Altered Functional Connectivity Density in Type 2 Diabetes Mellitus with and without Mild Cognitive Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical and Neuropsychological Data
2.3. Resting-State fMRI Data Acquisition
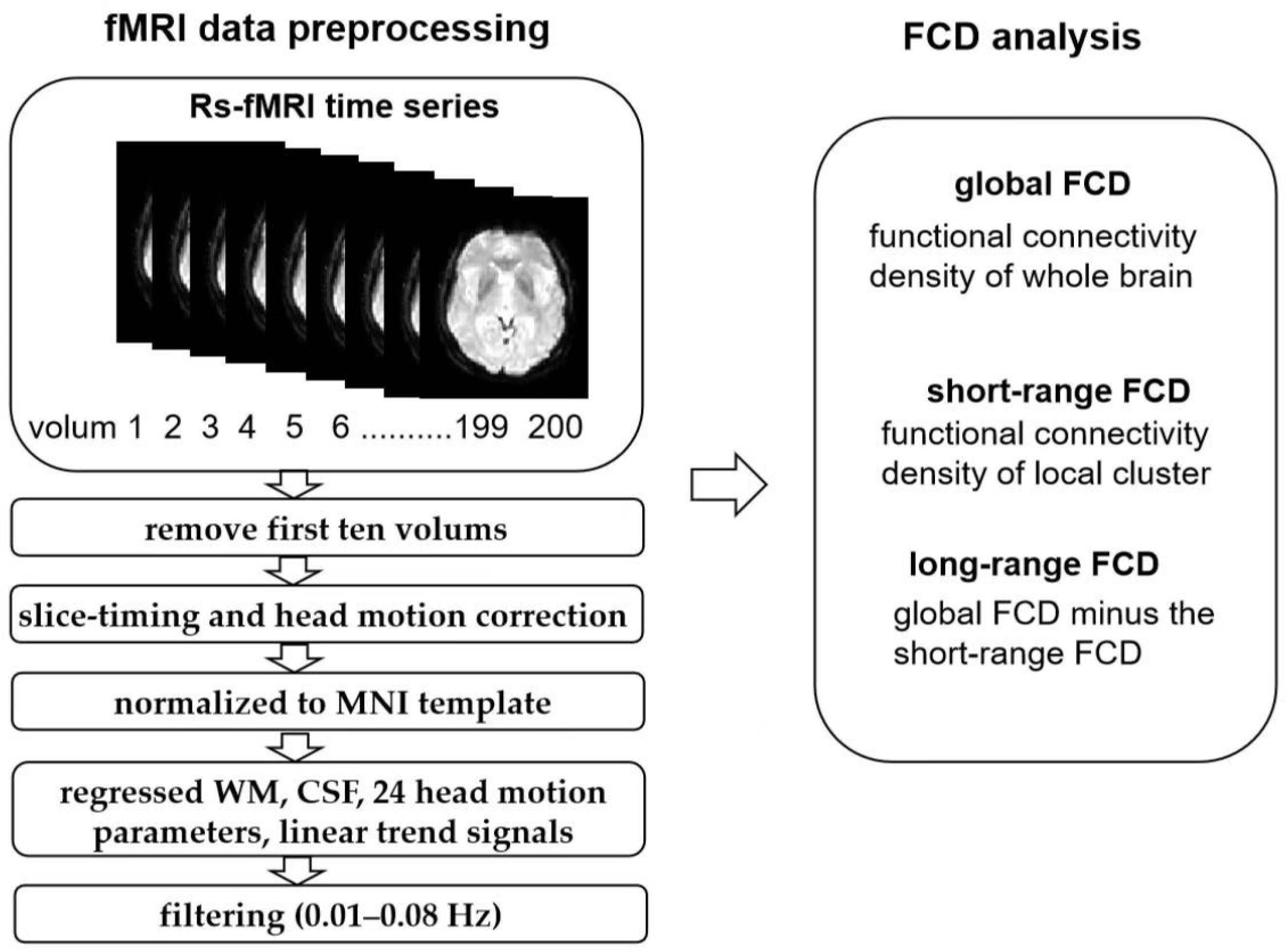
2.4. Resting-State fMRI Data Analysis
2.5. Statistical Analysis
3. Results
3.1. Comparison of Clinical and Neuropsychological Data
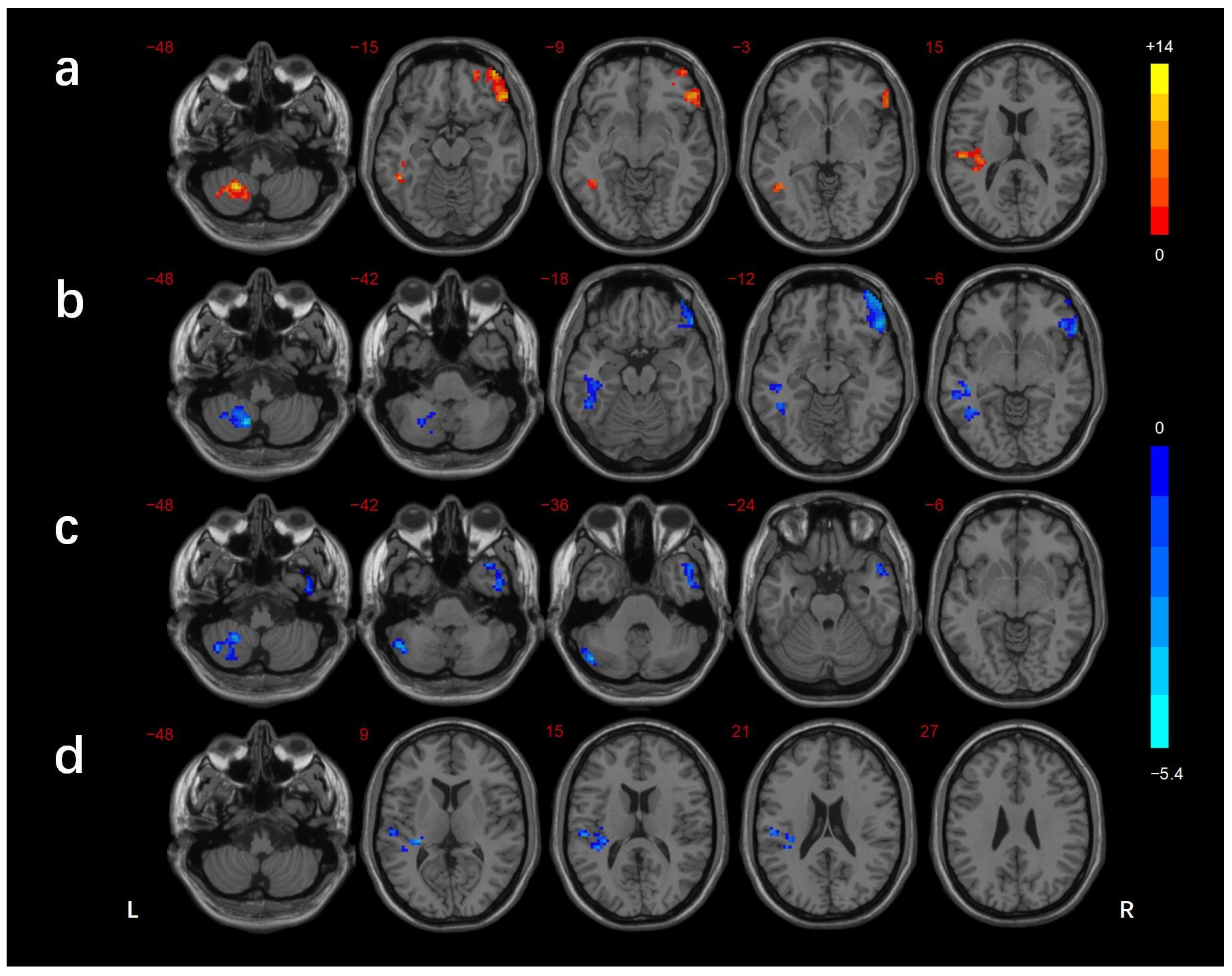
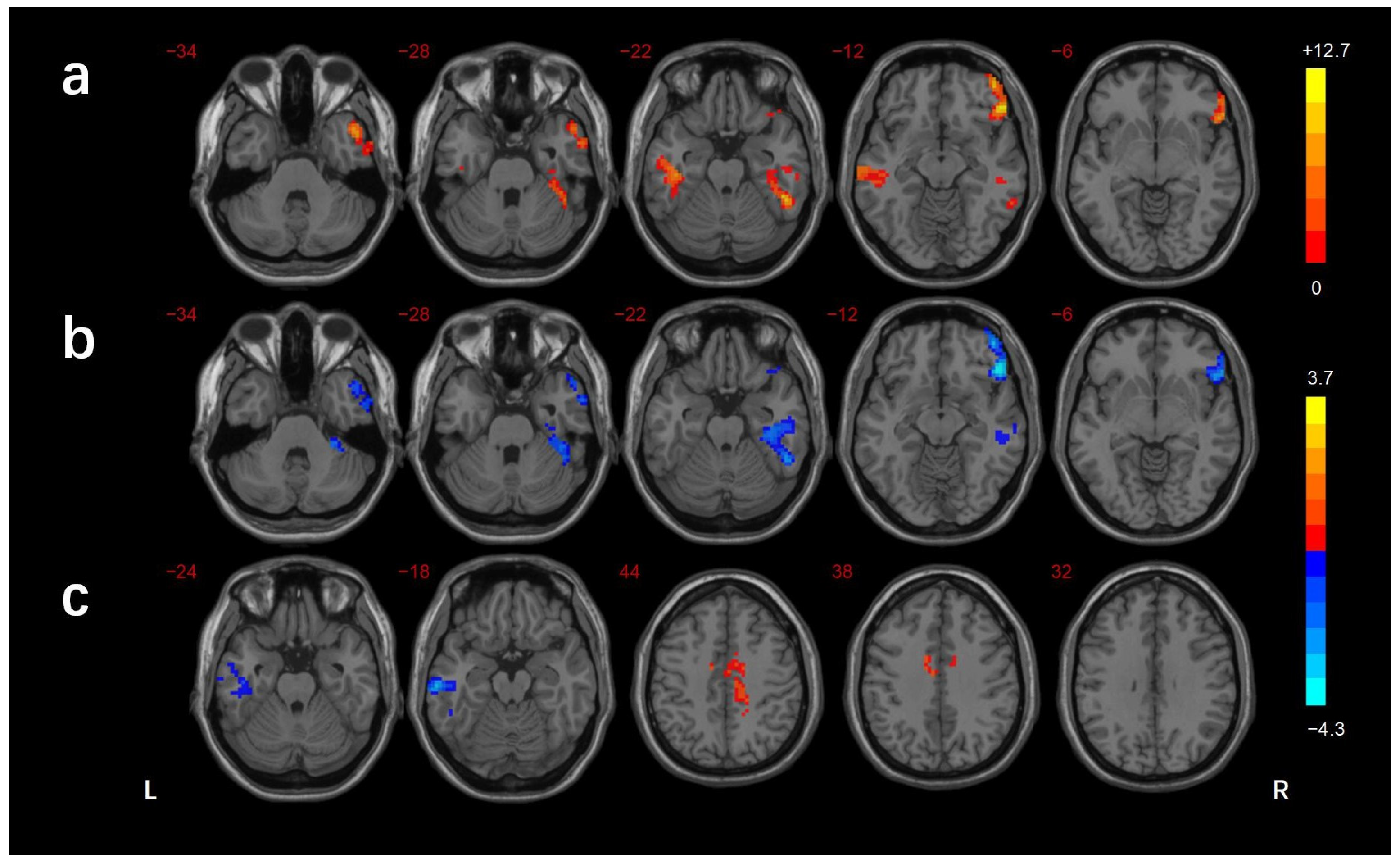
3.2. FCD Analysis
3.3. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, H.; Formica, M.; Harris, E.; Daly, R.M. Brain functional alterations in Type 2 Diabetes—A systematic review of fMRI studies. Front. Neuroendocrinol. 2017, 47, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Koekkoek, P.S.; Kappelle, L.J.; van den Berg, E.; Rutten, G.E.; Biessels, G.J. Cognitive function in patients with diabetes mellitus: Guidance for daily care. Lancet Neurol. 2015, 14, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C.; Hua, S.; Liao, H.; Wang, M.; Xiong, Y.; Cao, F. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract. 2017, 124, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.A. Brain injury with diabetes mellitus: Evidence, mechanisms and treatment implications. Expert Rev. Clin. Pharmacol. 2017, 10, 409–428. [Google Scholar] [CrossRef]
- Pandya, S.Y.; Clem, M.A.; Silva, L.M.; Woon, F.L. Does mild cognitive impairment always lead to dementia? A review. J. Neurol. Sci. 2016, 369, 57–62. [Google Scholar] [CrossRef]
- Dai, Z.; He, Y. Disrupted structural and functional brain connectomes in mild cognitive impairment and Alzheimer’s disease. Neurosci. Bull. 2014, 30, 217–232. [Google Scholar] [CrossRef]
- Benwell, C.S.Y.; Davila-Perez, P.; Fried, P.J.; Jones, R.N.; Travison, T.G.; Santarnecchi, E.; Pascual-Leone, A.; Shafi, M.M. EEG spectral power abnormalities and their relationship with cognitive dysfunction in patients with Alzheimer’s disease and type 2 diabetes. Neurobiol. Aging 2020, 85, 83–95. [Google Scholar] [CrossRef]
- Fried, P.J.; Pascual-Leone, A.; Bolo, N.R. Diabetes and the link between neuroplasticity and glutamate in the aging human motor cortex. Clin. Neurophysiol. 2019, 130, 1502–1510. [Google Scholar] [CrossRef]
- Takenoshita, N.; Fukasawa, R.; Ogawa, Y.; Shimizu, S.; Umahara, T.; Ishii, K.; Shimada, H.; Higuchi, M.; Suhara, T.; Hanyu, H. Amyloid and Tau Positron Emission Tomography in Suggested Diabetesrelated Dementia. Curr. Alzheimer Res. 2018, 15, 1062–1069. [Google Scholar] [CrossRef]
