Admission Serum Iron as an Independent Risk Factor for Postoperative Delayed Cerebral Ischemia Following Aneurysmal Subarachnoid Hemorrhage: A Propensity-Matched Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Clinical Management
2.4. Outcome Assessment
2.5. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Association of SI with Initial Clinical Status at Admission
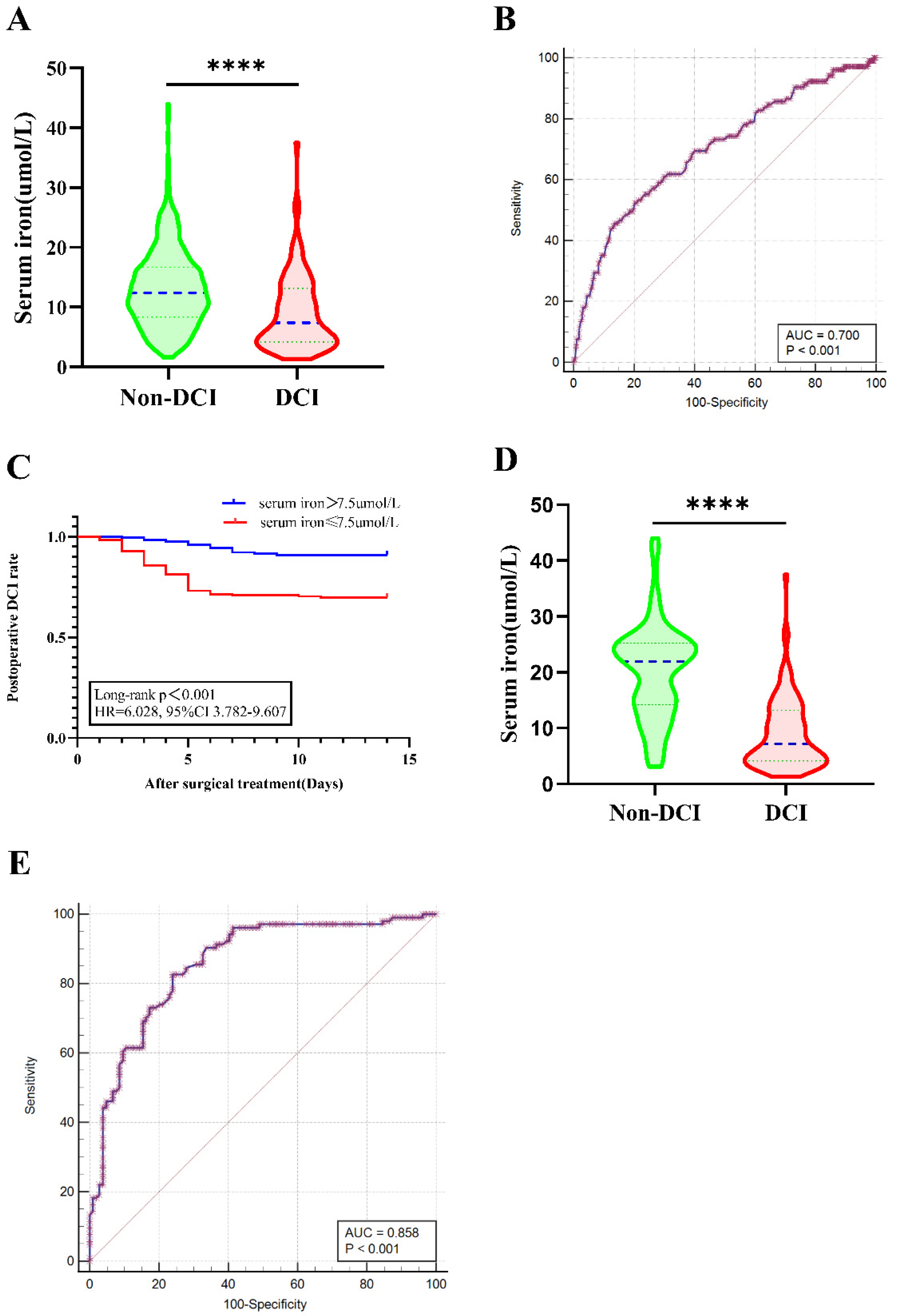
3.3. Association of Decreased SI with Postoperative DCI
3.4. Association of Decreased Serum Iron with 90-Day Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molyneux, A.J.; Kerr, R.S.; Birks, J.; Ranzi, N.; Yarnold, J.; Sneade, M.; ISAT Collaborators. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): Long-term follow-up. Lancet Neurol. 2009, 8, 427–433. [Google Scholar] [CrossRef]
- Lather, H.D.; Gornik, H.L.; Olin, J.W.; Gu, X.; Heidt, S.T.; Kim, E.S.H.; Kadian-Dodov, D.; Sharma, A.; Gray, B.; Jaff, M.R.; et al. Prevalence of Intracranial Aneurysm in Women With Fibromuscular Dysplasia: A Report From the US Registry for Fibromuscular Dysplasia. JAMA Neurol. 2017, 74, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L.; Schweizer, T.A. Spontaneous subarachnoid haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef]
- Osgood, M.L. Aneurysmal Subarachnoid Hemorrhage: Review of the Pathophysiology and Management Strategies. Curr. Neurol. Neurosci. Rep. 2021, 21, 50. [Google Scholar] [CrossRef]
- Macdonald, R.L. Delayed neurological deterioration after subarachnoid haemorrhage. Nat. Rev. Neurol. 2014, 10, 44–58. [Google Scholar] [CrossRef]
- Vergouwen, M.D.; Ilodigwe, D.; Macdonald, R.L. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke 2011, 42, 924–929. [Google Scholar] [CrossRef]
- De Rooij, N.K.; Rinkel, G.J.; Dankbaar, J.W.; Frijns, C.J. Delayed cerebral ischemia after subarachnoid hemorrhage: A systematic review of clinical, laboratory, and radiological predictors. Stroke 2013, 44, 43–54. [Google Scholar] [CrossRef]
- Leclerc, J.L.; Garcia, J.M.; Diller, M.A.; Carpenter, A.-M.; Kamat, P.K.; Hoh, B.L.; Doré, S. A Comparison of Pathophysiology in Humans and Rodent Models of Subarachnoid Hemorrhage. Front. Mol. Neurosci. 2018, 11, 71. [Google Scholar] [CrossRef]
- Lawton, M.T.; Vates, G.E. Subarachnoid Hemorrhage. N. Engl. J. Med. 2017, 377, 257–266. [Google Scholar] [CrossRef]
- Wang, W.X.; Springer, J.E.; Hatton, K.W. MicroRNAs as Biomarkers for Predicting Complications following Aneurysmal Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2021, 22, 9492. [Google Scholar] [CrossRef]
- Vergouwen, M.D.; de Haan, R.J.; Vermeulen, M.; Roos, Y.B. Effect of statin treatment on vasospasm, delayed cerebral ischemia, and functional outcome in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis update. Stroke 2010, 41, e47–e52. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, J.L.; Blackburn, S.; Neal, D.; Mendez, N.V.; Wharton, J.A.; Waters, M.F.; Doré, S. Haptoglobin phenotype predicts the development of focal and global cerebral vasospasm and may influence outcomes after aneurysmal subarachnoid hemorrhage. Proc. Natl. Acad. Sci. USA 2015, 112, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Carr, K.R.; Zuckerman, S.L.; Mocco, J. Inflammation, Cerebral Vasospasm, and Evolving Theories of Delayed Cerebral Ischemia. Neurol. Res. Int. 2013, 2013, 506584. [Google Scholar] [CrossRef] [PubMed]
