Sensorimotor Uncertainty of Immersive Virtual Reality Environments for People in Pain: Scoping Review
Abstract
1. Introduction
1.1. Pain Conceptualisation and Its Relationship with the Environment
1.2. Dynamics of Perception, Action, and Stress Responses
1.3. Precision, Uncertainty, and Sensorimotor Behaviour
1.4. Virtual Reality and Its Role in Pain Perception
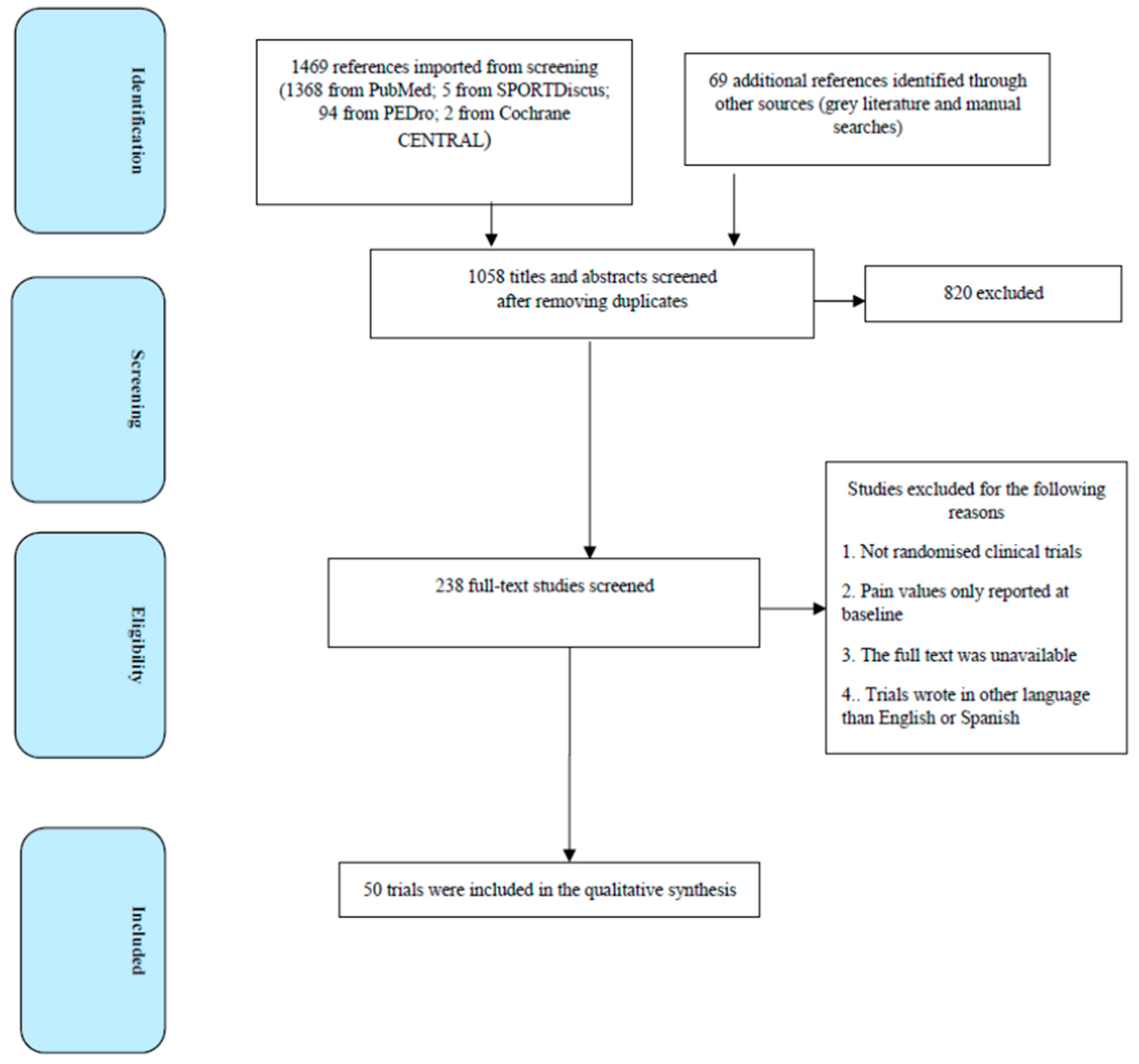
2. Methods
2.1. Study Design
2.2. Search
2.3. Selection of Articles
2.4. Data Summary and Synthesis
3. Results
3.1. Uncertainty about Sensory Information
3.2. Uncertainty about Current State
3.3. Uncertainty about Transition Rules
3.4. Uncertainty about Outcomes
| Type of Sensorimotor Uncertainty | Immersive Virtual Reality Conflict | Correspondence with Motor Decision Making Stage |
|---|---|---|
| Uncertainty about sensory information | Visuotactile incongruence [40,41,43,67] Visuo-auditory incongruence [45,46,47] Visuo-vestibular incongruence [50,51] | Multisensory integration |
| Uncertainty about current state | Visuo-proprioceptive incongruence [42,52,68,69] Peripersonal space (immersion and presence) [29,58,70] Body illusions [54,55,56,71] | Body embodiment |
| Uncertainty about transition rule | Visuomotor incongruence [59,60,61,62,72] Virtual mirror therapy [73,74,75,76,77,78,79] | Motor performance |
| Uncertainty about outcome | Sensorimotor conflicts [39,65,80,81,82,83] Movement accuracy [63,64,66,84,85,86,87,88] | Reinforcement learning |
4. Discussion
4.1. Limitations of the Study
4.2. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Date and Author | Type of Sensorimotor Uncertainty | Immersive VR Conflict | Correspondence with Motor Decision Making Stage | Main Findings |
| Rubo et al., 2019 [40] | Uncertainty about Sensory information | Visuo-tactile incongruence | Multisensory inte-gration | Action-oriented, unconscious body schema relies more heavily on tactile information compared to more explicit aspects of body ownership |
| Bekrater-Bodmann et al., 2014 [41] | Uncertainty about Sensory information | Visuo-tactile incongruence | Multisensory inte-gration | The temporal limits of the induction of limb ownership related to multisensory body-related input, suggesting their involvement in the processing of bodily awareness through the integration of visual and tactile events |
| Sano et al., 2016 [43] | Uncertainty about Sensory information | Visuo-tactile incongruence | Multisensory inte-gration | The tactile feedback improves the immediate pain intensity through rehabilitation using our virtual reality system |
| Mattsson et al., 2022 [67] | Uncertainty about Sensory information | Visuo-tactile incongruence | Multisensory inte-gration | Visuo-tactile temporal correlations have a stronger influence on body ownership than visuo-vestibular correlations and that ownership boosts self-motion perception |
| Naef et al., 2022 [45] | Uncertainty about Sensory information | Visuo-auditory incongruence | Multisensory inte-gration | The use of audiovisual VR stimulation is more effective at inducing a relaxation response compared to no artificial sensory inputs, or the independent inputs. |
| Khan et al., 2020 [46] | Uncertainty about Sensory information | Visuo-auditory incongruence | Multisensory inte-gration | Avatar’s movements can be used to influence a person’s own motion, but should include relevant auditory cues congruent with the movement to ensure a suitable level of entrainment is achieved. |