- Gao, S.; Chen, Y.; Sang, F.; Yang, Y.; Xia, J.; Li, X.; Zhang, J.; Chen, K.; Zhang, Z. White Matter Microstructural Change Contributes to Worse Cognitive Function in Patients with Type 2 Diabetes. Diabetes 2019, 68, 2085–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Zhang, S.; Shi, J.; Fan, Y.; Zhang, Q.; Zhu, W. Application of neurite orientation dispersion and density imaging to characterize brain microstructural abnormalities in type-2 diabetics with mild cognitive impairment. J. Magn. Reason. Imaging 2019, 50, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Sui, Y.; Xu, Z.; Zhang, Q.; Karaman, M.M.; Cai, K.; Anderson, T.M.; Zhu, W.; Wang, J.; Zhou, X.J. A Diffusion Tensor Imaging Study on White Matter Abnormalities in Patients with Type 2 Diabetes Using Tract-Based Spatial Statistics. AJNR Am. J. Neuroradiol. 2016, 37, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Tian, T.; Fan, Y.; Yang, S.; Xiong, X.; Zhang, Q.; Zhu, W. Diffusion Tensor Imaging Reveals Altered Topological Efficiency of Structural Networks in Type-2 Diabetes Patients with and without Mild Cognitive Impairment. J. Magn. Reason. Imaging 2022, 55, 917–927. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Qiu, M.; Liu, K.; Li, Y.; Zuo, Z.; Yin, X.; Lai, Y.; Fang, J.; Tong, H.; et al. Alterations of Brain Structural Network Connectivity in Type 2 Diabetes Mellitus Patients with Mild Cognitive Impairment. Front. Aging Neurosci. 2020, 12, 615048. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Zhang, J.; Liu, C.; Yuan, Q.; Yin, X.; Wei, L.; Cui, J.; Tao, R.; Wei, P.; et al. Gray matter volume abnormalities in type 2 diabetes mellitus with and without mild cognitive impairment. Neurosci. Lett. 2014, 562, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Q.; Xu, Z.P.; Xiong, Y.; Zhan, Y.F.; Guo, L.Y.; Zhang, S.; Jiang, R.F.; Yao, Y.H.; Qin, Y.Y.; Wang, J.Z.; et al. Altered Intranetwork and Internetwork Functional Connectivity in Type 2 Diabetes Mellitus with and without Cognitive Impairment. Sci. Rep. 2016, 6, 32980. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Chen, X.; Zhao, X.; Fan, Y.; Zhang, Q.; Zhu, W. Altered regional homogeneity and functional brain networks in Type 2 diabetes with and without mild cognitive impairment. Sci. Rep. 2020, 10, 21254. [Google Scholar] [CrossRef]
- Zhang, D.; Lei, Y.; Gao, J.; Qi, F.; Yan, X.; Ai, K.; Zhe, X.; Cheng, M.; Wang, M.; Su, Y.; et al. Right Frontoinsular Cortex: A Potential Imaging Biomarker to Evaluate T2DM-Induced Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 674288. [Google Scholar] [CrossRef]
- Zuo, X.N.; Ehmke, R.; Mennes, M.; Imperati, D.; Castellanos, F.X.; Sporns, O.; Milham, M.P. Network centrality in the human functional connectome. Cereb. Cortex 2012, 22, 1862–1875. [Google Scholar] [CrossRef]
- Sepulcre, J.; Liu, H.; Talukdar, T.; Martincorena, I.; Yeo, B.T.; Buckner, R.L. The organization of local and distant functional connectivity in the human brain. PLoS Comput. Biol. 2010, 6, e1000808. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, D.; Volkow, N.D. Functional connectivity density mapping. Proc. Natl. Acad. Sci. USA 2010, 107, 9885–9890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Wu, H.; Chen, S.; Ge, H.; Yan, Z.; Xue, C.; Qi, W.; Yuan, Q.; Liang, X.; Lin, X.; et al. Differential Abnormality in Functional Connectivity Density in Preclinical and Early-Stage Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 879836. [Google Scholar] [CrossRef] [PubMed]
