, G.; Falcicchia, C.; Lovisari, F.; Kokaia, M.; Bell, W.J.; Fradet, T.; Barbieri, M.; Wahlberg, L.U.; Emerich, D.F.; Simonato, M. Long-Term, Targeted Delivery of GDNF from Encapsulated Cells Is Neuroprotective and Reduces Seizures in the Pilocarpine Model of Epilepsy. J. Neurosci. 2019, 39, 2144–2156. [Google Scholar] [CrossRef] [Green Version]
- Naldini, L. Gene therapy returns to centre stage. Nature 2015, 526, 351–360. [Google Scholar] [CrossRef]
- Turovskaya, M.V.; Gaidin, S.G.; Vedunova, M.V.; Babaev, A.A.; Turovsky, E.A. BDNF Overexpression Enhances the Preconditioning Effect of Brief Episodes of Hypoxia, Promoting Survival of GABAergic Neurons. Neurosci. Bull. 2020, 36, 733–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binder, D.K.; Boison, D.; Eid, T.; Frankel, W.N.; Mingorance, A.; Smith, B.N.; Dacks, P.A.; Whittemore, V.; Poduri, A. Epilepsy Benchmarks Area II: Prevent Epilepsy and Its Progression. Epilepsy Curr. 2020, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Rayaprolu, V.; Kruse, S.; Kant, R.; Venkatakrishnan, B.; Movahed, N.; Brooke, D.; Lins, B.; Bennett, A.; Potter, T.; McKenna, R.; et al. Comparative analysis of adeno-associated virus capsid stability and dynamics. J. Virol. 2013, 87, 13150–13160. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.T.; Airavaara, M.; Harvey, B.K. Viral vectors for neurotrophic factor delivery: A gene therapy approach for neurodegenerative diseases of the CNS. Pharmacol. Res. 2010, 61, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mays, L.E.; Wang, L.; Lin, J.; Bell, P.; Crawford, A.; Wherry, E.J.; Wilson, J.M. AAV8 induces tolerance in murine muscle as a result of poor APC transduction, T cell exhaustion, and minimal MHCI upregulation on target cells. Mol. Ther. 2014, 22, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Park, C.H.; Kim, J.; Namgung, E.; Lee, D.W.; Kim, G.H.; Kim, M.; Kim, N.; Kim, T.D.; Kim, S.; Lyoo, I.K.; et al. The BDNF Val66Met Polymorphism Affects the Vulnerability of the Brain Structural Network. Front. Hum. Neurosci. 2017, 11, 400. [Google Scholar] [CrossRef]
- de Assis, G.G.; Hoffman, J.R. The BDNF Val66Met Polymorphism is a Relevant, But not Determinant, Risk Factor in the Etiology of Neuropsychiatric Disorders–Current Advances in Human Studies: A Systematic Review. Brain Plast. 2022, 1–10. [Google Scholar] [CrossRef]
- Giarratana, A.O.; Teng, S.; Reddi, S.; Zheng, C.; Adler, D.; Thakker-Varia, S.; Alder, J. BDNF Val66Met Genetic Polymorphism Results in Poor Recovery Following Repeated Mild Traumatic Brain Injury in a Mouse Model and Treatment With AAV-BDNF Improves Outcomes. Front. Neurol. 2019, 10, 1175. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, B.; Butler, J.; Felker, G.M.; Ponikowski, P.; Voors, A.A.; Pogoda, J.M.; Provost, R.; Guerrero, J.; Hajjar, R.J.; Zsebo, K.M. Prevalence of AAV1 neutralizing antibodies and consequences for a clinical trial of gene transfer for advanced heart failure. Gene Ther. 2016, 23, 313–319. [Google Scholar] [CrossRef]
- Mishchenko, T.A.; Zhidkova, N.M.; Urazov, M.D.; Golushkova, A.D.; Kustova, A.O.; Lukovnikova, L.B.; Terentieva, K.A.; Babaev, A.A.; Vedunova, M.V. The Influence of Chronic Prenatal Hypoxia on the Functional State of Mice and Their Adaptation to Audiogenic Seizures. Opera Med. Physiol. 2022, 9, 42–53. [Google Scholar] [CrossRef]