| Liu et al., 2020 [47] | Uncertainty about Sensory information | Visuo-auditory incongruence | Multisensory inte-gration | Depth localization of a visual object in virtual reality can be altered by a spatially incongruent sound, and provide a potential approach that we can adopt a spatially incongruent sound as a cue to reduce the depth compression in VR |
| Ng et al., 2020 [50] | Uncertainty about Sensory information | Visuo-vestibular incongruence | Multisensory inte-gration | When users are placed under a visual-vestibular synchronised condition, their subjective miserable score of cybersickness decreased while their comfort level of the overall experience increased |
| Y So et al., 2001 [51] | Uncertainty about Sensory information | Visuo-vestibular incongruence | Multisensory inte-gration | The nausea and vection ratings increased significantly with speeds increasing from 3 m/s to 10 m/s. At speeds exceeding 10 m/s, the ratings stabilized. Navigation speeds were found to significantly affect the onset times of vection and nausea but did not affect their rates of increase with duration of exposure. Navigation speed had a significant influence on only the oculomotor subscore of SSQ |
| Ichinose et al., 2017 [42] | Uncertainty about Current state | Visuo-proprioceptive incongruence | Body embodiment | The analgesic effect of visual feedback during phantom limb movement is significantly improved by applying somatosensory feedback to the cheek on the affected side |
| Fossataro et al., 2020 [52] | Uncertainty about Current state | Visuo-proprioceptive incongruence | Body embodiment | When vision and proprioception are congruent (i.e., real and virtual hand coincide), a space-dependent modulation of the visual enhancement of touch (VET) effect occurs (with faster responses when visual stimuli are near to than far from the stimulated hand). Contrarily, when vision and proprioception are incongruent (i.e., a discrepancy between real and virtual hand is present), a comparable VET effect is observed when visual stimuli occur near to the real hand and when they occur far from it, but close to the virtual hand |
| Alemanno et al., 2019 [68] | Uncertainty about Current state | Visuo-proprioceptive incongruence | Body embodiment | Teaching patients to execute correct movements with the painful body parts to regain a correct body image, based on the augmented multisensory feedback (auditory, visual) provided by the VR shows significant reductions in all pain rating scale scores (p < 0.05); significant improvements of QoL in the domains of physical functioning, physical role functioning, bodily pain, vitality, and social role functioning; improvements in cognitive functions (p < 0.05); improvements in functional scales (p < 0.05) and mood (p = 0.04). |
| Limanowski et al., 2020 [69] | Uncertainty about Current state | Visuo-proprioceptive incongruence | Body embodiment | Endogenous attention can balance the gain of visual versus proprioceptive brain areas, thus contextualizing their influence on multisensory areas representing the body for action |
| Cooper et al., 2018 [58] | Uncertainty about Current state | Peripersonal space (immersion and presence) | Body embodiment | Participants performed best and felt an increased sense of immersion and involvement, collectively referred to as ’presence’, when substitute multimodal sensory feedback was provided. Significant main effects of audio and tactile cues on task performance and on participants’ subjective ratings were found. A significant negative relationship was found between the objective (overall completion times) and subjective (ratings of presence) performance measures |