- Bullmore, E.; Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, L.; Duan, S.; Yin, X.; Yang, W.; Shi, Y.; Zhang, J.; Wang, J. Disrupted Balance of Long- and Short-Range Functional Connectivity Density in Type 2 Diabetes Mellitus: A Resting-State fMRI Study. Front. Neurosci. 2018, 12, 875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Chen, Z.; Wei, Y.; Tang, F.; Nong, X.; Li, C.; Yu, B.; Duan, G.; Su, J.; Mai, W.; et al. Fusion analysis of gray matter and white matter in subjective cognitive decline and mild cognitive impairment by multimodal CCA-joint ICA. Neuroimage Clin. 2021, 32, 102874. [Google Scholar] [CrossRef]
- Xue, C.; Sun, H.; Hu, G.; Qi, W.; Yue, Y.; Rao, J.; Yang, W.; Xiao, C.; Chen, J. Disrupted Patterns of Rich-Club and Diverse-Club Organizations in Subjective Cognitive Decline and Amnestic Mild Cognitive Impairment. Front. Neurosci. 2020, 14, 575652. [Google Scholar] [CrossRef]
- Reinke, C.; Buchmann, N.; Fink, A.; Tegeler, C.; Demuth, I.; Doblhammer, G. Diabetes duration and the risk of dementia: A cohort study based on German health claims data. Age Ageing 2022, 51, afab231. [Google Scholar] [CrossRef]
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, D.G.; Shokri-Kojori, E.; Volkow, N.D. Temporal Evolution of Brain Functional Connectivity Metrics: Could 7 Min of Rest be Enough? Cereb. Cortex 2017, 27, 4153–4165. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D. Aging and functional brain networks. Mol. Psychiatry 2012, 17, 549–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Wang, X.; Yang, Q.; Wu, F.; Tang, L.; Wang, J.; Li, C. Topological alterations of the brain functional network in type 2 diabetes mellitus patients with and without mild cognitive impairment. Front. Aging Neurosci. 2022, 14, 834319. [Google Scholar] [CrossRef] [PubMed]
- Gunturkun, O.; Strockens, F.; Ocklenburg, S. Brain Lateralization: A Comparative Perspective. Physiol. Rev. 2020, 100, 1019–1063. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Nestor, P.J.; Rogers, T.T. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 2007, 8, 976–987. [Google Scholar] [CrossRef]
- Binder, J.R.; Desai, R.H. The neurobiology of semantic memory. Trends Cogn. Sci. 2011, 15, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Tune, S.; Asaridou, S.S. Stimulating the Semantic Network: What Can TMS Tell Us about the Roles of the Posterior Middle Temporal Gyrus and Angular Gyrus? J. Neurosci. 2016, 36, 4405–4407. [Google Scholar] [CrossRef] [Green Version]
