The Photic Stimulation Has an Impact on the Reproduction of 10 s Intervals Only in Healthy Controls but Not in Patients with Schizophrenia: The EEG Study
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Stimuli and Paradigm of the Study
2.3. The Task of Time Production
2.4. Analysis of Time Perception
- Real time (Treal).
- 2.
- Normalized time (Tnorm), i.e., the original values of time intervals were normalized for each participant using mean and std Treal.
- 3.
- The error of time perception (Terr): ).
2.5. EEG Registration
2.6. EEG Preprocessing
2.7. Data Analysis
2.7.1. Power Spectral Density (PSD)
2.7.2. Fractal Dimension (FD)
2.7.3. Envelope Mean Frequency (EMF)
2.7.4. Hjorth Complexity
2.7.5. Statistical Analysis
3. Results
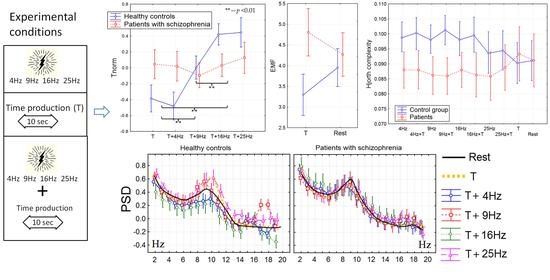
3.1. The Time Perception
3.2. The Power Spectral Density
3.3. Non-Linear Features of EEG
3.4. The Correlation Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Portnova, G.V.; Sysoeva, O.V.; Ivanitskiĭ, A. The influence of auditory rhythmic stimulation on the subjective time flow and the reaction time in cognitive tasks. Zhurnal Vyss. Nervn. Deiatelnosti Im. IP Pavlov. 2010, 60, 419–429. [Google Scholar]
- Stetson, C.; Fiesta, M.P.; Eagleman, D.M. Does time really slow down during a frightening event? PLoS ONE 2007, 2, e1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minkwitz, J.; Trenner, M.U.; Sander, C.; Olbrich, S.; Sheldrick, A.J.; Hegerl, U.; Himmerich, H. Time perception at different EEG-vigilance levels. Behav. Brain Funct. 2012, 8, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiles, J.D.; Bird, S.R.; Hopkins, J.; Riley, M. Effect of caffeinated coffee on running speed, respiratory factors, blood lactate and perceived exertion during 1500-m treadmill running. Br. J. Sports Med. 1992, 26, 116–120. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.M.; Weinstein, D.; Vega, T.; Kayser, A.S. Dopamine, time perception, and future time perspective. Psychopharmacology 2018, 235, 2783–2793. [Google Scholar] [CrossRef] [Green Version]
- Tinklenberg, J.R.; Roth, W.T.; Kopell, B.S. Marijuana and ethanol: Differential effects on time perception, heart rate, and subjective response. Psychopharmacology 1976, 49, 275–279. [Google Scholar] [CrossRef]
- Ueda, N.; Maruo, K.; Sumiyoshi, T. Positive symptoms and time perception in schizophrenia: A meta-analysis. Schizophr. Res. Cogn. 2018, 13, 3–6. [Google Scholar] [CrossRef]
- Gómez, J.; Marín-Méndez, J.J.; Molero, P.; Atakan, Z.; Ortuño, F. Time perception networks and cognition in schizophrenia: A review and a proposal. Psychiatry Res. 2014, 220, 737–744. [Google Scholar] [CrossRef]
- Thoenes, S.; Oberfeld, D. Meta-analysis of time perception and temporal processing in schizophrenia: Differential effects on precision and accuracy. Clin. Psychol. Rev. 2017, 54, 44–64. [Google Scholar] [CrossRef]
- Bonnot, O.; de Montalembert, M.; Kermarrec, S.; Botbol, M.; Walter, M.; Coulon, N. Are impairments of time perception in schizophrenia a neglected phenomenon? J. Physiol.-Paris 2011, 105, 164–169. [Google Scholar] [CrossRef]
- Schwarz, M.A.; Winkler, I.; Sedlmeier, P. The heart beat does not make us tick: The impacts of heart rate and arousal on time perception. Atten. Percept. Psychophys. 2013, 75, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Kingma, B.R.; Roijendijk, L.M.; Van Maanen, L.; Van Rijn, H.; Van Beurden, M.H. Time perception and timed decision task performance during passive heat stress. Temperature 2021, 8, 53–63. [Google Scholar] [CrossRef]