- Loftspring, M.C. Iron and early brain injury after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2010, 30, 1791–1792. [Google Scholar] [CrossRef]
- Helbok, R.; Rass, V.; Kofler, M.; Talasz, H.; Schiefecker, A.; Gaasch, M.; Scherfler, C.; Pfausler, B.; Thomé, C.; Beer, R.; et al. Intracerebral Iron Accumulation may be Associated with Secondary Brain Injury in Patients with Poor Grade Subarachnoid Hemorrhage. Neurocrit. Care 2022, 36, 171–179. [Google Scholar] [CrossRef]
- Bulters, D.; Gaastra, B.; Zolnourian, A.H.; Alexander, S.; Ren, D.; Blackburn, S.L.; Borsody, M.; Dore, S.; Galea, J.; Iihara, K.; et al. Haemoglobin scavenging in intracranial bleeding: Biology and clinical implications. Nat. Rev. Neurol. 2018, 14, 416–432. [Google Scholar] [CrossRef]
- Bakhshayesh, B.; Hosseininezhad, M.; Nazanin Seyed Saadat, S.; Moien Ansar, M.; Ramezani, H.; Mohammad Seyed Saadat, S. Iron Overload is Associated with Perihematoma Edema Growth Following Intracerebral Hemorrhage that may Contribute to In-hospital Mortality and Long-term Functional Outcome. Curr. Neurovasc. Res. 2014, 11, 248–253. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, H.; Li, Y.; Weng, Y.; Xu, K.; Zhou, L.; Lin, H.; Sun, T.; Cheng, D.; Shen, J.; et al. Serum Alkaline Phosphatase Level is Associated with Angiographic Vasospasm, Delayed Cerebral Ischemia-Caused Clinical Deterioration, and Functional Outcome After Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2019, 31, 466–475. [Google Scholar] [CrossRef]
- Dreier, J.P.; Major, S.; Pannek, H.W.; Woitzik, J.; Scheel, M.; Wiesenthal, D.; Martus, P.; Winkler, M.K.; Hartings, J.A.; Fabricius, M.; et al. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain 2012, 135, 259–275. [Google Scholar] [CrossRef]
- Suwatcharangkoon, S.; Meyers, E.; Falo, C.; Schmidt, J.M.; Agarwal, S.; Claassen, J.; Mayer, S.A. Loss of Consciousness at Onset of Subarachnoid Hemorrhage as an Important Marker of Early Brain Injury. JAMA Neurol. 2016, 73, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef] [PubMed]
- Vergouwen, M.D.; van Geloven, N.; de Haan, R.J.; Kruyt, N.D.; Vermeulen, M.; Roos, Y.B. Increased cortisol levels are associated with delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neurocrit. Care 2010, 12, 342–345. [Google Scholar] [CrossRef]