- Whitney, C.; Jefferies, E.; Kircher, T. Heterogeneity of the left temporal lobe in semantic representation and control: Priming multiple versus single meanings of ambiguous words. Cereb. Cortex 2011, 21, 831–844. [Google Scholar] [CrossRef] [Green Version]
- Whitney, C.; Kirk, M.; O’Sullivan, J.; Lambon Ralph, M.A.; Jefferies, E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cereb. Cortex 2011, 21, 1066–1075. [Google Scholar] [CrossRef] [Green Version]
- Davey, J.; Cornelissen, P.L.; Thompson, H.E.; Sonkusare, S.; Hallam, G.; Smallwood, J.; Jefferies, E. Automatic and Controlled Semantic Retrieval: TMS Reveals Distinct Contributions of Posterior Middle Temporal Gyrus and Angular Gyrus. J. Neurosci. 2015, 35, 15230–15239. [Google Scholar] [CrossRef] [Green Version]
- Marneras, C.; Messinis, L.; Ntoskou, A.; Nasios, G.; Martzoukou, M.; Papathanasopoulos, P. Quality of Life in Type 2 Diabetes Mellitus Patients with Neuropsychological Deficits. Adv. Exp. Med. Biol. 2020, 1196, 41–61. [Google Scholar]
- Marseglia, A.; Fratiglioni, L.; Laukka, E.J.; Santoni, G.; Pedersen, N.L.; Backman, L.; Xu, W. Early Cognitive Deficits in Type 2 Diabetes: A Population-Based Study. J. Alzheimer’s Dis. 2016, 53, 1069–1078. [Google Scholar] [CrossRef]
- Ferreira, F.S.; Pereira, J.M.S.; Reis, A.; Sanches, M.; Duarte, J.V.; Gomes, L.; Moreno, C.; Castelo-Branco, M. Early visual cortical structural changes in diabetic patients without diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2113–2118. [Google Scholar] [CrossRef]
- Xia, W.; Wang, S.; Sun, Z.; Bai, F.; Zhou, Y.; Yang, Y.; Wang, P.; Huang, Y.; Yuan, Y. Altered baseline brain activity in type 2 diabetes: A resting-state fMRI study. Psychoneuroendocrinology 2013, 38, 2493–2501. [Google Scholar] [CrossRef] [PubMed]
- Striemer, C.L.; Chouinard, P.A.; Goodale, M.A.; de Ribaupierre, S. Overlapping neural circuits for visual attention and eye movements in the human cerebellum. Neuropsychologia 2015, 69, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Weiner, K.S.; Zilles, K. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia 2016, 83, 48–62. [Google Scholar] [CrossRef] [Green Version]
- Bokde, A.L.; Lopez-Bayo, P.; Meindl, T.; Pechler, S.; Born, C.; Faltraco, F.; Teipel, S.J.; Moller, H.J.; Hampel, H. Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain 2006, 129, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- DiCarlo, J.J.; Zoccolan, D.; Rust, N.C. How does the brain solve visual object recognition? Neuron 2012, 73, 415–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serre, T. Models of visual categorization. Wiley Interdiscip. Rev. Cogn. Sci. 2016, 7, 197–213. [Google Scholar] [CrossRef]
- Conway, B.R. The Organization and Operation of Inferior Temporal Cortex. Annu. Rev. Vis. Sci. 2018, 4, 381–402. [Google Scholar] [CrossRef]
- Martinaud, O. Visual agnosia and focal brain injury. Rev. Neurol. 2017, 173, 451–460. [Google Scholar] [CrossRef]