- Hsieh, C.K.; Liao, W.C.; Yu, M.C.; Hung, Y.P. Interacting with the past: Creating a time perception journey experience using kinect-based breath detection and deterioration and recovery simulation technologies. J. Comput. Cult. Herit. (JOCCH) 2014, 7, 1. [Google Scholar] [CrossRef]
- Portnova, G.V.; Balashova, E.Y.; Vartanov, A.V. Fenomen “kognitivnogo zakhvatyvaniya” pri otsenivanii vremennykh intervalov (The phenomenon of “cognitive capture” in the estimation of time intervals). Psikhologicheskii Zhurnal (Psychol. J.) 2006, 27, 67–80. [Google Scholar]
- Cheng, R.K.; Tipples, J.; Narayanan, N.S.; Meck, W.H. Clock speed as a window into dopaminergic control of emotion and time perception. Timing Time Percept. 2016, 4, 99–122. [Google Scholar] [CrossRef]
- Hagura, N.; Kanai, R.; Orgs, G.; Haggard, P. Ready steady slow: Action preparation slows the subjective passage of time. Proc. R. Soc. B Biol. Sci. 2012, 279, 4399–4406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portnova, G. Age changes of EEG during photo-and auditory low-frequency stimulation and assessment of fatigue. In Proceedings of the 2018 IEEE 31st International Symposium on Computer-Based Medical Systems (CBMS), Karlstad, Sweden, 18–21 June 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–5. [Google Scholar]
- Cochrane, M.; Petch, I.; Pickering, A.D. Do measures of schizotypal personality provide non-clinical analogues of schizophrenic symptomatology? Psychiatry Res. 2010, 176, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Polner, B.; Faiola, E.; Urquijo, M.F.; Meyhöfer, I.; Steffens, M.; Rónai, L.; Koutsouleris, N.; Ettinger, U. The network structure of schizotypy in the general population. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 635–645. [Google Scholar] [CrossRef] [Green Version]
- Klucharev, V.; Munneke, M.A.; Smidts, A.; Fernández, G. Downregulation of the posterior medial frontal cortex prevents social conformity. J. Neurosci. 2011, 31, 11934–11940. [Google Scholar] [CrossRef] [Green Version]
- Berns, G.S.; Chappelow, J.; Zink, C.F.; Pagnoni, G.; Martin-Skurski, M.E.; Richards, J. Neurobiological correlates of social conformity and independence during mental rotation. Biol. Psychiatry 2005, 58, 245–253. [Google Scholar] [CrossRef]
- Whitehead, K.; Gollwitzer, S.; Millward, H.; Wehner, T.; Scott, C.; Diehl, B. The additional lateralizing and localizing value of the postictal EEG in frontal lobe epilepsy. Clin. Neurophysiol. 2016, 127, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Rubboli, G.; Parra, J.; Seri, S.; Takahashi, T.; Thomas, P. EEG diagnostic procedures and special investigations in the assessment of photosensitivity. Epilepsia 2004, 45, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Fylan, F.; Edson, A.S.; Harding, G.F.A. Clinical significance of EEG abnormalities during photic stimulation in patients with photosensitive epilepsy. Epilepsia 1999, 40, 370–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diehl, B.; Stodieck, S.R.; Diehl, R.R.; Ringelstein, E.B. The photic driving EEG response and photoreactive cerebral blood flow in the posterior cerebral artery in controls and in patients with epilepsy. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Portnova, G.V.; Atanov, M.S. Nonlinear EEG parameters of emotional perception in patients with moderate traumatic brain injury, coma, stroke and schizophrenia. AIMS Neurosci. 2018, 5, 221. [Google Scholar] [CrossRef]
