Influence of Nutritional Ketosis Achieved through Various Methods on Plasma Concentrations of Brain Derived Neurotropic Factor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study 1: Ketone Salt Supplementation with Acute Exercise
2.2. Study 2: Prolonged Ketogenic Diet and Resistance Training
2.3. Study 3: Ketogenic Diet, with and without Ketone Salts, during Weight Loss
| Methods | Experiment | ||
|---|---|---|---|
| Study 1 | Study 2 | Study 3 | |
| Sample Size | 12 M/12 F | 25 M/4 F | 19 M/18 F |
| Duration | Cross-sectional | 12 weeks | 6 weeks |
| Structured Exercise | Yes | Yes | No |
| Controlled Feeding | No | No | Yes |
| Weight Loss | No | KD only | Yes |
| Ketone Supplements | Yes | No | Yes |
| Cross-over | Yes | No | No |
| Randomized | Yes | No | Yes |
| Double Blind | Yes | No | Yes |
| Control Group | Yes | Yes | Yes |
2.4. Blood BHB and Plasma BDNF Analysis
2.5. Statistical Analysis
3. Results
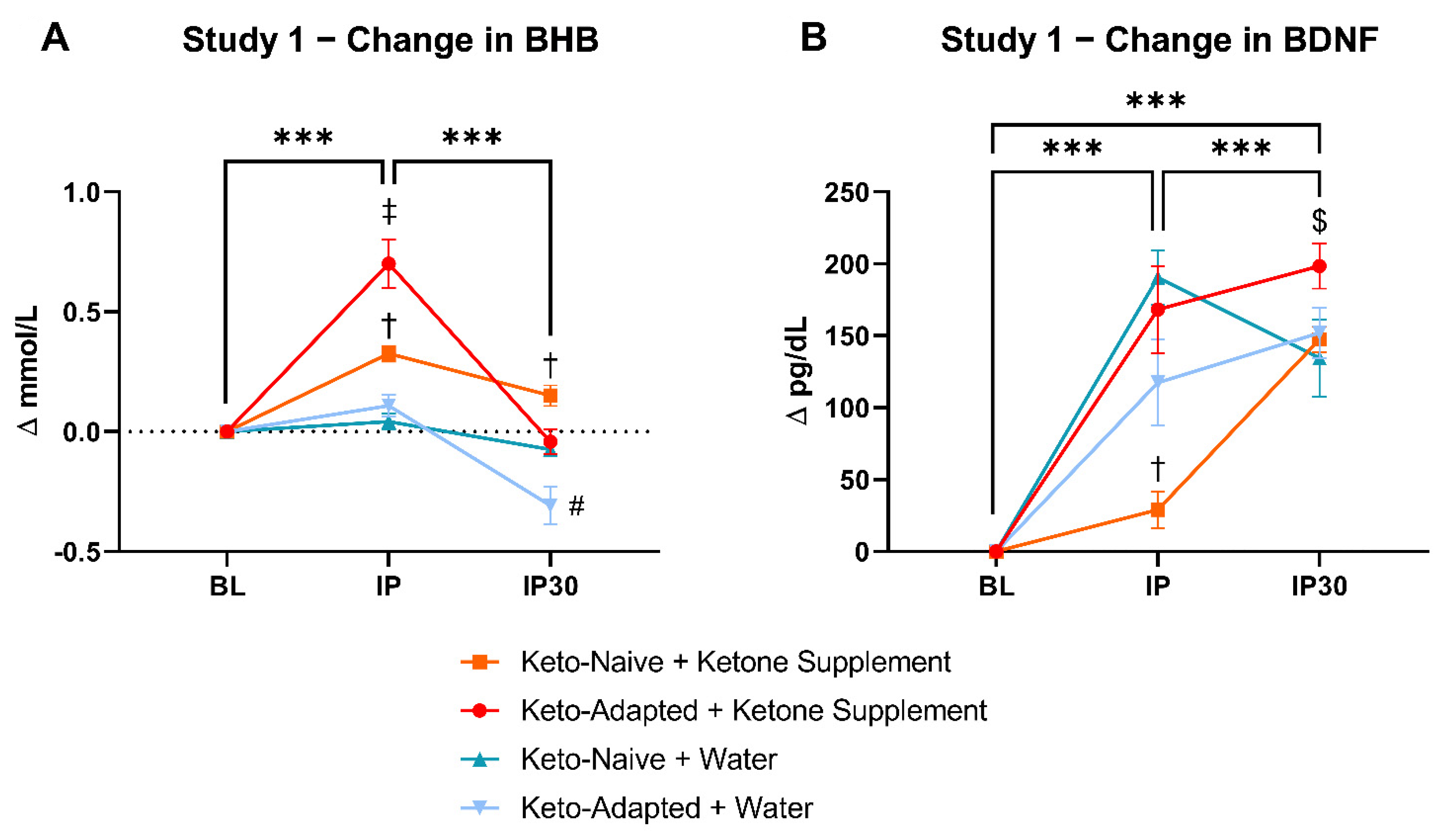
3.1. Study 1
3.1.1. Fasting/Resting BHB and BDNF
3.1.2. Post-Exercise BHB and BDNF
3.1.3. Metabolic and Hormonal Reponses
3.2. Study 2
3.2.1. BHB and BDNF
3.2.2. Metabolic and Hormonal Responses
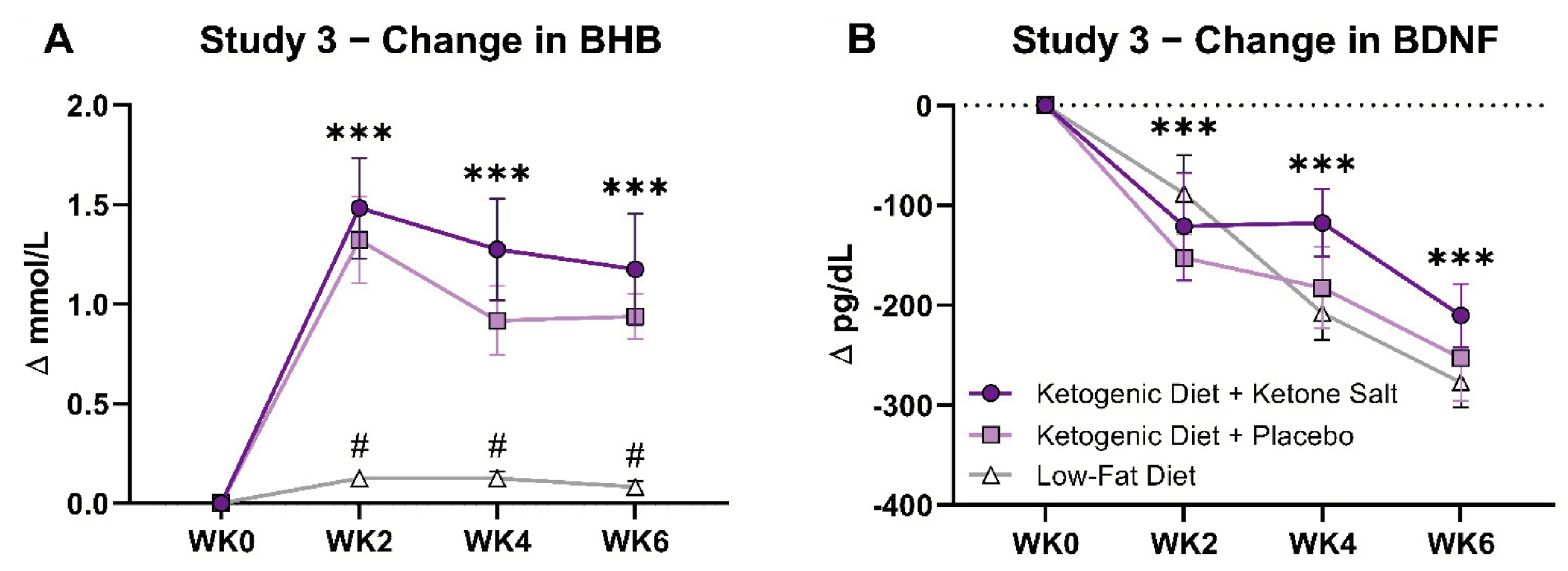
3.3. Study 3
3.3.1. BHB and BDNF
3.3.2. Metabolic and Hormonal Responses
3.4. Study 1 vs. Study 2 vs. Study 3
4. Discussion
4.1. Exercise and BDNF
4.2. Ketones and BDNF
4.3. Weight Loss and BDNF
4.4. Exploratory Effects of Different Markers of Health on BDNF
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rios, M.; Fan, G.; Fekete, C.; Kelly, J.; Bates, B.; Kuehn, R.; Lechan, R.M.; Jaenisch, R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 2001, 15, 1748–1757. [Google Scholar] [CrossRef]
- Hernandez, A.R.; Hernandez, C.M.; Campos, K.; Truckenbrod, L.; Federico, Q.; Moon, B.; McQuail, J.; Maurer, A.P.; Bizon, J.L.; Burke, S.N. A Ketogenic Diet Improves Cognition and Has Biochemical Effects in Prefrontal Cortex That Are Dissociable From Hippocampus. Front. Aging Neurosci. 2018, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Parodi, L.R.; Amgalan, A.; Sultan, S.F.; Antal, B.; Sun, X.; Skiena, S.; Lithen, A.; Adra, N.; Ratai, E.-M.; Weistuch, C.; et al. Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc. Natl. Acad. Sci. USA 2020, 117, 6170–6177. [Google Scholar] [CrossRef] [PubMed]
- Phinney, S.D.; Horton, E.S.; Sims, E.A.H.; Hanson, J.S.; Danforth, E., Jr.; Lagrange, B.M. Capacity for Moderate Exercise in Obese Subjects after Adaptation to a Hypocaloric, Ketogenic Diet. J. Clin. Investig. 1980, 66, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Volk, B.M.; Kunces, L.J.; Freidenreich, D.J.; Kupchak, B.R.; Saenz, C.; Artistizabal, J.C.; Fernandez, M.L.; Bruno, R.S.; Maresh, C.M.; Kraemer, W.J.; et al. Effects of Step-Wise Increases in Dietary Carbohydrate on Circulating Saturated Fatty Acids and Palmitoleic Acid in Adults with Metabolic Syndrome. PLoS ONE 2014, 9, e113605. [Google Scholar] [CrossRef]