- Cui, Y.; Jiao, Y.; Chen, Y.C.; Wang, K.; Gao, B.; Wen, S.; Ju, S.; Teng, G.J. Altered spontaneous brain activity in type 2 diabetes: A resting-state functional MRI study. Diabetes 2014, 63, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.L.; Chen, Y.L.; Leu, J.G.; Jaw, F.S.; Lee, C.H.; Tsai, Y.F.; Hsu, C.Y.; Bai, C.H.; Leemans, A. Microstructural white matter abnormalities in type 2 diabetes mellitus: A diffusion tensor imaging study. Neuroimage 2012, 59, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; An, J.; Tan, X.; Zeng, L.L.; Shen, H.; Qiu, S.; Hu, D. Changes in the cerebellar and cerebro-cerebellar circuit in type 2 diabetes. Brain Res. Bull. 2017, 130, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Centomo, H.; Termoz, N.; Savoie, S.; Beliveau, L.; Prince, F. Postural control following a self-initiated reaching task in type 2 diabetic patients and age-matched controls. Gait Posture 2007, 25, 509–514. [Google Scholar] [CrossRef]
- Allet, L.; Armand, S.; de Bie, R.A.; Pataky, Z.; Aminian, K.; Herrmann, F.R.; de Bruin, E.D. Gait alterations of diabetic patients while walking on different surfaces. Gait Posture 2009, 29, 488–493. [Google Scholar] [CrossRef]
- Ochoa, N.; Gogola, G.R.; Gorniak, S.L. Contribution of tactile dysfunction to manual motor dysfunction in type II diabetes. Muscle Nerve 2016, 54, 895–902. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Li, C.; Wang, Y.S.; Yang, Z.; Zuo, X.N. Segregation between the parietal memory network and the default mode network: Effects of spatial smoothing and model order in ICA. Sci. Bull. 2016, 61, 1844–1854. [Google Scholar]
- Donaldson, D.I.; Wheeler, M.E.; Petersen, S.E. Remember the source: Dissociating frontal and parietal contributions to episodic memory. J. Cogn. Neurosci. 2010, 22, 377–391. [Google Scholar] [CrossRef] [Green Version]
- Elman, J.A.; Cohn-Sheehy, B.I.; Shimamura, A.P. Dissociable parietal regions facilitate successful retrieval of recently learned and personally familiar information. Neuropsychologia 2013, 51, 573–583. [Google Scholar] [CrossRef]
- Henson, R.N.; Rugg, M.D.; Shallice, T.; Dolan, R.J. Confidence in recognition memory for words: Dissociating right prefrontal roles in episodic retrieval. J. Cogn. Neurosci. 2000, 12, 913–923. [Google Scholar] [CrossRef] [Green Version]
- Konishi, S.; Wheeler, M.E.; Donaldson, D.I.; Buckner, R.L. Neural correlates of episodic retrieval success. Neuroimage 2000, 12, 276–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, B.J.; Buckner, R.L. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J. Neurosci. 