Is Cortical Theta-Gamma Phase-Amplitude Coupling Memory-Specific?
Abstract
:1. Introduction
2. Methods
2.1. Participants
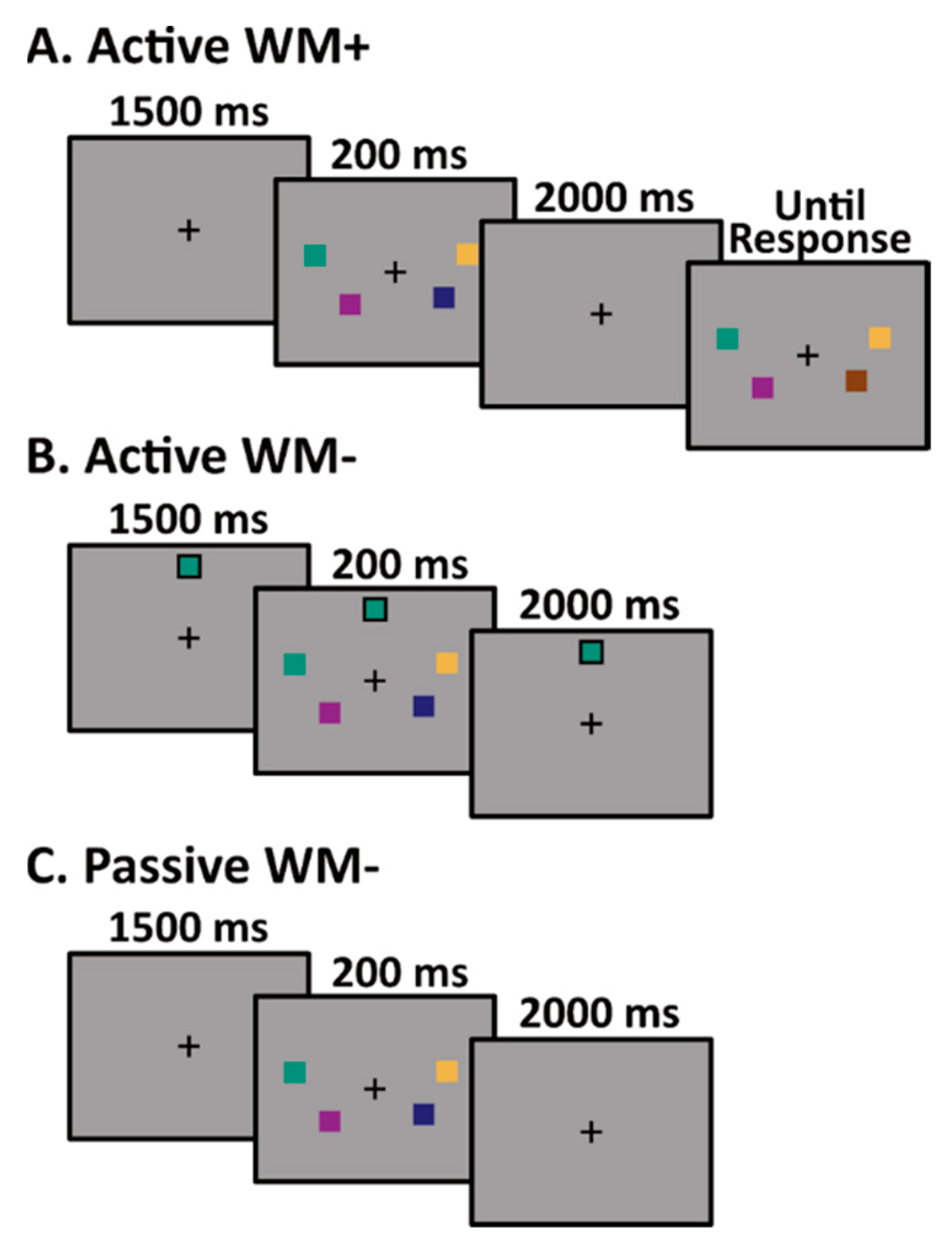
2.2. Experimental Paradigm
2.3. EEG Recording and Pre-Processing
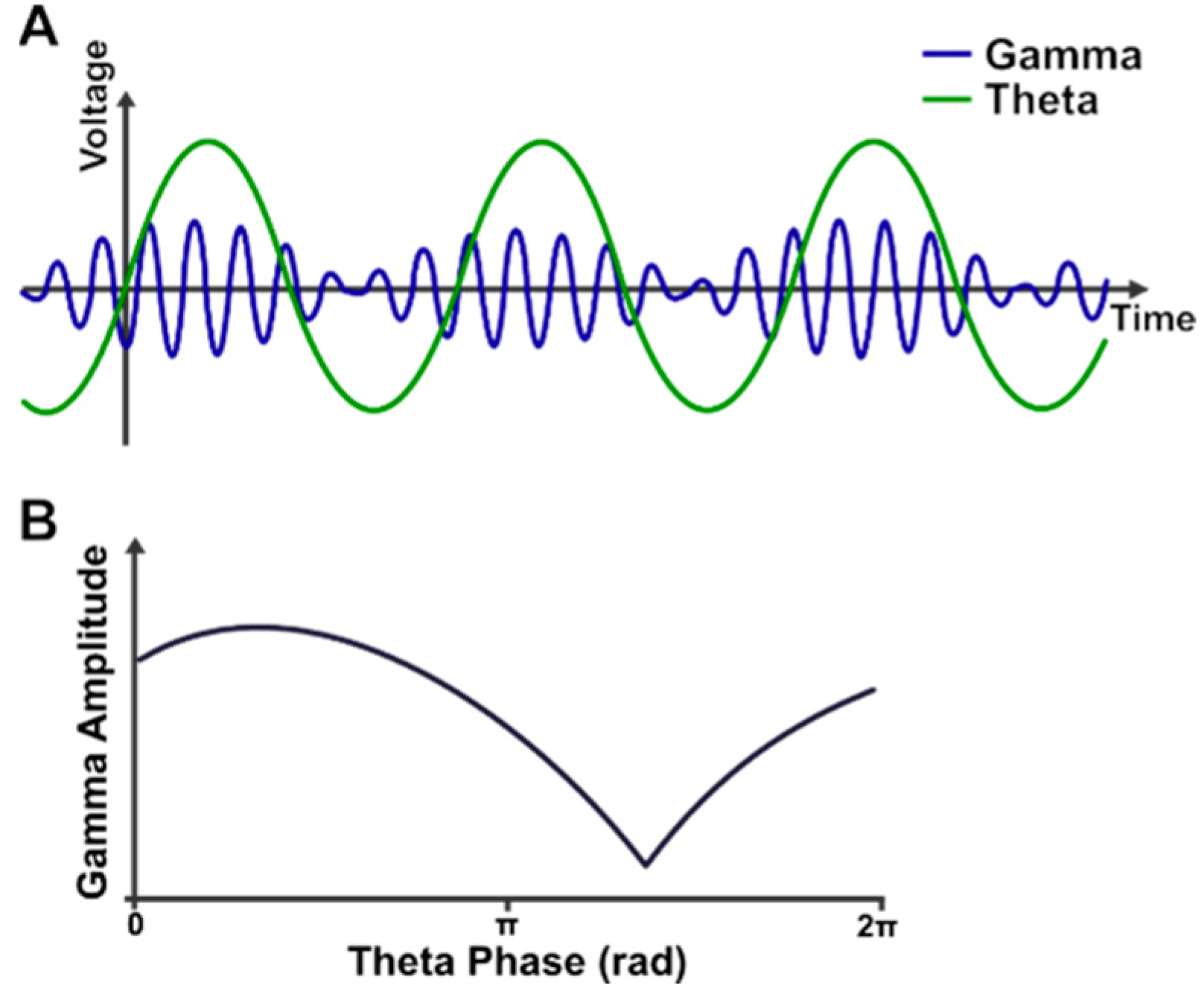
2.4. Theta-Gamma Phase-Amplitude Coupling
2.5. Statistical Analyses
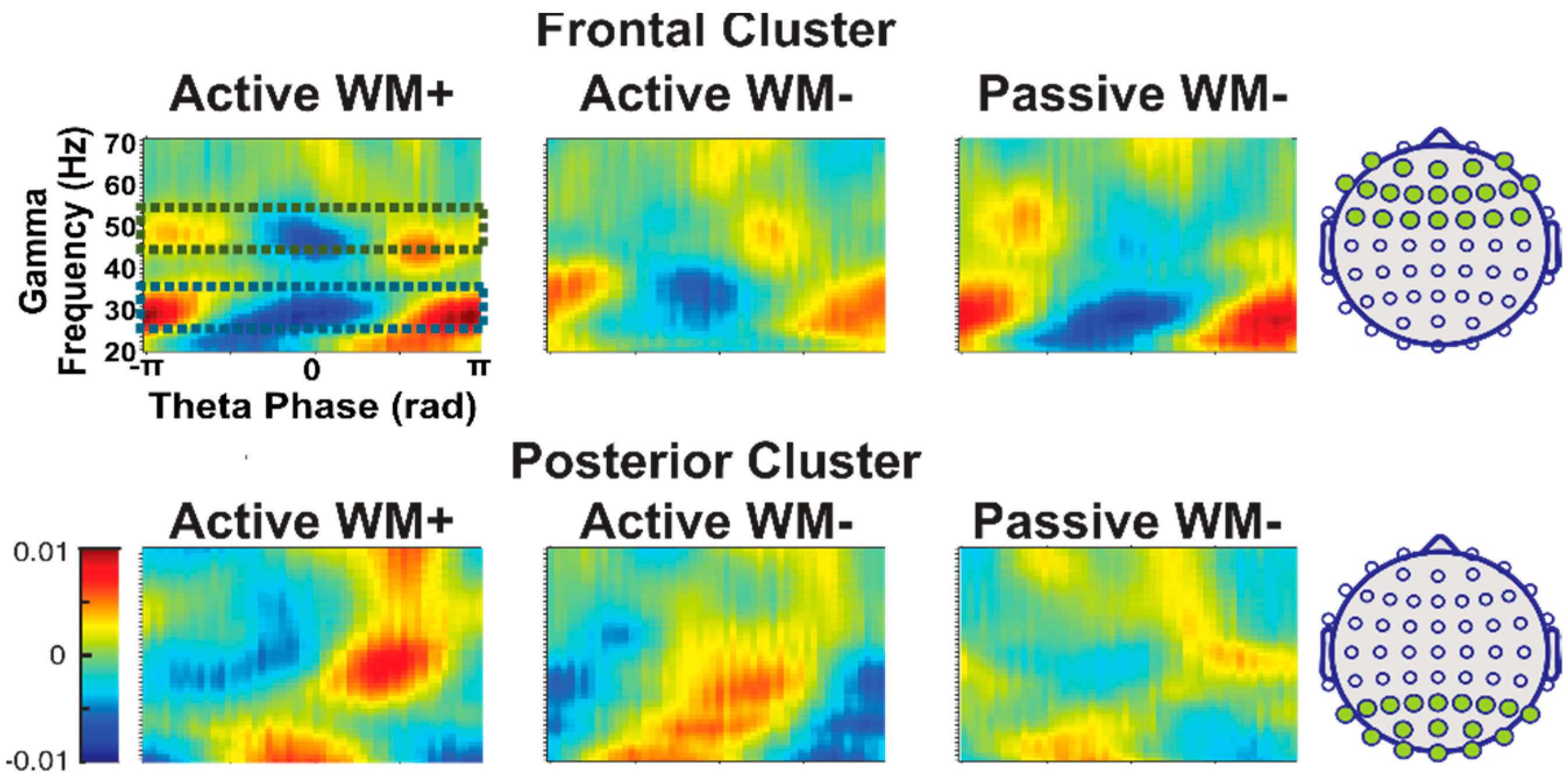
3. Results
3.1. Effects of Task and Time Window
3.2. Focused Contrast Analysis
3.3. Effects of Cluster and Frequency Bands
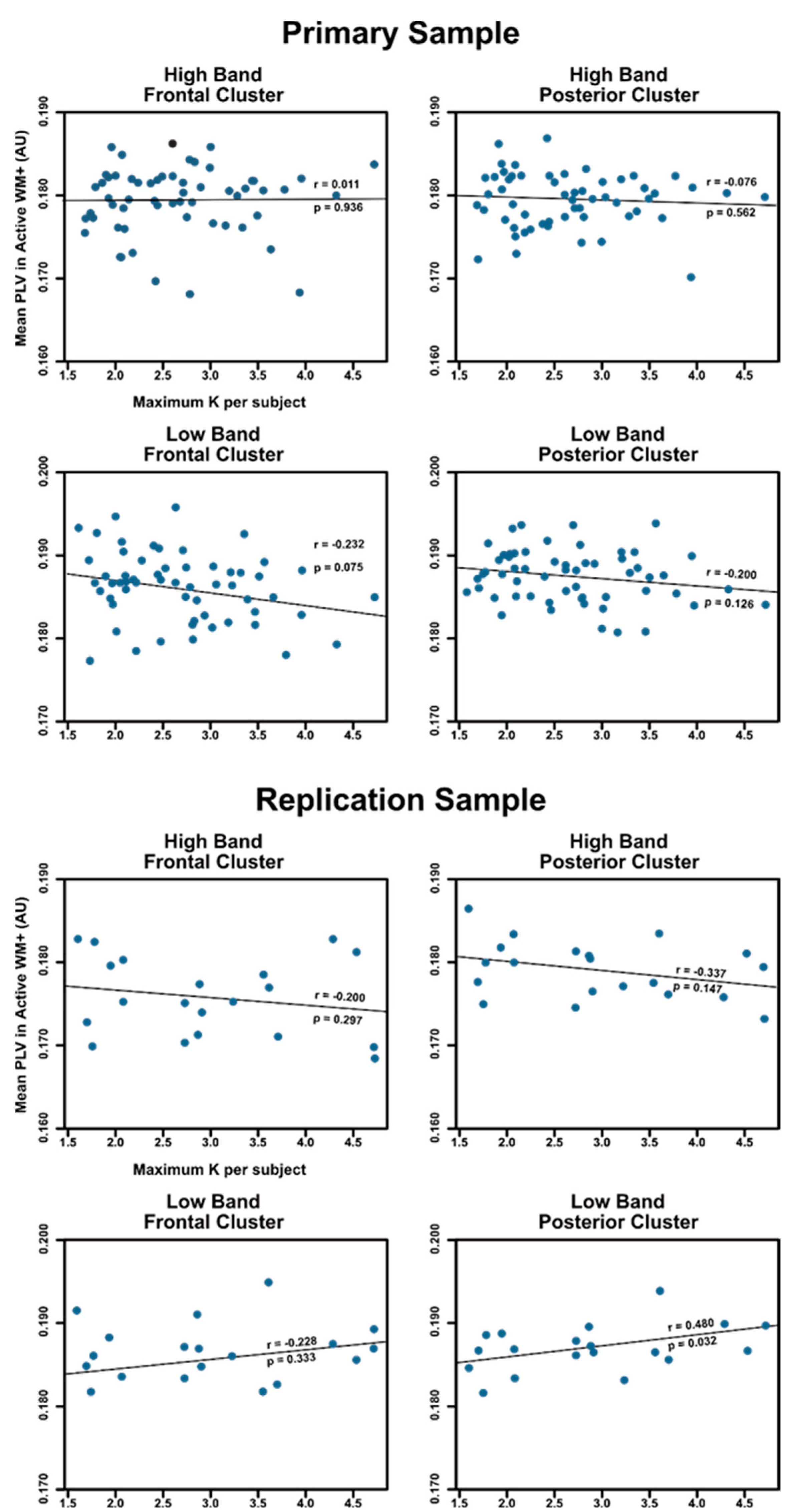
3.4. Relationship between PLV and Working Memory Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baddeley, A.D.; Hitch, G. Working Memory. In Psychology of Learning and Motivation; Academic Press: New York, NY, USA, 1974; Volume 8, pp. 47–89. ISBN 978-0-12-543308-2/1-84872-001-7. [Google Scholar]
- Baddeley, A. The Central Executive: A Concept and Some Misconceptions. J. Int. Neuropsychol. Soc. 1998, 4, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Grimley, M.; Banner, G. Working Memory, Cognitive Style, and Behavioural Predictors of GCSE Exam Success. Educ. Psychol. 2008, 28, 341–351. [Google Scholar] [CrossRef]
- Engle, R.W.; Tuholski, S.W.; Laughlin, J.E.; Conway, A.R.A. Working Memory, Short-Term Memory, and General Fluid Intelligence: A Latent-Variable Approach. J. Exp. Psychol. Gen. 1999, 128, 309–331. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Awh, E.; Vogel, E.K. Discrete Capacity Limits in Visual Working Memory. Curr. Opin. Neurobiol. 2010, 20, 177–182. [Google Scholar] [CrossRef]
- Gold, J.M.; Barch, D.M.; Feuerstahler, L.M.; Carter, C.S.; MacDonald, A.W.; Ragland, J.D.; Silverstein, S.M.; Strauss, M.E.; Luck, S.J. Working Memory Impairment Across Psychotic Disorders. Schizophr. Bull. 2019, 45, 804–812. [Google Scholar] [CrossRef]