| Scandola et al., 2020 [70] | Uncertainty about Current state | Peripersonal space (immersion and presence) | Body embodiment | The presence of motor feedback was necessary for the recovery of Peripersonal space (PPS) representation, both when the motor feedback was congruent and when it was incongruent with the visual feedback. In contrast, visuo-motor incongruence led to an inhibition of PPS representation in the control group |
| Hoffman et al., 2021 [29] | Uncertainty about Current state | Peripersonal space (immersion and presence) | Body embodiment | Interacting with virtual objects via embodied avatar hands (i.e., avatar VR)significantly increased the participant’s illusion of “being there” in the virtual world, increased VR analgesia, andincreased fun during the pain stimulus. |
| Matamala-Gomez M et al., 2020 [54] | Uncertainty about Current state | Body illusions | Body embodiment | Positive relationship between the level of ownership over the distorted and reddened-distorted virtual arms with the level of pain/discomfort, but not in the normal control arm |
| Matamala-Gomez M et al., 2021 [55] | Uncertainty about Current state | Body illusions | Body embodiment | Patients with chronic pain can achieve levels of ownership and agency over a virtual arm similar to healthy participants. This multisensory interventions by manipulating the body representation throughVR can modulate pain perception |
| Martini et al., 2013 [56] | Uncertainty about Current state | Body illusions | Body embodiment | Influence of skin color on pain perception. This top-down modulation of pain through visual input suggests a potential use of embodied virtual bodies for pain therapy |
| Pyasik et al., 2020 [72] | Uncertainty about Current state | Body illusions | Body embodiment | Subjective ownership of the own hand (OH) was stronger than of the fake hand (FH) in congruent location after synchronous stimulation. It was also present after asynchronous stimulation, being stronger when the virtual OH was subjectively more similar to the real hand. The results suggest that the detailed appearance of the body might act as an additional component in the construction of body ownership. |
| D’Angelo M et al., 2018 [59] | Uncertainty about Transition Rule | Visuo-motor incongruence | Motor performance | Body schema and peripersonal space are affected by the dynamic between intentional body movements and expected consequences in space |
| Brugada-Ramentol et al., 2019 [60] | Uncertainty about Transition Rule | Visuo-motor incongruence | Motor performance | Congruent active control enhanced and maintained the reported sense of ownership. Incongruent active control, achieved by adding noise to the trajectory of the movement, decreased both reported sense of agency and ownership. |
| Kokkinara E et al., 2015 [61] | Uncertainty about Transition Rule | Visuo-motor incongruence | Motor performance | Spatiotemporal manipulation of 2 and 4 times faster can significantly change participants’ proprioceptive judgments of a virtual object’s size and the agency of the movements. |
| Osumi M et al., 2017 [62] | Uncertainty about Transition Rule | Visuo-motor incongruence | Motor performance | Using a bimanual coordination task correlated with alleviation of phantom limb pain |
| Buetler K et al., 2022 [73] | Uncertainty about Transition Rule | Visuo-motor incongruence | Motor performance | The reported illusion strength was associated with and faster movement initiations, indicating that participants may have physically mirrored and compensated for the body characteristics of the avatar |