2004, 24, 10084–10092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakral, P.P.; Yu, S.S.; Rugg, M.D. The hippocampus is sensitive to the mismatch in novelty between items and their contexts. Brain Res. 2015, 1602, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, G.; Bonni, S.; Pellicciari, M.C.; Casula, E.P.; Mancini, M.; Esposito, R.; Ponzo, V.; Picazio, S.; Di Lorenzo, F.; Serra, L.; et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage 2018, 169, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Skouras, S.; Falcon, C.; Tucholka, A.; Rami, L.; Sanchez-Valle, R.; Llado, A.; Gispert, J.D.; Molinuevo, J.L. Mechanisms of functional compensation, delineated by eigenvector centrality mapping, across the pathophysiological continuum of Alzheimer’s disease. Neuroimage Clin. 2019, 22, 101777. [Google Scholar] [CrossRef]
- Antal, B.; McMahon, L.P.; Sultan, S.F.; Lithen, A.; Wexler, D.J.; Dickerson, B.; Ratai, E.M.; Mujica-Parodi, L.R. Type 2 diabetes mellitus accelerates brain aging and cognitive decline: Complementary findings from UK Biobank and meta-analyses. eLife 2022, 11, e73138. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.R.; Spreng, R.N. Executive functions and neurocognitive aging: Dissociable patterns of brain activity. Neurobiol. Aging 2012, 33, 826.e1–826.e13. [Google Scholar] [CrossRef]
- Breitling, C.; Zaehle, T.; Dannhauer, M.; Tegelbeckers, J.; Flechtner, H.H.; Krauel, K. Comparison between conventional and HD-tDCS of the right inferior frontal gyrus in children and adolescents with ADHD. Clin. Neurophysiol. 2020, 131, 1146–1154. [Google Scholar] [CrossRef]
- Verbruggen, F.; Aron, A.R.; Stevens, M.A.; Chambers, C.D. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proc. Natl. Acad. Sci. USA 2010, 107, 13966–13971. [Google Scholar] [CrossRef] [Green Version]
- Schaum, M.; Pinzuti, E.; Sebastian, A.; Lieb, K.; Fries, P.; Mobascher, A.; Jung, P.; Wibral, M.; Tuscher, O. Right inferior frontal gyrus implements motor inhibitory control via beta-band oscillations in humans. eLife 2021, 10, e61679. [Google Scholar] [CrossRef]
- Siponkoski, S.T.; Martinez-Molina, N.; Kuusela, L.; Laitinen, S.; Holma, M.; Ahlfors, M.; Jordan-Kilkki, P.; Ala-Kauhaluoma, K.; Melkas, S.; Pekkola, J.; et al. Music Therapy Enhances Executive Functions and Prefrontal Structural Neuroplasticity after Traumatic Brain Injury: Evidence from a Randomized Controlled Trial. J. Neurotrauma 2020, 37, 618–634. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, L.; Liu, J. The whole-brain voxel-based morphometry study in early stage of T2DM patients. Brain Behav. 2022, 12, e2497. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Lai, M.Y.; Huang, J.J.; Kang, M.; Ma, M.M.; Li, K.A.; Lian, J.G.; Wang, Z.; Yin, D.Z.; Wang, Y.F. Compensatory Hippocampal Connectivity in Young Adults With Early-Stage Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 3025–3038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, M.; Gao, J.; Huang, Y.; Qi, F.; Lei, Y.; Ai, K.; Yan, X.; Cheng, M.; Su, Y.; et al. Altered Functional Connectivity of Insular Subregions in Type 2 Diabetes Mellitus. Front. Neurosci. 2021, 15, 676624. [Google Scholar] [CrossRef] [PubMed]