- González-Ortega, I.; de los Mozos, V.; Echeburúa, E.; Mezo, M.; Besga, A.; Ruiz de Azúa, S.; González-Pinto, A.; Gutierrez, M.; Zorrilla, I.; González-Pinto, A. Working Memory as a Predictor of Negative Symptoms and Functional Outcome in First Episode Psychosis. Psychiatry Res. 2013, 206, 8–16. [Google Scholar] [CrossRef]
- Lisman, J.E.; Jensen, O. The Theta-Gamma Neural Code. Neuron 2013, 77, 1002–1016. [Google Scholar] [CrossRef]
- Lisman, J.E.; Idiart, M.A.P. Storage of 7 ± 2 Short-Term Memories in Oscillatory Subcycles. Science 1995, 267, 1512–1515. [Google Scholar] [CrossRef]
- Lisman, J. Working Memory: The Importance of Theta and Gamma Oscillations. Curr. Biol. 2010, 20, R490–R492. [Google Scholar] [CrossRef]
- Axmacher, N.; Henseler, M.M.; Jensen, O.; Weinreich, I.; Elger, C.E.; Fell, J. Cross-Frequency Coupling Supports Multi-Item Working Memory in the Human Hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 3228–3233. [Google Scholar] [CrossRef] [Green Version]
- Bahramisharif, A.; Jensen, O.; Jacobs, J.; Lisman, J. Serial Representation of Items during Working Memory Maintenance at Letter-Selective Cortical Sites. PLoS Biol. 2018, 16, e2003805. [Google Scholar] [CrossRef]
- Chaieb, L.; Leszczynski, M.; Axmacher, N.; Höhne, M.; Elger, C.E.; Fell, J. Theta-Gamma Phase-Phase Coupling during Working Memory Maintenance in the Human Hippocampus. Cogn. Neurosci. 2015, 6, 149–157. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Heise, K.F.; Gruber, W.R.; Holz, E.; Karim, A.A.; Glennon, M.; Gerloff, C.; Birbaumer, N.; Hummel, F.C. Brain Oscillatory Substrates of Visual Short-Term Memory Capacity. Curr. Biol. 2009, 19, 1846–1852. [Google Scholar] [CrossRef]