- Portnova, G.V. Lack of a sense of threat and higher emotional lability in patients with chronic microvascular ischemia as measured by non-linear EEG parameters. Front. Neurol. 2020, 11, 122. [Google Scholar] [CrossRef]
- Elbert, T.; Lutzenberger, W.; Rockstroh, B.; Berg, P.; Cohen, R. Physical aspects of the EEG in schizophrenics. Biol. Psychiatry 1992, 32, 595–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Kumar, A.; Trikha, A.; Anand, S. Electroencephalogram based quantitative estimation of pain for balanced anaesthesia. Measurement 2015, 59, 296–301. [Google Scholar] [CrossRef]
- Zappasodi, F.; Olejarczyk, E.; Marzetti, L.; Assenza, G.; Pizzella, V.; Tecchio, F. Fractal dimension of EEG activity senses neuronal impairment in acute stroke. PLoS ONE 2014, 9, e100199. [Google Scholar] [CrossRef] [Green Version]
- Soghoyan, G.; Ledovsky, A.; Nekrashevich, M.; Martynova, O.; Polikanova, I.; Portnova, G.; Rebreikina, A.; Sysoeva, O.; Sharaev, M. A Toolbox and Crowdsourcing Platform for Automatic Labeling of Independent Components in Electroencephalography. Front. Neuroinformatics 2021, 15, 720229. [Google Scholar] [CrossRef] [PubMed]
- Allman, M.J.; Meck, W.H. Pathophysiological distortions in time perception and timed performance. Brain 2012, 135, 656–677. [Google Scholar] [CrossRef] [Green Version]
- Peterburs, J.; Nitsch, A.M.; Miltner, W.H.R.; Straube, T. Impaired representation of time in schizophrenia is linked to positive symptoms and cognitive demand. PLoS ONE 2013, 8, e67615. [Google Scholar] [CrossRef]
- Seeman, P.; Johannes, S.; Jiang-Fan, C.; Henry, S.; Perreault, M.; McKnight, G.S.; Roder, J.C.; Remi, Q.; Patricia, B.; Srivastava, L.K.; et al. Psychosis pathways converge via D2High dopamine receptors. Synapse 2006, 60, 319–346. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.C. Intention as a component of the alpha-rhythm response to mental activity. Int. J. Psychophysiol. 1996, 24, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Meichsner, J.H.; Zou, S.; Zhao, L. Correlation between alpha rhythm and cognitive processes. In Proceedings of the 2010 3rd International Conference on Biomedical Engineering and Informatics, Yantai, China, 16–18 October 2010; IEEE: Piscataway, NJ, USA, 2010; Volume 2, pp. 823–827. [Google Scholar]
- Portnova, G.V.; Ukraintseva, Y.V.; Liaukovich, K.M.; Martynova, O.V. Association of the retrospective self-report ratings with the dynamics of EEG. Heliyon 2019, 5, e02533. [Google Scholar] [CrossRef] [Green Version]
- Rusalova, M.N.; Kislova, O.O.; Strelnikova, G.V. Dynamics of activation processes in people with different abilities to recognize emotional expressions in speech. Neurosci. Behav. Physiol. 2012, 42, 447–455. [Google Scholar] [CrossRef]
- Fink, A.; Schwab, D.; Papousek, I. Sensitivity of EEG upper alpha activity to cognitive and affective creativity interventions. Int. J. Psychophysiol. 2011, 82, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Baldauf, D.; Burgard, E.; Wittmann, M. Time perception as a workload measure in simulated car driving. Appl. Ergon. 2009, 40, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Hancock, P.A.; Vercruyssen, M.; Rodenburg, G.J. The effect of gender and time-of-day on time perception and mental workload. Curr. Psychol. 1992, 11, 203–225. [Google Scholar] [CrossRef]