| Barton et al., 2014 [74] | Uncertainty about Transition Rule | Virtual mirror therapy | Motor performance | Dynamic morphing using Virtual Mirror Box resulted in a compromise between mirrored movement of the intact side and gait events of the virtual limbs matched with physical events of the impaired side |
| Hsu et al., 2022 [75] | Uncertainty about Transition Rule | Virtual mirror therapy | Motor performance | Virtual reality Mirror Therapy had the same effects in restoring the upper extremity motor function as actual Mirror Therapy |
| Murray et al., 2007 [78] | Uncertainty about Transition Rule | Virtual mirror therapy | Motor performance | All participants reported the transferal of sensations into the muscles and joints of the phantom limb, and a decrease in phantom pain during at least one of the sessions |
| Weber et al., 2019 [79] | Uncertainty about Transition Rule | Virtual mirror therapy | Motor performance | Motor outcomes did not achieve statistical significance using Immersive VR mirror therapy |
| Mazzola et al., 2020 [80] | Uncertainty about Transition Rule | Virtual mirror therapy | Motor performance | There was no significant difference in time between the mirrored and virtual-normal conditions |
| Naylor et al., 2021 [39] | Uncertainty about Outcome | Sensorimotor conflicts | Reinforcement learning | Expectations derived from tactile material cues exert a more substantial influence on heaviness perception, compared to visual material cues |
| Harvie et al., 2015 [65] | Uncertainty about Outcome | Sensorimotor conflicts | Reinforcement learning | Visual-proprioceptive information modulated the threshold for movement-evoked pain |
| Berger et al., 2022 [81] | Uncertainty about Outcome | Sensorimotor conflicts | Reinforcement learning | Positive correlation between the extent of the outward drift of the participants’ arm and the perceived reachability of distal objects |
| Gordon et al., 2019 [82] | Uncertainty about Outcome | Sensorimotor conflicts | Reinforcement learning | Effects on pain threshold were present for type of visuo-tactile stimulation but not type of movement |
| Bourdin et al., 2019 [83] | Uncertainty about Outcome | Sensorimotor conflicts | Reinforcement learning | Altered visual feedback through body ownership illusions can influence motor performance |
| Spitzley et al., 2022 [63] | Uncertainty about Outcome | Movement accuracy | Reinforcement learning | When available, vision was relied upon more heavily than proprioception for task completion |
| Dagenais et al., 2021 [64] | Uncertainty about Outcome | Movement accuracy | Reinforcement learning | Altering visual feedback did not influence pain during a reaching task, and both groups adapted similarly to it |
| Matamala-Gómez et al., 2022 [66] | Uncertainty about Outcome | Movement accuracy | Reinforcement learning | Functional recovery was correlated with the ownership and agency scores over the virtual arm. Larger range of joint movements and lower disability |
| Odermatt et al., 2021 [85] | Uncertainty about Outcome | Movement accuracy | Reinforcement learning | Congruency of information create subjective body ownership and is associated with faster reaction times |
| Harvie et al., 2017 [86] | Uncertainty about Outcome | Movement accuracy | Reinforcement learning | Altered visual feedback caused a kinaesthetic drift in the direction of the visually suggested movement |
| Yamada et al., 2021 [87] | Uncertainty about Outcome | Movement accuracy | Reinforcement learning | Better performance, specifically greater accuracy and lower one-dimensional bias in the anteroposterior direction when adopting an external attentional focus |