- Haegens, N.M.; Gathier, C.S.; Horn, J.; Coert, B.A.; Verbaan, D.; van den Bergh, W.M. Induced Hypertension in Preventing Cerebral Infarction in Delayed Cerebral Ischemia After Subarachnoid Hemorrhage. Stroke 2018, 49, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Juvela, S. Plasma endothelin concentrations after aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2000, 92, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Al-Mufti, F.; Amuluru, K.; Damodara, N.; Dodson, V.; Roh, D.; Agarwal, S.; Meyers, P.M.; Connolly, E.S.; Schmidt, M.J.; Claassen, J.; et al. Admission neutrophil-lymphocyte ratio predicts delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. J. Neurointerv. Surg. 2019, 11, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Zheng, S.F.; Shang-Guan, H.C.; Kang, D.Z.; Chen, G.R.; Yao, P.S. Lower Iron Levels Predict Acute Hydrocephalus Following Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2019, 126, e907–e913. [Google Scholar] [CrossRef]
- Platz, J.; Guresir, E.; Wagner, M.; Seifert, V.; Konczalla, J. Increased risk of delayed cerebral ischemia in subarachnoid hemorrhage patients with additional intracerebral hematoma. J. Neurosurg. 2017, 126, 504–510. [Google Scholar] [CrossRef]
- Classen, J.; Bernardini, G.L.; Kreiter, K.; Bates, J.; Du, Y.E.; Copeland, D.; Connolly, E.S.; Mayer, S.A. Effect of Cisternal and Ventricular Blood on Risk of Delayed Cerebral Ischemia After Subarachnoid Hemorrhage The Fisher Scale Revisited. Stroke 2001, 32, 2012–2020. [Google Scholar] [CrossRef]
- Xi, G.; Keep, R.F.; Hoff, J.T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006, 5, 53–63. [Google Scholar] [CrossRef]
- Morgan, M.; Singhal, D.; Anderson, B. Quantitative assessment of blood-brain barrier damage during microdialysis. J. Pharmacol. Exp. Ther. 1996, 277, 1167–1176. [Google Scholar]
- Gomes, J.A.; Selim, M.; Cotleur, A.; Hussain, M.S.; Toth, G.; Koffman, L.; Asi, K.; Provencio, J.J. Brain iron metabolism and brain injury following subarachnoid hemorrhage: iCeFISH-pilot (CSF iron in SAH). Neurocrit. Care 2014, 21, 285–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinsberg, L.W.; Alexander, S.A.; Crago, E.A.; Minster, R.L.; Poloyac, S.M.; Weeks, D.E.; Conley, Y.P. Genetic Variability in the Iron Homeostasis Pathway and Patient Outcomes After Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2020, 33, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Heinsberg, L.W.; Arockiaraj, A.I.; Crago, E.A.; Ren, D.; Shaffer, J.R.; Sherwood, P.R.; Sereika, S.M.; Weeks, D.E.; Conley, Y.P. Genetic Variability and Trajectories of DNA Methylation May Support a Role for HAMP in Patient Outcomes After Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2020, 32, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Lai, P.M.R.; Castro, V.M.; Yu, S.; Dligach, D.; Finan, S.; Gainer, V.; Shadick, N.A.; Savova, G.; Murphy, S.; et al. Decreased Total Iron Binding Capacity May Correlate with Ruptured Intracranial Aneurysms. Sci. Rep. 2019, 9, 6054. [Google Scholar] [CrossRef]
- Lee, J.Y.; Keep, R.F.; He, Y.; Sagher, O.; Hua, Y.; Xi, G. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J. Cereb. Blood Flow Metab. 2010, 30, 1793–1803. [Google Scholar] [CrossRef]