- Portnova, G.V.; Girzhova, I.N.; Martynova, O.V. Residual and compensatory changes of resting-state EEG in successful recovery after moderate TBI. Brain Sci. Adv. 2020, 6, 364–378. [Google Scholar] [CrossRef]
- Lappe, C.; Trainor, L.J.; Herholz, S.C.; Pantev, C. Cortical plasticity induced by short-term multimodal musical rhythm training. PLoS ONE 2011, 6, e21493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tichko, P.; Large, E.W. Modeling infants’ perceptual narrowing to musical rhythms: Neural oscillation and Hebbian plasticity. Ann. N. Y. Acad. Sci. 2019, 1453, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Mégevand, P.; Troncoso, E.; Quairiaux, C.; Muller, D.; Michel, C.M.; Kiss, J.Z. Long-term plasticity in mouse sensorimotor circuits after rhythmic whisker stimulation. J. Neurosci. 2009, 29, 5326–5335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servaas, M.N.; Kos, C.; Gravel, N.; Renken, R.J.; Marsman, J.B.C.; Van Tol, M.J.; Aleman, A. Rigidity in motor behavior and brain functioning in patients with schizophrenia and high levels of apathy. Schizophr. Bull. 2019, 45, 542–551. [Google Scholar] [CrossRef] [PubMed]




| Time Parameters | Valid N | Mean | S.D. | Valid N | Mean | S.D. | |
|---|---|---|---|---|---|---|---|
| PANSS | 22 | 102.17 | 15.69 | - | |||
| Treal | T | 22 | 11,279 | 2689 | 24 | 9677 | 1939 |
| 4 Hz | 22 | 11,841 | 2971 | 24 | 9765 | 2344 | |
| 9 Hz | 22 | 10,836 | 2569 | 24 | 10,068 | 2181 | |
| 16 Hz | 22 | 11,155 | 2346 | 24 | 10,321 | 1821 | |
| 25 Hz | 22 | 11,539 | 2475 | 24 | 10,231 | 2149 | |
| Tnorm | T | 22 | 0.05 | 0.81 | 24 | −0.39 | 0.88 |
| 4 Hz | 22 | 0.02 | 0.91 | 24 | −0.48 | 0.84 | |
| 9 Hz | 22 | 0.00 | 0.64 | 24 | 0.00 | 0.80 | |
| 16 Hz | 22 | 0.03 | 0.67 | 24 | 0.42 | 0.67 | |
| 25 Hz | 22 | 0.13 | 1.02 | 24 | 0.44 | 0.82 | |
| Terr | T | 22 | 294.76 | 256.18 | 24 | 158.79 | 115.88 |
| 4 Hz | 22 | 358.10 | 390.80 | 24 | 198.63 | 126.83 | |
| 9 Hz | 22 | 268.05 | 250.11 | 24 | 176.21 | 128.68 | |
| 16 Hz | 22 | 286.32 | 208.14 | 24 | 178.83 | 152.75 | |
| 25 Hz | 22 | 333.00 | 336.31 | 24 | 164.38 | 140.49 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portnova, G.V.; Maslennikova, A.V. The Photic Stimulation Has an Impact on the Reproduction of 10 s Intervals Only in Healthy Controls but Not in Patients with Schizophrenia: The EEG Study. Brain Sci. 2023, 13, 112. https://doi.org/10.3390/brainsci13010112
Portnova GV, Maslennikova AV. The Photic Stimulation Has an Impact on the Reproduction of 10 s Intervals Only in Healthy Controls but Not in Patients with Schizophrenia: The EEG Study. Brain Sciences. 2023; 13(1):112. https://doi.org/10.3390/brainsci13010112
Chicago/Turabian StylePortnova, Galina V., and Aleksandra V. Maslennikova. 2023. "The Photic Stimulation Has an Impact on the Reproduction of 10 s Intervals Only in Healthy Controls but Not in Patients with Schizophrenia: The EEG Study" Brain Sciences 13, no. 1: 112. https://doi.org/10.3390/brainsci13010112
APA StylePortnova, G. V., & Maslennikova, A. V. (2023). The Photic Stimulation Has an Impact on the Reproduction of 10 s Intervals Only in Healthy Controls but Not in Patients with Schizophrenia: The EEG Study. Brain Sciences, 13(1), 112. https://doi.org/10.3390/brainsci13010112