- Jensen, N.J.; Nilsson, M.; Ingerslev, J.S.; Olsen, D.A.; Fenger, M.; Svart, M.; Moller, N.; Zander, M.; Miskowiak, K.; Rungby, J. Effects of β-hydroxybutyrate on cognition in patients with type 2 diabetes. Eur. J. Endocrinol. 2020, 182, 233–242. [Google Scholar] [CrossRef]
- Evans, M.; Egan, B. Intermittent Running and Cognitive Performance after Ketone Ester Ingestion. Med. Sci. Sport. Exerc. 2018, 50, 2330–2338. [Google Scholar] [CrossRef]
- Gyorkos, A.; Baker, M.H.; Miutz, L.N.; Lown, D.A.; Jones, M.A.; Houghton-Rahrig, L.D. Carbohydrate-restricted Diet and Exercise Increase Brain-derived Neurotrophic Factor and Cognitive Function: A Randomized Crossover Trial. Cureus 2019, 11, e5604. [Google Scholar] [CrossRef]
- Taylor, M.K.; Sullivan, D.K.; Mahnken, J.D.; Burns, J.; Swerdlow, R.H. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 4, 28–36. [Google Scholar] [CrossRef]
- Lee, J.; Duan, W.; Mattson, M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 2002, 82, 1367–1375. [Google Scholar] [CrossRef]
- Brownlow, M.L.; Jung, S.H.; Moore, R.J.; Bechmann, N.; Jankord, R. Nutritional Ketosis Affects Metabolism and Behavior in Sprague-Dawley Rats in Both Control and Chronic Stress Environments. Front. Mol. Neurosci. 2017, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Mohorko, N.; Černelič-Bizjak, M.; Poklar-Vatovec, T.; Grom, G.; Kenig, S.; Petelin, A.; Jenko-Pražnikar, Z. Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr. Res. 2018, 62, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Myette-Côté, É; Little, J.P. The Effect of Exogenous Ketone Monoester Ingestion on Plasma BDNF During an Oral Glucose Tolerance Test. Front. Physiol. 2020, 11, 1094. [Google Scholar] [CrossRef]
- Walsh, J.J.; Neudorf, H.; Little, J.P. 14-Day Ketone Supplementation Lowers Glucose and Improves Vascular Function in Obesity: A Randomized Crossover Trial. J. Clin. Endocrinol. Metab. 2021, 106, e1738–e1754. [Google Scholar] [CrossRef] [PubMed]
- Kackley, M.L.; Short, J.A.; Hyde, P.N.; LaFountain, R.A.; Buga, A.; Miller, V.J.; Dickerson, R.M.; Sapper, T.N.; Barnhart, E.C.; Krishnan, D.; et al. A Pre-Workout Supplement of Ketone Salts, Caffeine, and Amino Acids Improves High-Intensity Exercise Performance in Keto-Naïve and Keto-Adapted Individuals. J. Am. Coll. Nutr. 2020, 39, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.J.; Cox, P.J.; Evans, R.D.; Santer, P.; Miller, J.J.; Faull, O.K.; Magor-Elliott, S.; Hiyama, S.; Stirling, M.; Clarke, K. On the Metabolism of Exogenous Ketones in Humans. Front. Physiol. 2017, 8, 848. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef]