- Osher, Y.; Dobron, A.; Belmaker, R.H.; Bersudsky, Y.; Dwolatzky, T. Computerized testing of neurocognitive function in euthymic bipolar patients compared to those with mild cognitive impairment and cognitively healthy controls. Psychother. Psychosom. 2011, 80, 298–303. [Google Scholar] [CrossRef]
- Eshkoor, S.A.; Hamid, T.A.; Mun, C.Y.; Ng, C.K. Mild cognitive impairment and its management in older people. Clin. Interv. Aging 2015, 10, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Leff, A.P.; Schofield, T.M.; Crinion, J.T.; Seghier, M.L.; Grogan, A.; Green, D.W.; Price, C.J. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: Evidence from 210 patients with stroke. Brain 2009, 132, 3401–3410. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Zhang, Z. Exploring Structural and Functional Brain Changes in Mild Cognitive Impairment: A Whole Brain ALE Meta-Analysis for Multimodal MRI. ACS Chem. Neurosci. 2019, 10, 2823–2829. [Google Scholar] [CrossRef]
- Wei, H.; Kong, M.; Zhang, C.; Guan, L.; Ba, M.; Alzheimer’s Disease Neuroimaging Initiative. The structural MRI markers and cognitive decline in prodromal Alzheimer’s disease: A 2-year longitudinal study. Quant. Imaging Med. Surg. 2018, 8, 1004–1019. [Google Scholar] [CrossRef]
- Kircher, T.T.; Weis, S.; Freymann, K.; Erb, M.; Jessen, F.; Grodd, W.; Heun, R.; Leube, D.T. Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J. Neurol. Neurosurg. Psychiatry 2007, 78, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Bokde, A.L.; Karmann, M.; Born, C.; Teipel, S.J.; Omerovic, M.; Ewers, M.; Frodl, T.; Meisenzahl, E.; Reiser, M.; Moller, H.J.; et al. Altered brain activation during a verbal working memory task in subjects with amnestic mild cognitive impairment. J. Alzheimers Dis. 2010, 21, 103–118. [Google Scholar] [CrossRef] [PubMed]


| HC (n = 40) | DMCN (n = 37) | DMCI (n = 40) | F/χ2 | p | |
|---|---|---|---|---|---|
| Age (years) | 54.80 ± 5.35 | 54.03 ± 5.86 | 55.33 ± 6.60 | 0.460 | 0.633 |
| Sex (male/female) | 27/13 | 26/11 | 24/16 | 0.978 | 0.613 # |
| Duration (years) | - | 9.05 ± 5.60 | 9.48 ± 5.44 | - | 0.739 & |
| Education (years) | 14.35 ± 2.82 | 14.08 ± 2.20 | 13.18 ± 2.71 a | 2.229 | 0.112 |
| BMI (kg/m2) | 24.41 ± 2.93 | 25.05 ± 2.67 | 24.75 ± 2.77 | 0.456 | 0.635 |
| Systolic blood pressure (mmHg) | 124.62 ± 12.27 | 126.14 ± 18.77 | 127.28 ± 16.82 | 0.175 | 0.840 |
| Diastolic blood pressure (mmHg) | 85.47 ± 9.40 | 81.38 ± 9.92 | 80.68 ± 13.79 | 0.944 | 0.393 |
| FBG (mmol/L) | 5.19 ± 0.81 | 8.78 ± 2.85 a | 8.21 ± 2.825 a | 19.977 | <0.001 * |
| PBG (mmol/L)2 | - | 12.76 ± 3.20 | 12.14 ± 3.78 | - | 0.498 & |
| HbA1c (%) | 5.62 ± 0.51 | 8.26 ± 1.66 a | 7.90 ± 2.37 a | 25.336 | <0.001 * |
| TG (mmol/L) | 1.83 ± 1.25 | 2.55 ± 4.46 | 1.86 ± 0.86 | 0.858 | 0.427 |
| TC (mmol/L) | 4.87 ± 0.95 | 4.65 ± 1.56 | 4.52 ± 1.20 | 0.741 | 0.479 |