- Munoz-Bravo, C.; Gutierrez-Bedmar, M.; Gomez-Aracena, J.; Garcia-Rodriguez, A.; Navajas, J.F. Iron: Protector or risk factor for cardiovascular disease? Still controversial. Nutrients 2013, 5, 2384–2404. [Google Scholar] [CrossRef]
- Hokari, M.; Uchida, K.; Shimbo, D.; Gekka, M.; Asaoka, K.; Itamoto, K. Acute systematic inflammatory response syndrome and serum biomarkers predict outcomes after subarachnoid hemorrhage. J. Clin. Neurosci. 2020, 78, 108–113. [Google Scholar] [CrossRef]
- Ahn, S.-H.; Savarraj, J.P.J.; Parsha, K.; Hergenroeder, G.W.; Chang, T.R.; Kim, D.H.; Kitagawa, R.S.; Blackburn, S.L.; Choi, H.A. Inflammation in delayed ischemia and functional outcomes after subarachnoid hemorrhage. J. Neuroinflamm. 2019, 16, 213. [Google Scholar] [CrossRef]
- McMahon, C.J.; Hopkins, S.; Vail, A.; King, A.T.; Smith, D.; Illingworth, K.J.; Clark, S.; Rothwell, N.J.; Tyrrell, P.J. Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage. J. Neurointerv. Surg. 2013, 5, 512–517. [Google Scholar] [CrossRef]
- Ma, X.; Lan, F.; Zhang, Y. Associations between C-reactive protein and white blood cell count, occurrence of delayed cerebral ischemia and poor outcome following aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. Acta Neurol. Belg. 2021, 121, 1311–1324. [Google Scholar] [CrossRef]
- Cai, L.; Zeng, H.; Tan, X.; Wu, X.; Qian, C.; Chen, G. The Role of the Blood Neutrophil-to-Lymphocyte Ratio in Aneurysmal Subarachnoid Hemorrhage. Front. Neurol. 2021, 12, 671098. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Grote, A. Interleukin 6 and Aneurysmal Subarachnoid Hemorrhage. A Narrative Review. Int. J. Mol. Sci. 2021, 22, 4133. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Tian, D.-C.; Li, Z.-G.; Ducruet, A.F.; Lawton, M.T.; Shi, F.-D. Global brain inflammation in stroke. Lancet Neurol. 2019, 18, 1058–1066. [Google Scholar] [CrossRef]
- Tombini, M.; Squitti, R.; Cacciapaglia, F.; Ventriglia, M.; Assenza, G.; Benvenga, A.; Pellegrino, G.; Campana, C.; Assenza, F.; Siotto, M.; et al. Inflammation and iron metabolism in adult patients with epilepsy: Does a link exist? Epilepsy Res. 2013, 107, 244–252. [Google Scholar] [CrossRef] [PubMed]
- de la Ossa, N.P.; Sobrino, T.; Silva, Y.; Blanco, M.; Millán, M.; Gomis, M.; Agulla, J.; Araya, P.; Reverté, S.; Serena, J.; et al. Iron-related brain damage in patients with intracerebral hemorrhage. Stroke 2010, 41, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.; Harr, K.; Murphy, D.; Walsh, M.; de Wit, M.; Deutsch, C.; Bonde, R. Serum Iron Analytes in Healthy and Diseased Florida Manatees (Trichechus manatus latirostris). J. Comp. Pathol. 2019, 173, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, A.; Watanabe, M.; Kazumi, T.; Fukuo, K. Association of Low Serum Iron Levels with Low-grade Inflammation and Hyperadiponectinemia in Community-living Elderly Women. J. Atheroscler. Thromb. 2013, 20, 670–677. [Google Scholar] [CrossRef]
- Muñoz, M.; Romero, A.; Morales, M.; Campos, A.; García-Erce, J.A.; Ramírez, G. Iron metabolism, inflammation and anemia in critically ill patients. A cross-sectional study. Nutr. Hosp. 2005, 20, 115–120. [Google Scholar]