- Altar, C. Neurotrophins and depression. Trends Pharmacol. Sci. 1999, 20, 59–62. [Google Scholar] [CrossRef]
- Heyman, E.; Gamelin, F.-X.; Goekint, M.; Piscitelli, F.; Roelands, B.; Leclair, E.; Di Marzo, V.; Meeusen, R. Intense exercise increases circulating endocannabinoid and BDNF levels in humans—Possible implications for reward and depression. Psychoneuroendocrinology 2012, 37, 844–851. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002, 25, 295–301. [Google Scholar] [CrossRef]
- Church, D.D.; Hoffman, J.R.; Mangine, G.T.; Jajtner, A.R.; Townsend, J.; Beyer, K.S.; Wang, R.; La Monica, M.B.; Fukuda, D.; Stout, J.R. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J. Appl. Physiol. 2016, 121, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Buga, A.; Kackley, M.L.; Crabtree, C.D.; Sapper, T.N.; Mccabe, L.; Fell, B.; LaFountain, R.A.; Hyde, P.N.; Martini, E.R.; Bowman, J.; et al. The Effects of a 6-Week Controlled, Hypocaloric Ketogenic Diet, With and Without Exogenous Ketone Salts, on Body Composition Responses. Front. Nutr. 2021, 8, 618520. [Google Scholar] [CrossRef] [PubMed]
- LaFountain, R.A.; Miller, V.J.; Barnhart, E.C.; Hyde, P.N.; Crabtree, C.D.; McSwiney, F.T.; Beeler, M.K.; Buga, A.; Sapper, T.N.; Short, J.A.; et al. Extended Ketogenic Diet and Physical Training Intervention in Military Personnel. Mil. Med. 2019, 184, e538–e547. [Google Scholar] [CrossRef]
- Miller, V.J.; LaFountain, R.A.; Barnhart, E.; Sapper, T.S.; Short, J.; Arnold, W.D.; Hyde, P.N.; Crabtree, C.D.; Kackley, M.L.; Kraemer, W.J.; et al. A ketogenic diet combined with exercise alters mitochondrial function in human skeletal muscle while improving metabolic health. Am. J. Physiol. Metab. 2020, 319, E995–E1007. [Google Scholar] [CrossRef]
- Crabtree, C.; Kackley, M.; Buga, A.; Fell, B.; LaFountain, R.; Hyde, P.; Sapper, T.; Kraemer, W.; Scandling, D.; Simonetti, O.; et al. Comparison of Ketogenic Diets with and without Ketone Salts versus a Low-Fat Diet: Liver Fat Responses in Overweight Adults. Nutrients 2021, 13, 966. [Google Scholar] [CrossRef]
- Chen, O.; Blonquist, T.; Mah, E.; Sanoshy, K.; Beckman, D.; Nieman, K.; Winters, B.; Anthony, J.; Verdin, E.; Newman, J.; et al. Tolerability and Safety of a Novel Ketogenic Ester, Bis-Hexanoyl (R)-1,3-Butanediol: A Randomized Controlled Trial in Healthy Adults. Nutrients 2021, 13, 2066. [Google Scholar] [CrossRef]