- Kaplan, R.; Bush, D.; Bonnefond, M.; Bandettini, P.A.; Barnes, G.R.; Doeller, C.F.; Burgess, N. Medial Prefrontal Theta Phase Coupling during Spatial Memory Retrieval. Hippocampus 2014, 24, 656–665. [Google Scholar] [CrossRef]
- Muthukumaraswamy, S. High-Frequency Brain Activity and Muscle Artifacts in MEG/EEG: A Review and Recommendations. Front. Hum. Neurosci. 2013, 7, 138. [Google Scholar] [CrossRef]
- Park, J.Y.; Jhung, K.; Lee, J.; An, S.K. Theta–Gamma Coupling during a Working Memory Task as Compared to a Simple Vigilance Task. Neurosci. Lett. 2013, 532, 39–43. [Google Scholar] [CrossRef]
- Rajji, T.K.; Zomorrodi, R.; Barr, M.S.; Blumberger, D.M.; Mulsant, B.H.; Daskalakis, Z.J. Ordering Information in Working Memory and Modulation of Gamma by Theta Oscillations in Humans. Cereb. Cortex 2016, 27, bhv326. [Google Scholar] [CrossRef]
- Goodman, M.S.; Kumar, S.; Zomorrodi, R.; Ghazala, Z.; Cheam, A.S.M.; Barr, M.S.; Daskalakis, Z.J.; Blumberger, D.M.; Fischer, C.; Flint, A.; et al. Theta-Gamma Coupling and Working Memory in Alzheimer’s Dementia and Mild Cognitive Impairment. Front. Aging Neurosci. 2018, 10, 101. [Google Scholar] [CrossRef]
- Barr, M.S.; Rajji, T.K.; Zomorrodi, R.; Radhu, N.; George, T.P.; Blumberger, D.M.; Daskalakis, Z.J. Impaired Theta-Gamma Coupling during Working Memory Performance in Schizophrenia. Schizophr. Res. 2017, 189, 104–110. [Google Scholar] [CrossRef]
- Sauseng, P.; Griesmayr, B.; Freunberger, R.; Klimesch, W. Control Mechanisms in Working Memory: A Possible Function of EEG Theta Oscillations. Neurosci. Biobehav. Rev. 2010, 34, 1015–1022. [Google Scholar] [CrossRef]