- Bross, R.; Zitterkoph, J.; Pithia, J.; Benner, D.; Rambod, M.; Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Association of serum total iron-binding capacity and its changes over time with nutritional and clinical outcomes in hemodialysis patients. Am. J. Nephrol. 2009, 29, 571–581. [Google Scholar] [CrossRef]
- Krisai, P.; Leib, S.; Aeschbacher, S.; Kofler, T.; Assadian, M.; Maseli, A.; Todd, J.; Estis, J.; Risch, M.; Risch, L.; et al. Relationships of iron metabolism with insulin resistance and glucose levels in young and healthy adults. Eur. J. Intern. Med. 2016, 32, 31–37. [Google Scholar] [CrossRef]
- Mehta, P.J.; Chapman, S.; Jayam-Trouth, A.; Kurukumbi, M. Acute ischemic stroke secondary to iron deficiency anemia: A case report. Case Rep. Neurol. Med. 2012, 2012, 487080. [Google Scholar] [CrossRef] [PubMed]
- Ayling, O.G.S.; Ibrahim, G.M.; Alotaibi, N.M.; Gooderham, P.A.; Macdonald, R.L. Anemia After Aneurysmal Subarachnoid Hemorrhage Is Associated With Poor Outcome and Death. Stroke 2018, 49, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Gulyani, S.; Earley, C.J.; Cutler, R.G.; Mattson, M.P.; Rifkind, J.M. Iron-deficiency anaemia enhances red blood cell oxidative stress. Free Radic. Res. 2008, 42, 824–829. [Google Scholar] [CrossRef]
- Sabri, M.; Ai, J.; Lass, E.; D’Abbondanza, J.; Macdonald, R.L. Genetic elimination of eNOS reduces secondary complications of experimental subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2013, 33, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Aladag, M.A.; Turkoz, Y.; Parlakpinar, H.; Gul, M. Nebivolol attenuates cerebral vasospasm both by increasing endothelial nitric oxide and by decreasing oxidative stress in an experimental subarachnoid haemorrhage. Br. J. Neurosurg. 2017, 31, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Su, Z.; Zha, L.; Zhu, Z.; Liu, W.; Sun, Y.; Yu, P.; Wang, Y.; Zhang, G.; Zhang, Z. Tetramethylpyrazine Nitrone Reduces Oxidative Stress to Alleviate Cerebral Vasospasm in Experimental Subarachnoid Hemorrhage Models. Neuromol. Med. 2019, 21, 262–274. [Google Scholar] [CrossRef]
- Dodd, W.S.; Laurent, D.; Dumont, A.S.; Hasan, D.M.; Jabbour, P.M.; Starke, R.M.; Hosaka, K.; Polifka, A.J.; Hoh, B.L.; Chalouhi, N. Pathophysiology of Delayed Cerebral Ischemia After Subarachnoid Hemorrhage: A Review. J. Am. Heart Assoc. 2021, 10, e021845. [Google Scholar] [CrossRef]



| Before Propensity-Score Matching | After Propensity-Score Matching | |||||
|---|---|---|---|---|---|---|
| Characteristics | Non-DCI | DCI | p Value | Non-DCI | DCI | p Value |
| (n = 625) | (n = 105) | (n = 104) | (n = 104) | |||
| Age, yrs, mean ± SD | 54.72 ± 11.47 | 55.65 ± 11.42 | 0.440 | 55.34 ± 11.22 | 55.76 ± 11.42 | 0.788 |
| Gender (n, %) | 0.513 | 1.00 | ||||
| Male | 235 (37.6) | 43 (41.0) | 43 (41.3) | 43 (41.3) | ||
| Female | 390 (62.4) | 62 (59.0) | 61 (58.7) | 61 (58.7) | ||