| Aoyagi et al., 2021 [88] | Uncertainty about Outcome | Movement accuracy | Reinforcement learning | Sense of agency can be enhanced by modifying feedback to motor tasks according to the goal of the task, even when visual feedback is discrepant from the actual body movements |
References
- Stilwell, P.; Harman, K. An enactive approach to pain: Beyond the biopsychosocial model. Phenom Cogn. Sci. 2019, 18, 637–665. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.; Crombez, G. Behavioral Conceptualization and Treatment of Chronic Pain. Annu. Rev. Clin. Psychol. 2020, 16, 187–212. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Smith, M.; Adhia, D. Pain and the Triple Network Model. Front. Neurol. 2022, 13, 757241. [Google Scholar] [CrossRef] [PubMed]
- Coninx, S.; Stilwell, P. Pain and the field of affordances: An enactive approach to acute and chronic pain. Synthese 2021, 199, 7835–7863. [Google Scholar] [CrossRef]
- Cormack, B.; Stilwell, P.; Coninx, S.; Gibson, J. The biopsychosocial model is lost in translation: From misrepresentation to an enactive modernization. Physiother. Theory Pract. 2022, 39, 2273–2288. [Google Scholar] [CrossRef]
- Zaman, J.; Van Oudenhove, L.; Vlaeyen, J.W.S. Uncertainty in a context of pain: Disliked but also more painful? Pain 2021, 162, 995–998. Available online: https://journals.lww.com/pain/Fulltext/2021/04000/Uncertainty_in_a_context_of_pain__disliked_but.3.aspx (accessed on 25 January 2023). [CrossRef]
- Peters, A.; McEwen, B.S.; Friston, K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog. Neurobiol. 2017, 156, 164–188. [Google Scholar] [CrossRef] [PubMed]
- Koolhaas, J.; Bartolomucci, A.; Buwalda, B.; de Boer, S.; Flügge, G.; Korte, S.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef]
- Battaglia, P.W.; Schrater, P.R. Humans Trade Off Viewing Time and Movement Duration to Improve Visuomotor Accuracy in a Fast Reaching Task. J. Neurosci. 2007, 27, 6984–6994. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.R.; Dolan, R.J. Knowing how much you don’t know: A neural organization of uncertainty estimates. Nat. Rev. Neurosci. 2012, 13, 572–586. [Google Scholar] [CrossRef]
- Loued-Khenissi, L.; Martin-Brevet, S.; Schumacher, L.; Corradi-Dell’acqua, C. The effect of uncertainty on pain decisions for self and others. Eur. J. Pain 2022, 26, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, A.; Mary, P.M. When feeling is more im- portant than seeing in sensorimotor adaptation. I-Manag. J. Power Syst. Eng. 2014, 2, 11–16. [Google Scholar]
- van Beers, R.J.; Baraduc, P.; Wolpert, D.M. Role of uncertainty in sensorimotor control. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.A.; Selen, L.P.J.; Wolpert, D.M. Noise in the nervous system. Nat. Rev. Neurosci. 2009, 9, 292–303. [Google Scholar] [CrossRef]
- Faisal, A.A.; Wolpert, D.M.; Field, G.D.; Uzzell, V.; Chichilnisky, E.J.; Rieke, F.; Brenner, E.; Smeets, J.B.J.; Arnoux, L.; Fromentin, S.; et al. Near Optimal Combination of Sensory and Motor Uncertainty in Time During a Naturalistic Perception-Action Task. J. Neurophysiol. 2009, 101, 1901–1912. [Google Scholar] [CrossRef]
- Hewitson, C.L.; Kaplan, D.M.; Crossley, M.J.; Haven, N.; Haven, N. Sensory uncertainty punctuates motor learning independently of movement error when both feedforward and feedback control processes are engaged. bioRxiv 2022. bioRxiv:01.506147. [Google Scholar]
- Sengupta, S.; Medendorp, W.P.; Praamstra, P.; Selen, L.P.J. Uncertainty modulated exploration in the trade-off between sensing and acting. PLoS ONE 2018, 13, e0199544. [Google Scholar] [CrossRef]