- Voytek, B.; Canolty, R.; Shestyuk, A.; Crone, N.; Parvizi, J.; Knight, R. Shifts in Gamma Phase–Amplitude Coupling Frequency from Theta to Alpha Over Posterior Cortex During Visual Tasks. Front. Hum. Neurosci. 2010, 4, 191. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.A.; Smith, D.; Albrecht, M.A.; Silverstein, S. Alpha-band Desynchronization Reflects Memory-specific Processes during Visual Change Detection. Psychophysiology 2019, 56, e13442. [Google Scholar] [CrossRef]
- Vogel, E.K.; Machizawa, M.G. Neural Activity Predicts Individual Differences in Visual Working Memory Capacity. Nature 2004, 428, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Leonard, C.J.; Kaiser, S.T.; Robinson, B.M.; Kappenman, E.S.; Hahn, B.; Gold, J.M.; Luck, S.J. Toward the Neural Mechanisms of Reduced Working Memory Capacity in Schizophrenia. Cereb. Cortex 2013, 23, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Lopez-Calderon, J.; Luck, S.J. ERPLAB: An Open-Source Toolbox for the Analysis of Event-Related Potentials. Front. Hum. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput. Intell. Neurosci. 2010, 2011, e156869. [Google Scholar] [CrossRef]
- Penny, W.D.; Duzel, E.; Miller, K.J.; Ojemann, J.G. Testing for Nested Oscillation. J. Neurosci. Methods 2008, 174, 50–61. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2022. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Lawrence, M.A. Ez: Easy Analysis and Visualization of Factorial Experiments. 2016. Available online: https://CRAN.R-project.org/package=ez (accessed on 19 July 2022).
- Morey, R.D.; Rouder, J.N. BayesFactor: Computation of Bayes Factors for Common Designs. 2021. Available online: https://CRAN.R-project.org/package=BayesFactor (accessed on 19 July 2022).
- Pinheiro, J.; Bates, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models. 2022. Available online: https://CRAN.R-project.org/package=nlme (accessed on 19 July 2022).