- Brunelli, A.; Dimauro, I.; Sgrò, P.; Emerenziani, G.P.; Magi, F.; Baldari, C.; Guidetti, L.; DI Luigi, L.; Parisi, P.; Caporossi, D. Acute Exercise Modulates BDNF and pro-BDNF Protein Content in Immune Cells. Med. Sci. Sport. Exerc. 2012, 44, 1871–1880. [Google Scholar] [CrossRef]
- Best, J.R.; Chiu, B.K.; Hsu, C.L.; Nagamatsu, L.S.; Liu-Ambrose, T. Long-Term Effects of Resistance Exercise Training on Cognition and Brain Volume in Older Women: Results from a Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2015, 21, 745–756. [Google Scholar] [CrossRef]
- Forti, L.N.; Van Roie, E.; Njemini, R.; Coudyzer, W.; Beyer, I.; Delecluse, C.; Bautmans, I. Dose-and gender-specific effects of resistance training on circulating levels of brain derived neurotrophic factor (BDNF) in community-dwelling older adults. Exp. Gerontol. 2015, 70, 144–149. [Google Scholar] [CrossRef]
- Walsh, J.J.; Scribbans, T.D.; Bentley, R.F.; Kellawan, J.M.; Gurd, B.; Tschakovsky, M.E. Neurotrophic growth factor responses to lower body resistance training in older adults. Appl. Physiol. Nutr. Metab. 2016, 41, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Yarrow, J.F.; White, L.J.; McCoy, S.C.; Borst, S.E. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci. Lett. 2010, 479, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Patchett, E.; Nally, R.; Kearns, R.; Larney, M.; Egan, B. Effect of acute ingestion of β-hydroxybutyrate salts on the response to graded exercise in trained cyclists. Eur. J. Sport Sci. 2018, 18, 376–386. [Google Scholar] [CrossRef]
- Morris, G.; Puri, B.K.; Maes, M.; Olive, L.; Berk, M.; Carvalho, A.F. The role of microglia in neuroprogressive disorders: Mechanisms and possible neurotherapeutic effects of induced ketosis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 99, 109858. [Google Scholar] [CrossRef] [PubMed]
- Rivell, A.; Mattson, M.P. Intergenerational Metabolic Syndrome and Neuronal Network Hyperexcitability in Autism. Trends Neurosci. 2019, 42, 709–726. [Google Scholar] [CrossRef]
- Norgren, J.; Daniilidou, M.; Kåreholt, I.; Sindi, S.; Akenine, U.; Nordin, K.; Rosenborg, S.; Ngandu, T.; Kivipelto, M.; Sandebring-Matton, A. Serum proBDNF Is Associated With Changes in the Ketone Body β-Hydroxybutyrate and Shows Superior Repeatability Over Mature BDNF: Secondary Outcomes From a Cross-Over Trial in Healthy Older Adults. Front. Aging Neurosci. 2021, 13, 716594. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-T.; Wang, J.-S.; Fu, C.-P.; Lin, S.-Y.; Sheu, W.H.-H. Relationship between body weight and the increment in serum brain-derived neurotrophic factor after oral glucose challenge in men with obesity and metabolic syndrome. Medicine 2016, 95, e5260. [Google Scholar] [CrossRef]