- Beck, J.M.; Ma, W.J.; Pitkow, X.; Latham, P.E.; Pouget, A. Not Noisy, Just Wrong: The Role of Suboptimal Inference in Behavioral Variability. Neuron 2012, 74, 30–39. [Google Scholar] [CrossRef]
- Gordon, E.M.; Chauvin, R.J.; Van, A.N.; Rajesh, A.; Nielsen, A.; Newbold, D.J.; Lynch, C.J.; Seider, N.A.; Krimmel, S.R.; Scheidter, K.M.; et al. A mind-body interface alternates with effector-specific regions in motor cortex Evan. bioRxiv 2022. bioRxiv:26.513940. [Google Scholar]
- Wolpert, D.M.; Landy, M.S. Motor control is decision-making. Curr. Opin. Neurobiol. 2012, 22, 996–1003. [Google Scholar] [CrossRef]
- Stappers, P.J.; Gaver, W.; Overbeeke, C.J. Beyond the limits of real-time realism: Moving from stimulation correspondence to information correspondence. In Virtual and Adaptive Environments; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Vittersø, A.D.; Halicka, M.; Buckingham, G.; Proulx, M.J.; Bultitude, J.H. The sensorimotor theory of pathological pain revisited. Neurosci. Biobehav. Rev. 2022, 139, 104735. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Armas, J.; Flores-Cortes, M.; Pineda-Galan, C.; Luque-Suarez, A.; La Touche, R. Role of Immersive Virtual Reality in Motor Behaviour Decision-Making in Chronic Pain Patients. Brain Sci. 2023, 13, 617. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.M.; Held, R. Visually directed pointing as a function of target distance, direction, and available cues. Percept Psychol. 1972, 12, 263–268. [Google Scholar] [CrossRef]
- van Beers, R.J.; Sittig, A.C.G.J. Integration of proprioceptive and visual position-information: An experimentally supported model. J. Neurophysiol. 1999, 81, 1355–1364. [Google Scholar] [CrossRef]
- Vallortigara, G. The Efference Copy Signal as a Key Mechanism for Consciousness. Front. Syst. Neurosci. 2021, 15, 765646. [Google Scholar] [CrossRef]
- Trost, Z.; France, C.; Anam, M.; Shum, C. Virtual reality approaches to pain: Toward a state of the science. Pain 2021, 162, 325–331. Available online: https://journals.lww.com/pain/Fulltext/2021/02000/Virtual_reality_approaches_to_pain__toward_a_state.2.aspx (accessed on 23 August 2022). [CrossRef]
- Kardong-Edgren, S.; Farra, S.L.; Alinier, G.; Young, H.M. A Call to Unify Definitions of Virtual Reality. Clin. Simul. Nurs. 2019, 31, 28–34. [Google Scholar] [CrossRef]
- Hoffman, H.G. Interacting with virtual objects via embodied avatar hands reduces pain intensity and diverts attention. Sci. Rep. 2021, 11, 10672. [Google Scholar] [CrossRef]
- Slater, M. Immersion and the illusion of presence in virtual reality. Br. J. Psychol. 2018, 109, 431–433. [Google Scholar] [CrossRef]
- Triberti, S.; Riva, G. Being Present in Action: A Theoretical Model About the “Interlocking” Between Intentions and Environmental Affordances. Front. Psychol. 2016, 6, 158981. [Google Scholar] [CrossRef]
- Gutierrez-Maldonado, J.; Gutierrez-Martinez, O.; Cabas-Hoyos, K. Interactive and passive virtual reality distraction: Effects on presence and pain intensity. Stud. Health Technol. Inform. 2011, 167, 69–73. [Google Scholar] [CrossRef]
- Baker, N.A.; Polhemus, A.H.; Ospina, E.H.; Feller, H.; Zenni, M.; Deacon, M.; DeGrado, G.; Basnet, S.; Driscoll, M. The State of Science in the Use of Virtual Reality in the Treatment of Acute and Chronic Pain: A Systematic Scoping Review. Clin. J. Pain. 2022, 38, 424–441. Available online: https://journals.lww.com/clinicalpain/Fulltext/2022/06000/The_State_of_Science_in_the_Use_of_Virtual_Reality.7.aspx (accessed on 16 March 2023). [CrossRef]