| Smoking (n, %) | 112 (17.9) | 16 (15.2) | 0.504 | 23 (22.1) | 16 (15.4) | 0.214 |
| Alcohol (n, %) | 62 (9.9) | 8 (7.6) | 0.459 | 9 (8.7) | 8 (7.7) | 0.800 |
| Medical history | ||||||
| Hypertension (n, %) | 307 (49.1) | 75 (71.4) | <0.001 | 81 (77.9) | 74 (71.2) | 0.218 |
| Diabetes (n, %) | 68 (10.9) | 13 (12.4) | 0.650 | 15 (14.4) | 13 (12.4) | 0.685 |
| Coronary heart disease (n, %) | 19 (3.0) | 2 (1.9) | 0.520 | 6 (5.8) | 2 (1.9) | 0.149 |
| Hyperlipidemia (n, %) | 119 (19.0) | 15 (14.3) | 0.244 | 25 (24.0) | 15 (14.4) | 0.079 |
| WFNS grade | <0.001 | 0.114 | ||||
| I–III | 535 (85.6) | 73 (69.5) | 82 (78.8) | 72 (69.2) | ||
| IV–V | 90 (14.4) | 32 (30.5) | 22 (21.2) | 32 (30.8) | ||
| Modified Fisher grade | <0.001 | 0.078 | ||||
| 1–2 | 482 (77.1) | 64 (61.0) | 75 (72.1) | 63 (60.6) | ||
| 3–4 | 143 (22.9) | 41 (39.0) | 29 (27.9) | 41 (39.4) | ||
| Aneurysm characteristics (n, %) | ||||||
| Multiple aneurysms | 115 (18.4) | 23 (21.9) | 0.396 | 17 (16.3) | 23 (22.1) | 0.291 |
| Single aneurysm location | 0.206 | 0.146 | ||||
| Anterior cerebral artery | 33 (6.5) | 2 (2.4) | 7 (18.0) | 2 (2.5) | ||
| Anterior communicating artery | 164 (32.2) | 30 (36.6) | 32 (36.8) | 29 (35.8) | ||
| Internal carotid artery | 76 (14.9) | 8 (9.8) | 15 (17.2) | 8 (9.9) | ||
| Middle cerebral artery | 104 (20.4) | 24 (29.3) | 14 (16.1) | 24 (29.6) | ||
| Posterior communicating artery | 109 (21.4) | 16 (19.5) | 15 (17.2) | 16 (19.8) | ||
| Others | 24 (4.7) | 2 (2.4) | 4 (4.6) | 2 (2.5) | ||
| Aneurysm size (n, %) | 0.405 | 0.487 | ||||
| <5 mm | 265 (42.4) | 41 (39.0) | 47 (45.2) | 41 (39.4) | ||
| 5–15 mm | 299 (47.8) | 53 (50.5) | 48 (46.2) | 52 (50.0) | ||
| 15–25 mm | 51 (8.2) | 7 (6.7) | 8 (7.7) | 7 (6.7) | ||
| >25 mm | 10 (1.6) | 4 (3.8) | 1 (1.0) | 4 (3.8) | ||
| Surgical methods (n, %) | 0.132 | 0.059 | ||||
| Clipping | 399 (63.8) | 75 (71.4) | 61 (58.7) | 74 (71.2) | ||
| Coiling | 226 (36.2) | 30 (28.6) | 43 (41.3) | 30 (28.8) | ||
| Time from admission to surgical treatment (n, %) | 0.018 | 0.874 | ||||
| ≤72 h | 396 (63.4) | 79 (75.2) | 77 (74.0) | 78 (75.0) | ||
| >72 h | 229 (36.6) | 26 (24.8) | 27 (26.0) | 26 (25.0) | ||
| Intracranial infection (n, %) | 45 (7.2) | 15 (14.3) | 0.014 | 18 (17.3) | 14 (13.5) | 0.442 |
| Hemoglobin, g/L, mean ± SD | 128.73 ± 17.97 | 130.13 ± 18.63 | 0.475 | 134.32 ± 17.14 | 129.88 ± 18.53 | 0.074 |
| Hematocrit, %, mean ± SD | 38.19 ± 4.86 | 38.56 ± 4.88 | 0.473 | 39.47 ± 4.74 | 38.49 ± 4.85 | 0.144 |
| Serum iron, μmol/L, mean ± SD | 13.11 ± 6.52 | 8.99 ± 6.26 | <0.001 | 20.13 ± 8.45 | 9.00 ± 6.29 | <0.001 |
| modified Rankin Scale (n, %) | <0.001 | <0.001 | ||||
| 0–2 | 539 (86.2) | 58 (55.2) | 94 (90.4) | 57 (54.8) | ||
| 3–6 | 86 (13.8) | 47 (44.8) | 10 (9.6) | 47 (45.2) | ||
| Unadjusted OR (95% CI) | Adjusted AOR (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
| Independent Variable | OR | Lower | Upper | p Value | OR | Lower | Upper | p Value |
| Hypertension | 0.386 | 0.246 | 0.607 | <0.001 | 0.452 | 0.281 | 0.726 | 0.001 |