- Levinger, I.; Goodman, C.; Matthews, V.; Hare, D.; Jerums, G.; Garnham, A.; Selig, S. BDNF, Metabolic Risk Factors, and Resistance Training in Middle-Aged Individuals. Med. Sci. Sport. Exerc. 2008, 40, 535–541. [Google Scholar] [CrossRef]
- Huang, C.-J.; Mari, D.C.; Whitehurst, M.; Slusher, A.; Wilson, A.; Shibata, Y. Brain-derived neurotrophic factor expression ex vivo in obesity. Physiol. Behav. 2014, 123, 76–79. [Google Scholar] [CrossRef]
- Giacobbo, B.L.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [Green Version]
- Meek, T.H.; Wisse, B.E.; Thaler, J.P.; Guyenet, S.J.; Matsen, M.E.; Fischer, J.D.; Taborsky, G.J.; Schwartz, M.W.; Morton, G.J. BDNF Action in the Brain Attenuates Diabetic Hyperglycemia via Insulin-Independent Inhibition of Hepatic Glucose Production. Diabetes 2013, 62, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C.-M. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.R.; Jacka, F.N.; Gama, C.S.; Berk, M.; Leitão-Azevedo, C.L.; de Abreu, M.G.B.; Lobato, M.I.; Andreazza, A.C.; Ceresér, K.M.; Kapczinski, F.; et al. Serum levels of brain-derived neurotrophic factor in schizophrenia on a hypocaloric diet. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1595–1598. [Google Scholar] [CrossRef]
- Alasantro, L.H.; Hicks, T.H.; Green-Krogmann, E.; Murphy, C. Metabolic syndrome and cognitive performance across the adult lifespan. PLoS ONE 2021, 16, e0249348. [Google Scholar] [CrossRef]
- Duman, R.S. Neurotrophic factors and regulation of mood: Role of exercise, diet and metabolism. Neurobiol. Aging 2005, 26, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Pineda, W.D.; Parra-Rojas, I.; Rodríguez-Ruíz, H.A.; Illades-Aguiar, B.; Matia-García, I.; Garibay-Cerdenares, O.L. The regulatory role of insulin in energy metabolism and leukocyte functions. J. Leukoc. Biol. 2021, 111, 197–208. [Google Scholar] [CrossRef]
- Henninger, J.; Eliasson, B.; Smith, U.; Rawshani, A. Identification of markers that distinguish adipose tissue and glucose and insulin metabolism using a multi-modal machine learning approach. Sci. Rep. 2021, 11, 17050. [Google Scholar] [CrossRef]
- Rozanska, O.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Brain-Derived Neurotrophic Factor and Diabetes. Int. J. Mol. Sci. 2020, 21, 841. [Google Scholar] [CrossRef]
- Mikkelsen, K.H.; Seifert, T.; Secher, N.H.; Grøndal, T.; van Hall, G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males. J. Clin. Endocrinol. Metab. 2015, 100, 636–643. [Google Scholar] [CrossRef]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.; Lindegaard, B.; Petersen, A.M.W.; Taudorf, S.; et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]