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000. [Google Scholar]
- Rouder, J.N.; Morey, R.D.; Morey, C.C.; Cowan, N. How to Measure Working Memory Capacity in the Change Detection Paradigm. Psychon. Bull. Rev. 2011, 18, 324–330. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 2000, 57, 289–300. [Google Scholar] [CrossRef]
- Luck, S.J.; Gaspelin, N. How to Get Statistically Significant Effects in Any ERP Experiment (and Why You Shouldn’t). Psychophysiology 2017, 54, 146–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Main ANOVA Results | |||||||
|---|---|---|---|---|---|---|---|
| Cluster | Frequency Band | Effect | dfs | F | p(GG) | η2 | BF10 |
| Frontal | High | Time Window | 1, 59 | 73,073 | <0.001 *** | 0.986 | >10374 |
| Frontal | High | Task | 1.97, 116.3 | 0.40 | 0.666 | 0.006 | 0.030 |
| Frontal | High | Timewindow × Task | 1.96, 115.4 | 0.27 | 0.757 | 0.002 | -- |
| Frontal | Low | Time Window | 1, 59 | 20,363 | <0.001 *** | 0.995 | >10355 |
| Frontal | Low | Task | 1.74, 102.8 | 3.12 | 0.055 | 0.044 | 0.031 |
| Frontal | Low | Timewindow × Task | 2.00, 118.0 | 2.57 | 0.081 | 0.003 | -- |
| Posterior | High | Time Window | 1, 59 | 5915 | <0.001 *** | 0.980 | >10374 |
| Posterior | High | Task | 1.97, 116.1 | 0.18 | 0.831 | 0.008 | 0.033 |
| Posterior | High | Timewindow × Task | 1.88, 111.1 | 1.77 | 0.177 | 0.000 | -- |
| Posterior | Low | Time Window | 1, 59 | 5625 | <0.001 *** | 0.990 | >10403 |
| Posterior | Low | Task | 1.91, 112.7 | 0.79 | 0.451 | 0.001 | 0.030 |
| Posterior | Low | Timewindow × Task | 1.91, 112.6 | 1.54 | 0.217 | 0.004 | -- |
| Contrast Analysis Results | ||||||
|---|---|---|---|---|---|---|