- Malloy, K.M.; Milling, L.S. The effectiveness of virtual reality distraction for pain reduction: A systematic review. Clin. Psychol. Rev. 2010, 30, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Scott, K.; Dukewich, M. Innovative Technology Using Virtual Reality in the Treatment of Pain: Does It Reduce Pain via Distraction, or Is There More to It? Pain Med. 2018, 19, 151–159. [Google Scholar] [CrossRef]
- Bascour-Sandoval, C.; Salgado-Salgado, S.; Gómez-Milán, E.; Fernández-Gómez, J.; Michael, G.A.; Gálvez-García, G. Pain and Distraction According to Sensory Modalities: Current Findings and Future Directions. Pain Pract. 2019, 19, 686–702. [Google Scholar] [CrossRef]
- Gold, J.I.; Belmont, K.A.; Thomas, D.A. The Neurobiology of Virtual Reality Pain Attenuation. CyberPsychol. Behav. 2007, 10, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Limanowski, J. Precision control for a flexible body representation. Neurosci. Biobehav. Rev. 2022, 134, 104401. [Google Scholar] [CrossRef] [PubMed]
- Naylor, C.E.; Proulx, M.J.; Buckingham, G. Using Immersive Virtual Reality to Examine How Visual and Tactile Cues Drive the Material-Weight Illusion. Atten. Percept. Psychophys. 2021, 84, 509–518. [Google Scholar] [CrossRef]
- Rubo, M.; Gamer, M. Visuo-tactile congruency influences the body schema during full body ownership illusion. Conscious. Cogn. 2019, 73, 102758. [Google Scholar] [CrossRef]
- Bekrater-Bodmann, R.; Foell, J.; Diers, M.; Kamping, S.; Rance, M.; Kirsch, P.; Trojan, J.; Fuchs, X.; Bach, F.; Çakmak, H.K.; et al. The Importance of Synchrony and Temporal Order of Visual and Tactile Input for Illusory Limb Ownership Experiences—An fMRI Study Applying Virtual Reality. PLoS ONE 2014, 9, e87013. [Google Scholar] [CrossRef]
- Ichinose, A.; Sano, Y.; Osumi, M.; Sumitani, M.; Kumagaya, S.-I.; Kuniyoshi, Y. Somatosensory Feedback to the Cheek During Virtual Visual Feedback Therapy Enhances Pain Alleviation for Phantom Arms. Neurorehabilit. Neural Repair 2017, 31, 717–725. [Google Scholar] [CrossRef]
- Sano, Y.; Wake, N.; Ichinose, A.; Osumi, M.; Oya, R.; Sumitani, M.; Kumagaya, S.-I.; Kuniyoshi, Y. Tactile feedback for relief of deafferentation pain using virtual reality system: A pilot study. J. Neuroeng. Rehabilitation 2016, 13, 61. [Google Scholar] [CrossRef]
- Gehrke, L.; Lopes, P.; Klug, M.; Akman, S.; Gramann, K. Neural sources of prediction errors detect unrealistic VR interactions. J. Neural Eng. 2022, 19, 036002. [Google Scholar] [CrossRef]
- Naef, A.C.; Jeitziner, M.-M.; Knobel, S.E.J.; Exl, M.T.; Müri, R.M.; Jakob, S.M.; Nef, T.; Gerber, S.M. Investigating the role of auditory and visual sensory inputs for inducing relaxation during virtual reality stimulation. Sci. Rep. 2022, 12, 17073. [Google Scholar] [CrossRef]
- Khan, O.; Ahmed, I.; Cottingham, J.; Rahhal, M.; Arvanitis, T.N.; Elliott, M.T. Timing and correction of stepping movements with a virtual reality avatar. PLoS ONE 2020, 15, e0229641. [Google Scholar] [CrossRef]
- Liu, D.; Rau, P.-L.P. Spatially incongruent sounds affect visual localization in virtual environments. Atten. Percept. Psychophys. 2020, 82, 2067–2075. [Google Scholar] [CrossRef]
- Weech, S.; Kenny, S.; Barnett-Cowan, M. Presence and Cybersickness in Virtual Reality Are Negatively Related: A Review. Front. Psychol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Li, X.; Luh, D.-B.; Xu, R.-H.; An, Y. Considering the Consequences of Cybersickness in Immersive Virtual Reality Rehabilitation: A Systematic Review and Meta-Analysis. Appl. Sci. 2023, 13, 5159. [Google Scholar] [CrossRef]