| WFNS grade (IV-V) | 2.606 | 1.626 | 4.176 | <0.001 | 0.791 | 0.456 | 1.369 | 0.402 |
| Modified Fisher grade (3–4) | 0.463 | 0.300 | 0.715 | 0.001 | 0.736 | 0.449 | 1.206 | 0.223 |
| Time from admission to surgical treatment(≤72 h) | 0.569 | 0.355 | 0.912 | 0.019 | 1.289 | 0.781 | 2.126 | 0.320 |
| Intracranial infection | 2.148 | 1.150 | 4.014 | 0.017 | 1.832 | 0.940 | 3.571 | 0.075 |
| Serum iron (≤7.5 μmol/L) | 0.232 | 0.151 | 0.356 | <0.001 | 0.281 | 0.177 | 0.448 | <0.001 |
| Before Propensity-Score Matching | After Propensity-Score Matching | |||||
|---|---|---|---|---|---|---|
| Characteristics | Good Outcome | Poor Outcome | p Value | Good Outcome | Poor Outcome | p Value |
| (n = 597) | (n = 133) | (n = 93) | (n = 93) | |||
| Age, yrs, mean ± SD | 54.17 ± 11.30 | 57.89 ± 11.69 | 0.001 | 56.53 ± 11.54 | 56.75 ± 11.57 | 0.894 |
| Gender (n, %) | 0.898 | 1.00 | ||||
| Male | 228 (38.2) | 50 (37.6) | 34 (36.6) | 34 (36.6) | ||
| Female | 369 (61.8) | 83 (62.4) | 59 (63.4) | 59 (63.4) | ||
| Smoking (n, %) | 110 (18.4) | 18 (10.5) | 0.180 | 20 (21.5) | 13 (14.0) | 0.179 |
| Alcohol (n, %) | 60 (10.1) | 10 (7.5) | 0.356 | 10 (10.8) | 7 (7.5) | 0.445 |
| Medical history | ||||||
| Hypertension (n, %) | 293 (49.1) | 89 (66.9) | <0.001 | 66 (71.0) | 61 (65.6) | 0.431 |
| Diabetes (n, %) | 63 (10.6) | 18 (13.5) | 0.322 | 13 (14.0) | 11 (11.8) | 0.662 |
| Coronary heart disease (n, %) | 16 (2.7) | 5 (3.8) | 0.501 | 1 (1.1) | 2 (2.2) | 0.561 |
| Hyperlipidemia (n, %) | 105 (17.6) | 29 (21.8) | 0.256 | 20 (21.5) | 23 (34.7) | 0.602 |
| WFNS grade | <0.001 | 1.00 | ||||
| I–III | 543 (90.1) | 65 (48.9) | 60 (64.5) | 60 (64.5) | ||
| IV–V | 54 (9.1) | 68 (51.1) | 33 (35.5) | 33 (35.5) | ||
| Modified Fisher grade | <0.001 | 0.305 | ||||
| 1–2 | 487 (81.6) | 59 (44.4) | 44 (47.3) | 51 (54.8) | ||
| 3–4 | 110 (18.4) | 74 (55.6) | 49 (52.7) | 42 (45.2) | ||
| Aneurysm characteristics (n, %) | ||||||
| Multiple aneurysms | 108 (18.1) | 30 (22.6) | 0.234 | 16 (17.2) | 22 (23.7) | 0.275 |
| Single aneurysm location | 0.196 | 0.648 | ||||
| Anterior cerebral artery | 28 (5.3) | 7 (6.8) | 5 (6.5) | 4 (5.6) | ||
| Anterior communicating artery | 158 (32.3) | 36 (35.0) | 27 (35.1) | 25 (35.2) | ||
| Internal carotid artery | 63 (12.9) | 21 (20.4) | 8 (10.4) | 13 (18.3) | ||
| Middle cerebral artery | 107 (21.9) | 21 (20.4) | 16 (20.8) | 16 (22.5) | ||
| Posterior communicating artery | 109 (22.3) | 16 (15.5) | 18 (23.4) | 12 (16.9) | ||
| Others | 24 (4.9) | 2 (1.9) | 3 (3.9) | 1 (1.4) | ||
| Aneurysm size (n, %) | 0.570 | 0.594 | ||||
| <5 mm | 246 (41.2) | 60 (45.1) | 37 (39.8) | 44 (47.3) | ||
| 5–15 mm | 292 (48.9) | 60 (45.1) | 50 (53.8) | 41 (44.1) | ||
| 15–25 mm | 49 (8.2) | 9 (6.8) | 4 (4.3) | 6 (6.5) | ||
| >25 mm | 10 (1.7) | 4 (3.0) | 2 (2.2) | 4 (2.2) | ||
| Surgical methods (n, %) | 0.260 | 0.117 | ||||
| Clipping | 396 (63.3) | 78 (58.6) | 68 (73.1) | 58 (62.4) | ||
| Coiling | 201 (33.7) | 55 (41.4) | 25 (26.9) | 35 (37.6) | ||