| Cluster | Frequency Band | Effect | df | t | p | Cohen’s d |
| Frontal | High | Time Window | 295 | 240 | <0.001 *** | >104 |
| Frontal | High | Task | 295 | 0.86 | 0.390 | 0.043 |
| Frontal | High | Timewindow × Task | 295 | −0.28 | 0.782 | 0.005 |
| Frontal | Low | Time Window | 295 | 210 | <0.001 *** | >104 |
| Frontal | Low | Task | 295 | 0.66 | 0.512 | 0.025 |
| Frontal | Low | Timewindow × Task | 295 | 1.37 | 0.171 | 0.109 |
| Posterior | High | Time Window | 295 | 240 | <0.001 *** | >104 |
| Posterior | High | Task | 295 | −0.85 | 0.395 | 0.042 |
| Posterior | High | Timewindow × Task | 295 | 0.68 | 0.495 | 0.027 |
| Posterior | Low | Time Window | 295 | 269 | <0.001 *** | >104 |
| Posterior | Low | Task | 295 | 0.66 | 0.509 | 0.025 |
| Posterior | Low | Timewindow × Task | 295 | 0.31 | 0.406 | 0.006 |
| Pairwise Comparisons for Electrode Cluster | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cluster | PLV | Time Window | Frequency Band | Task | df | t | p | FDR | Cohen’s d | |
| Mean | SD | |||||||||
| Frontal | 0.105 | 0.005 | Pre-Stimulus | High | Active WM+ | 59 | −0.61 | 0.54 | n.s. | −0.08 |
| Posterior | 0.109 | 0.004 | ||||||||
| Frontal | 0.106 | 0.005 | Pre-Stimulus | High | Active WM- | 59 | −0.35 | 0.731 | n.s. | −0.05 |
| Posterior | 0.109 | 0.003 | ||||||||
| Frontal | 0.106 | 0.005 | Pre-Stimulus | High | Passive WM- | 59 | −1.19 | 0.24 | n.s. | −0.15 |
| Posterior | 0.109 | 0.003 | ||||||||
| Frontal | 0.114 | 0.002 | Pre-Stimulus | Low | Active WM+ | 59 | 1.11 | 0.271 | n.s. | 0.14 |
| Posterior | 0.116 | 0.003 | ||||||||
| Frontal | 0.114 | 0.002 | Pre-Stimulus | Low | Active WM- | 59 | −0.74 | 0.464 | n.s. | −0.10 |
| Posterior | 0.114 | 0.002 | ||||||||
| Frontal | 0.114 | 0.003 | Pre-Stimulus | Low | Passive WM- | 59 | 1.64 | 0.107 | n.s. | 0.21 |