| Study | Condition | Timepoints | Change | Effect Size | RM ANOVA (p-Values) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Midpoints | Post | Post–Pre | d | Condition | Time | Interaction | |||
| Study 1 | KN + KS | 322 ± 10 # | 351 ± 12 | 469 ± 13 | 147 ± 5 | 3.7 | 0.011 | <0.001 | <0.001 | |
| KA + KS | 257 ± 15 | 423 ± 34 | 456 ± 18 | 198 ± 7 | 3.5 | |||||
| KN + WT | 235 ± 6 | 426 ± 22 | 370 ± 26 | 134 ± 8 | 2.0 | |||||
| KA + WT | 234 ± 17 | 355 ± 27 | 386 ± 16 | 152 ± 7 | 2.7 | |||||
| Study 2 | KD + KS | 603 ± 31 | 482 ± 42 | 486 ± 27 | 393 ± 19 | −210 ± 10 | 2.4 | 0.54 | <0.001 | 0.15 |
| KD + PL | 639 ± 27 | 487 ± 22 | 457 ± 30 | 386 ± 27 | −253 ± 11 | 2.6 | ||||
| LFD | 610 ± 28 | 522 ± 25 | 402 ± 23 | 332 ± 28 | −277 ± 11 | 2.9 | ||||
| Study 3 | KD | 325 ± 28 | 335 ± 20 | 10 ± 10 | 0.1 | 0.76 | 0.64 | 0.91 | ||
| MD | 319 ± 18 | 325 ± 19 | 6 ± 7 | 0.1 | ||||||
| Study | Condition | Study 1 | Study 2 | Study 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| KN + KS | KA + KS | KN + WT | KA + WT | KD | MD | KD + KS | KD + PL | LFD | ||
| Study 1 | KN + KS | |||||||||
| KA + KS | 0.9 | |||||||||
| KN + WT | 0.2 | 0.8 | ||||||||
| KA + WT | 0.1 | 0.8 | 0.2 | |||||||
| Study 2 | KD | 2.3 | 2.8 | 1.5 | 2.2 | |||||
| MD | 2.4 | 2.9 | 1.6 | 2.3 | 0.1 | |||||
| Study 3 | KD + KS | 1.1 | 0.2 | 1.0 | 1.0 | 2.9 | 3.0 | |||
| KD + PL | 1.4 | 0.7 | 1.3 | 1.3 | 2.8 | 2.9 | 0.5 | |||
| LFD | 1.7 | 1.0 | 1.5 | 1.6 | 3.1 | 3.2 | 0.8 | 0.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kackley, M.L.; Buga, A.; Crabtree, C.D.; Sapper, T.N.; McElroy, C.A.; Focht, B.C.; Kraemer, W.J.; Volek, J.S. Influence of Nutritional Ketosis Achieved through Various Methods on Plasma Concentrations of Brain Derived Neurotropic Factor. Brain Sci. 2022, 12, 1143. https://doi.org/10.3390/brainsci12091143
Kackley ML, Buga A, Crabtree CD, Sapper TN, McElroy CA, Focht BC, Kraemer WJ, Volek JS. Influence of Nutritional Ketosis Achieved through Various Methods on Plasma Concentrations of Brain Derived Neurotropic Factor. Brain Sciences. 2022; 12(9):1143. https://doi.org/10.3390/brainsci12091143
Chicago/Turabian StyleKackley, Madison L., Alex Buga, Chris D. Crabtree, Teryn N. Sapper, Craig A. McElroy, Brian C. Focht, William J. Kraemer, and Jeff S. Volek. 2022. "Influence of Nutritional Ketosis Achieved through Various Methods on Plasma Concentrations of Brain Derived Neurotropic Factor" Brain Sciences 12, no. 9: 1143. https://doi.org/10.3390/brainsci12091143