| LDL-C (mmol/L) | 2.74 ± 0.77 | 2.57 ± 0.66 | 2.42 ± 0.88 | 1.491 | 0.230 |
| MMSE score | 28.69 ± 1.49 | 28.68 ± 1.73 | 27.65 ± 1.42 a,b | 5.834 | 0.004 * |
| MoCA score | 26.90 ± 1.92 | 27.43 ± 1.06 | 22.95 ± 1.92 a,b | 81.722 | <0.001* |
| CDT score | 22.56 ± 6.58 | 22.50 ± 7.66 | 21.46 ± 8.12 | 0.260 | 0.772 |
| TMT-A score | 68.11 ± 26.93 | 74.68 ± 31.92 | 88.10 ± 28.85 a,b | 4.745 | 0.011 * |
| RAVLT immediate score | - | 46.29 ± 6.86 | 40.03 ± 6.59 | - | 0.001 & |
| RAVLT delay score | - | 9.71 ± 1.94 | 8.11 ± 2.10 | - | 0.004 & |
| Brain Regions | BA | Voxels (mm3) | Peak MNI Coordinates | f/t-Value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| ANOVA | ||||||
| L_cerebellar lobule VIII/Crus II | - | 104 | −24 | −54 | −48 | 13.916 |
| L_ITG/FG | 37 | 109 | −39 | −51 | −6 | 12.070 |
| R_IFG | 47 | 217 | 51 | 33 | −12 | 13.389 |
| L_STG | 13/41 | 116 | −36 | −30 | 9 | 12.689 |
| DMCN vs. HC | ||||||
| L_cerebellar lobule VIII/Crus I/II | - | 159 | −48 | −72 | −33 | −4.836 |
| R_ MTG/ITG | 38/21 | 175 | 51 | 12 | −30 | −4.089 |
| DMCI vs. HC | ||||||
| L_cerebellar lobule VIII | - | 125 | −15 | −63 | −48 | −5.304 |
| L_MTG/ITG/FG | 37 | 245 | −45 | −60 | 0 | −4.382 |
| R_IFG | 47 | 253 | 51 | 33 | −15 | −4.695 |
| DMCI vs. DMCN | ||||||
| L_STG | 41 | 124 | −30 | −30 | 9 | −3.784 |
| Brain Regions | BA | Voxels (mm3) | Peak MNI Coordinates | f/t-Value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| ANOVA | ||||||
| R_MTG/ITG | 21/38 | 194 | 51 | 9 | −39 | 10.514 |
| R_MTG/ITG/FG/cerebellar lobule VI | 37/20 | 187 | 54 | −48 | −18 | 11.278 |
| L_ITG | 21 | 155 | −60 | −21 | −15 | 10.434 |
| R_IFG | 47 | 206 | 51 | 33 | −15 | 12.651 |
| DMCN vs. HC | ||||||
| L_MTG/ITG | 21 | 188 | −66 | −24 | −15 | −3.975 |
| B_MCC/R_precuneus | 24 | 188 | 15 | −24 | 42 | 3.665 |
| DMCI vs. HC | ||||||
| R_MTG/ITG | 21/38 | 185 | 51 | 12 | −45 | −4.018 |
| R_MTG/FG/cerebellar lobule VI | 37/20 | 299 | 39 | −24 | −18 | −3.908 |
| R_IFG | 47 | 229 | 48 | 30 | −12 | −4.243 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Liu, S.; Huang, Y.; Gao, J.; Liu, W.; Liu, W.; Ai, K.; Lei, X.; Zhang, X. Altered Functional Connectivity Density in Type 2 Diabetes Mellitus with and without Mild Cognitive Impairment. Brain Sci. 2023, 13, 144. https://doi.org/10.3390/brainsci13010144
Zhang D, Liu S, Huang Y, Gao J, Liu W, Liu W, Ai K, Lei X, Zhang X. Altered Functional Connectivity Density in Type 2 Diabetes Mellitus with and without Mild Cognitive Impairment. Brain Sciences. 2023; 13(1):144. https://doi.org/10.3390/brainsci13010144
Chicago/Turabian StyleZhang, Dongsheng, Shasha Liu, Yang Huang, Jie Gao, Weirui Liu, Wanting Liu, Kai Ai, Xiaoyan Lei, and Xiaoling Zhang. 2023. "Altered Functional Connectivity Density in Type 2 Diabetes Mellitus with and without Mild Cognitive Impairment" Brain Sciences 13, no. 1: 144. https://doi.org/10.3390/brainsci13010144