| Posterior | 0.115 | 0.002 | ||||||||
| Frontal | 0.176 | 0.005 | Post-Stimulus | High | Active WM+ | 59 | −1.4 | 0.168 | n.s. | −0.18 |
| Posterior | 0.179 | 0.004 | ||||||||
| Frontal | 0.177 | 0.006 | Post-Stimulus | High | Active WM- | 59 | −1.51 | 0.134 | n.s. | −0.19 |
| Posterior | 0.180 | 0.004 | ||||||||
| Frontal | 0.177 | 0.005 | Post-Stimulus | High | Passive WM- | 59 | −0.86 | 0.578 | n.s. | −0.11 |
| Posterior | 0.179 | 0.004 | ||||||||
| Frontal | 0.186 | 0.006 | Post-Stimulus | Low | Active WM+ | 59 | 0.66 | 0.51 | n.s. | 0.09 |
| Posterior | 0.187 | 0.003 | ||||||||
| Frontal | 0.184 | 0.005 | Post-Stimulus | Low | Active WM- | 59 | −0.45 | 0.658 | n.s. | −0.06 |
| Posterior | 0.186 | 0.003 | ||||||||
| Frontal | 0.185 | 0.006 | Post-Stimulus | Low | Passive WM- | 59 | −1.91 | 0.06 | n.s. | −0.25 |
| Posterior | 0.187 | 0.003 | ||||||||
| Pairwise Comparisons for Frequency Band. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | PLV | Time window | Cluster | Task | df | t | p | FDR | Cohen’s d | |
| Mean | SD | |||||||||
| High | 0.105 | 0.005 | Pre-Stimulus | Frontal | Active WM+ | 59 | −9.8 | <0.001 | * | −1.27 |
| Low | 0.114 | 0.002 | ||||||||
| High | 0.106 | 0.005 | Pre-Stimulus | Frontal | Active WM- | 59 | −10.2 | <0.001 | * | −1.32 |
| Low | 0.114 | 0.002 | ||||||||
| High | 0.106 | 0.005 | Pre-Stimulus | Frontal | Passive WM- | 59 | −12.2 | <0.001 | * | −1.58 |
| Low | 0.114 | 0.003 | ||||||||
| High | 0.109 | 0.004 | Pre-Stimulus | Posterior | Active WM+ | 59 | −10.5 | <0.001 | * | −1.36 |
| Low | 0.116 | 0.003 | ||||||||
| High | 0.109 | 0.003 | Pre-Stimulus | Posterior | Active WM- | 59 | −12.4 | <0.001 | * | −1.60 |
| Low | 0.114 | 0.002 | ||||||||