- Mitroshina, E.V.; Mishchenko, T.A.; Usenko, A.V.; Epifanova, E.A.; Yarkov, R.S.; Gavrish, M.S.; Babaev, A.A.; Vedunova, M.V. AAV-Syn-BDNF-EGFP virus construct exerts neuroprotective action on the hippocampal neural network during hypoxia in vitro. Int. J. Mol. Sci. 2018, 19, 2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Ash, R.T.; Ceballos-Diaz, C.; Levites, Y.; Golde, T.E.; Smirnakis, S.M.; Jankowsky, J.L. Viral transduction of the neonatal brain delivers controllable genetic mosaicism for visualising and manipulating neuronal circuits in vivo. Eur. J. Neurosci. 2013, 37, 1203–1220. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Grunke, S.D.; Levites, Y.; Golde, T.E.; Jankowsky, J.L. Intracerebroventricular viral injection of the neonatal mouse brain for persistent and widespread neuronal transduction. J. Vis. Exp. 2014, 15, 51863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semiokhina, A.F.; Fedotova, I.B.; Poletaeva, I.I. Rats of Krushinsky–Molodkina strain: Studies of audiogenic epilepsy, vascular pathology and behavior. Zhurnal Vyss. Nervn. Deyatelnosti Im. IP Pavlov. 2006, 56, 298–316. [Google Scholar]
- Borisova, E.; Epifanova, E.; Tutukova, S.; Belousova, I.; Zhidkova, N.; Rusanova, A.; Salina, V.; Turovsky, E.; Turovskaya, M.; Tarabykin, V. Identification of novel mutations controlling cerebral cortex malformations caused by ENU-induced mutagenesis in the mouse. CoBpeMeHHыe MexHoлoгuu B MeдuцuHe 2018, 10, 70–76. [Google Scholar] [CrossRef]
- Novozhilova, M.; Mishchenko, T.; Kondakova, E.; Lavrova, T.; Gavrish, M.; Aferova, S.; Franceschi, C.; Vedunova, M. Features of age-related response to sleep deprivation: In vivo experimental studies. Aging 2021, 13, 19108–19126. [Google Scholar] [CrossRef]
- Beni-Adani, L.; Gozes, I.; Cohen, Y.; Assaf, Y.; Steingart, R.A.; Brenneman, D.E.; Eizenberg, O.; Trembolver, V.; Shohami, E. A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J. Pharmacol. Exp. Ther. 2001, 296, 57–63. [Google Scholar]
- Gavrish, M.S.; Urasov, M.D.; Mishchenko, T.A.; Turubanova, V.D.; Krut, V.G.; Babaev, A.A.; Vedunova, M.V.; Mitroshina, E.V. Neuroprotective effects of brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) overexpression in experimental model of acute hypobaric hypoxia in vivo. Int. J. Mol. Sci. 2022. [Google Scholar]
- Farrell, J.S.; Wolff, M.D.; Teskey, G.C. Neurodegeneration and Pathology in Epilepsy: Clinical and Basic Perspectives. Adv. Neurobiol. 2017, 15, 317–334. [Google Scholar]
- Sirven, J.I. Epilepsy: A Spectrum Disorder. Cold Spring Harb. Perspect. Med. 2015, 5, a022848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pracucci, E.; Pillai, V.; Lamers, D.; Parra, R.; Landi, S. Neuroinflammation: A Signature or a Cause of Epilepsy? Int. J. Mol. Sci. 2021, 22, 6981. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Galanopoulou, A.S. Epilepsy and epileptic syndrome. Adv. Exp. Med. Biol. 2012, 724, 99–113. [Google Scholar] [PubMed]
- Shlobin, N.A.; Sander, J.W. Learning from the comorbidities of epilepsy. Curr. Opin. Neurol. 2022, 35, 175–180. [Google Scholar] [CrossRef]
- Kerr, M.P. The impact of epilepsy on patients’ lives. Acta Neurol. Scand. 2012, 194, 1–9. [Google Scholar] [CrossRef]