| Time from admission to surgical treatment (n, %) | 0.370 | 0.633 | ||||
| ≤72 h | 384 (64.3) | 91 (68.4) | 66 (71.0) | 63 (67.7) | ||
| >72 h | 213 (35.7) | 42 (31.6) | 27 (29.0) | 30 (32.3) | ||
| Intracranial infection (n, %) | 48 (8.0) | 12 (9.0) | 0.709 | 8 (8.6) | 7 (7.5) | 0.788 |
| Hemoglobin, g/L, mean ± SD | 128.47 ± 17.69 | 131.02 ± 19.57 | 0.168 | 130.63 ± 16.48 | 130.71 ± 19.83 | 0.978 |
| Hematocrit, %, mean ± SD | 38.12 ± 4.81 | 38.79 ± 5.05 | 0.165 | 38.44 ± 4.43 | 38.74 ± 5.00 | 0.664 |
| Serum iron, μmol/L, mean ± SD | 13.40 ± 6.80 | 8.59 ± 3.96 | <0.001 | 17.32 ± 9.90 | 9.38 ± 4.00 | <0.001 |
| DCI (n, %) | 58 (9.7) | 47 (35.3) | <0.001 | 23 (24.7) | 22 (23.7) | 0.864 |
| Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | AOR (95% CI) | |||||||
| Independent Variable | OR | Lower | Upper | p Value | OR | Lower | Upper | p Value |
| Age | 1.029 | 1.012 | 1.047 | 0.001 | 1.024 | 1.004 | 1.045 | 0.020 |
| Hypertension | 0.476 | 0.321 | 0.707 | <0.001 | 0.868 | 0.536 | 1.406 | 0.564 |
| WFNS grade (IV–Ⅴ) | 0.095 | 0.061 | 0.148 | <0.001 | 0.178 | 0.107 | 0.295 | <0.001 |
| mFisher grade (3–4) | 0.180 | 0.121 | 0.269 | <0.001 | 0.394 | 0.245 | 0.635 | <0.001 |
| DCI | 0.197 | 0.126 | 0.308 | <0.001 | 0.254 | 0.149 | 0.434 | <0.001 |
| Serum iron (≤12.8 μmol/L) | 0.175 | 0.106 | 0.289 | <0.001 | 0.363 | 0.209 | 0.630 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-B.; Zheng, F.; Stavrinou, L.; Wang, H.-J.; Fan, W.-J.; Yao, P.-S.; Lin, Y.-X.; Goldbrunner, R.; Zheng, S.-F.; Stavrinou, P.; et al. Admission Serum Iron as an Independent Risk Factor for Postoperative Delayed Cerebral Ischemia Following Aneurysmal Subarachnoid Hemorrhage: A Propensity-Matched Analysis. Brain Sci. 2022, 12, 1183. https://doi.org/10.3390/brainsci12091183
Zhang Y-B, Zheng F, Stavrinou L, Wang H-J, Fan W-J, Yao P-S, Lin Y-X, Goldbrunner R, Zheng S-F, Stavrinou P, et al. Admission Serum Iron as an Independent Risk Factor for Postoperative Delayed Cerebral Ischemia Following Aneurysmal Subarachnoid Hemorrhage: A Propensity-Matched Analysis. Brain Sciences. 2022; 12(9):1183. https://doi.org/10.3390/brainsci12091183
Chicago/Turabian StyleZhang, Yi-Bin, Feng Zheng, Lampis Stavrinou, Hao-Jie Wang, Wen-Jian Fan, Pei-Sen Yao, Yuan-Xiang Lin, Roland Goldbrunner, Shu-Fa Zheng, Pantelis Stavrinou, and et al. 2022. "Admission Serum Iron as an Independent Risk Factor for Postoperative Delayed Cerebral Ischemia Following Aneurysmal Subarachnoid Hemorrhage: A Propensity-Matched Analysis" Brain Sciences 12, no. 9: 1183. https://doi.org/10.3390/brainsci12091183
APA StyleZhang, Y.-B., Zheng, F., Stavrinou, L., Wang, H.-J., Fan, W.-J., Yao, P.-S., Lin, Y.-X., Goldbrunner, R., Zheng, S.-F., Stavrinou, P., & Kang, D.-Z. (2022). Admission Serum Iron as an Independent Risk Factor for Postoperative Delayed Cerebral Ischemia Following Aneurysmal Subarachnoid Hemorrhage: A Propensity-Matched Analysis. Brain Sciences, 12(9), 1183. https://doi.org/10.3390/brainsci12091183