| High | 0.109 | 0.003 | Pre-Stimulus | Posterior | Passive WM- | 59 | −10.4 | <0.001 | * | −1.34 |
| Low | 0.115 | 0.002 | ||||||||
| High | 0.176 | 0.005 | Post-Stimulus | Frontal | Active WM+ | 59 | −6.9 | <0.001 | * | −0.89 |
| Low | 0.186 | 0.006 | ||||||||
| High | 0.177 | 0.006 | Post-Stimulus | Frontal | Active WM- | 59 | −8.8 | <0.001 | * | −1.14 |
| Low | 0.184 | 0.005 | ||||||||
| High | 0.177 | 0.005 | Post-Stimulus | Frontal | Passive WM- | 59 | −4.1 | <0.001 | * | −0.53 |
| Low | 0.185 | 0.006 | ||||||||
| High | 0.179 | 0.004 | Post-Stimulus | Posterior | Active WM+ | 59 | −10.4 | <0.001 | * | −1.34 |
| Low | 0.187 | 0.003 | ||||||||
| High | 0.180 | 0.004 | Post-Stimulus | Posterior | Active WM- | 59 | −12.9 | <0.001 | * | −1.67 |
| Low | 0.186 | 0.003 | ||||||||
| High | 0.179 | 0.004 | Post-Stimulus | Posterior | Passive WM- | 59 | −12.9 | <0.001 | * | −1.67 |
| Low | 0.187 | 0.003 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papaioannou, O.; Crespo, L.P.; Clark, K.; Ogbuagu, N.N.; Alliende, L.M.; Silverstein, S.M.; Erickson, M.A. Is Cortical Theta-Gamma Phase-Amplitude Coupling Memory-Specific? Brain Sci. 2022, 12, 1131. https://doi.org/10.3390/brainsci12091131
Papaioannou O, Crespo LP, Clark K, Ogbuagu NN, Alliende LM, Silverstein SM, Erickson MA. Is Cortical Theta-Gamma Phase-Amplitude Coupling Memory-Specific? Brain Sciences. 2022; 12(9):1131. https://doi.org/10.3390/brainsci12091131
Chicago/Turabian StylePapaioannou, Orestis, Laura P. Crespo, Kailey Clark, Nicole N. Ogbuagu, Luz Maria Alliende, Steven M. Silverstein, and Molly A. Erickson. 2022. "Is Cortical Theta-Gamma Phase-Amplitude Coupling Memory-Specific?" Brain Sciences 12, no. 9: 1131. https://doi.org/10.3390/brainsci12091131