- Iughetti, L.; Lucaccioni, L.; Fugetto, F.; Predieri, B.; Berardi, A.; Ferrari, F. Brain-derived neurotrophic factor and epilepsy: A systematic review. Neuropeptides 2018, 72, 23–29. [Google Scholar] [CrossRef]
- Li, S.; Xu, B.; Martin, D.; Racine, R.J.; Fahnestock, M. Glial cell line-derived neurotrophic factor modulates kindling and activation-induced sprouting in hippocampus of adult rats. Exp. Neurol. 2002, 178, 49–58. [Google Scholar] [CrossRef]
- Vedunova, М.; Sakharnova, T.; Mitroshina, E.; Shishkina, T.; Astrakhanova, T.; Mukhina, I. Antihypoxic and neuroprotective properties of BDNF and GDNF in vitro and in vivo under hypoxic conditions. Sovrem. Tehnol. V Med. 2014, 6, 38–45. [Google Scholar]


| A: Parameters of locomotor activity | |||||
| Mouse Group | Distance Traveled in the Arena, cm | Time in the Arena Center, s | Number of Upright Postures | ||
| Periphery | Center | Total | |||
| Sham | 841.2 ± 247.4 | 105.2 ± 28.7 | 973.3 ± 161.3 | 67.7 ± 34.8 | 29.8 ± 5.7 |
| PBS | 1526.2 ± 172.5 | 120.1 ± 11.5 | 1421.5 ± 162.8 | 22.9 ± 12.9 | 29.4 ± 4.5 |
| AAV-BDNF-eGFP | 1005.5 ± 174.0 | 130.7 ± 43.5 | 1001.26 ± 160.8 | 32.7 ± 16.5 | 20.4 ± 5.4 |
| AAV-GDNF-eGFP | 762.1 ± 242.8 | 92.7 ± 30.6 | 817.6 ± 158.1 | 44.4 ± 31.1 | 16.7 ± 6.2 |
| B: Emotional status characteristics | |||||
| Mouse Group | Acts of Urination | Acts of Defecation | |||
| Sham | 1.6 ± 0.4 | 2.7 ± 0.6 | |||
| PBS | 2.6 ± 0.5 | 2.7 ± 0.8 | |||
| AAV-BDNF-eGFP | 2.1 ± 0.5 | 2.7 ± 0.4 | |||
| AAV-GDNF-eGFP | 1.7 ± 0.5 | 3.3 ± 0.9 | |||
| Mouse Group | Latent Period of Movement to the Dark Chamber Section, s | |
|---|---|---|
| Training Session | Retesting | |
| Sham | 40.9 ± 11.8 | 155.7 ± 9.3 |
| PBS | 32.1 ± 6.2 | 162.9 ± 9.1 |
| AAV-BDNF-eGFP | 45.1 ± 9.9 | 167.6 ± 9.6 |
| AAV-GDNF-eGFP | 50.9 ± 6.7 | 179.7 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kustova, A.O.; Gavrish, M.S.; Sergeeva, M.A.; Avlasenko, D.A.; Kiseleva, A.O.; Epifanova, E.A.; Babaev, A.A.; Mishchenko, T.A.; Vedunova, M.V. The Influence of Neurotrophic Factors BDNF and GDNF Overexpression on the Functional State of Mice and Their Adaptation to Audiogenic Seizures. Brain Sci. 2022, 12, 1039. https://doi.org/10.3390/brainsci12081039
Kustova AO, Gavrish MS, Sergeeva MA, Avlasenko DA, Kiseleva AO, Epifanova EA, Babaev AA, Mishchenko TA, Vedunova MV. The Influence of Neurotrophic Factors BDNF and GDNF Overexpression on the Functional State of Mice and Their Adaptation to Audiogenic Seizures. Brain Sciences. 2022; 12(8):1039. https://doi.org/10.3390/brainsci12081039
Chicago/Turabian StyleKustova, Angelina O., Maria S. Gavrish, Marina A. Sergeeva, Daria A. Avlasenko, Anna O. Kiseleva, Ekaterina A. Epifanova, Alexey A. Babaev, Tatiana A. Mishchenko, and Maria V. Vedunova. 2022. "The Influence of Neurotrophic Factors BDNF and GDNF Overexpression on the Functional State of Mice and Their Adaptation to Audiogenic Seizures" Brain Sciences 12, no. 8: 1039. https://doi.org/10.3390/brainsci12081039
APA StyleKustova, A. O., Gavrish, M. S., Sergeeva, M. A., Avlasenko, D. A., Kiseleva, A. O., Epifanova, E. A., Babaev, A. A., Mishchenko, T. A., & Vedunova, M. V. (2022). The Influence of Neurotrophic Factors BDNF and GDNF Overexpression on the Functional State of Mice and Their Adaptation to Audiogenic Seizures. Brain Sciences, 12(8), 1039. https://doi.org/10.3390/brainsci12081039