- Ng, A.K.; Chan, L.K.; Lau, H.Y. A study of cybersickness and sensory conflict theory using a motion-coupled virtual reality system. Displays 2020, 61, 101922. [Google Scholar] [CrossRef]
- So, R.H.Y.; Lo, W.T.; Ho, A.T.K. Effects of Navigation Speed on Motion Sickness Caused by an Immersive Virtual Environment. Hum. Factors 2001, 43, 452–461. [Google Scholar] [CrossRef]
- Fossataro, C.; Sebastiano, A.R.; Tieri, G.; Poles, K.; Galigani, M.; Pyasik, M.; Bruno, V.; Bertoni, T.; Garbarini, F. Immersive virtual reality reveals that visuo-proprioceptive discrepancy enlarges the hand-centred peripersonal space. Neuropsychologia 2020, 146, 107540. [Google Scholar] [CrossRef]
- Ho, J.T.; Krummenacher, P.; Lenggenhager, B. Not my body, not my pain? Pain perception and placebo analgesia in individuals with body integrity dysphoria. Cortex 2022, 153, 44–54. [Google Scholar] [CrossRef]
- Matamala-Gomez, M.; Nierula, B.; Donegan, T.; Slater, M.; Sanchez-Vives, M.V. Manipulating the Perceived Shape and Color of a Virtual Limb Can Modulate Pain Responses. J. Clin. Med. 2020, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Matamala-Gomez, M.; Gonzalez, A.M.D.; Slater, M.; Sanchez-Vives, M.V. Decreasing Pain Ratings in Chronic Arm Pain Through Changing a Virtual Body: Different Strategies for Different Pain Types. J. Pain 2019, 20, 685–697. [Google Scholar] [CrossRef]
- Martini, M.; Perez-Marcos, D.; Sanchez-Vives, M.V. What colour is my arm? Changes in skin colour of an embodied virtual arm modulates pain threshold. Front. Hum. Neurosci. 2013, 7, 438. [Google Scholar] [CrossRef] [PubMed]
- Colloca, L.; Raghuraman, N.; Wang, Y.; Akintola, T.; Brawn-Cinani, B.; Colloca, G.; Kier, C.; Varshney, A.; Murthi, S. Virtual reality: Physiological and behavioral mechanisms to increase individual pain tolerance limits. Pain 2020, 161, 2010–2021. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.; Milella, F.; Pinto, C.; Cant, I.; White, M.; Meyer, G. The effects of substitute multisensory feedback on task performance and the sense of presence in a virtual reality environment. PLoS ONE 2018, 13, e0191846. [Google Scholar] [CrossRef] [PubMed]
- D’angelo, M.; di Pellegrino, G.; Seriani, S.; Gallina, P.; Frassinetti, F. The sense of agency shapes body schema and peripersonal space. Sci. Rep. 2018, 8, 13847. [Google Scholar] [CrossRef]
- Brugada-Ramentol, V.; Clemens, I.; de Polavieja, G.G. Active control as evidence in favor of sense of ownership in the moving Virtual Hand Illusion. Conscious. Cogn. 2019, 71, 123–135. [Google Scholar] [CrossRef]
- Kokkinara, E.; Slater, M.; López-Moliner, J. The Effects of Visuomotor Calibration to the Perceived Space and Body, through Embodiment in Immersive Virtual Reality. ACM Trans. Appl. Percept. 2015, 13, 1–22. [Google Scholar] [CrossRef]
- Osumi, M.; Ichinose, A.; Sumitani, M.; Wake, N.; Sano, Y.; Yozu, A.; Kumagaya, S.; Kuniyoshi, Y.; Morioka, S. Restoring movement representation and alleviating phantom limb pain through short-term neurorehabilitation with a virtual reality system. Eur. J. Pain 2017, 21, 140–147. [Google Scholar] [CrossRef]
- Spitzley, K.A.; Karduna, A.R. Joint Position Accuracy Is Influenced by Visuoproprioceptive Congruency in Virtual Reality. J. Mot. Behav. 2022, 54, 92–101. [Google Scholar] [CrossRef]
- Dagenais, M.; Brun, C.; Ohayon, A.; Mercier, C. Virtual Reality in Fibromyalgia: Does Altering Visual Feedback Impact on Pain and Movement During Reaching? Front. Virtual Real. 2021, 2, 681034. [Google Scholar] [CrossRef]
- Harvie, D.S.; Broecker, M.; Smith, R.T.; Meulders, A.; Madden, V.J.; Moseley, G.L. Bogus Visual Feedback Alters Onset of Movement-Evoked Pain in People With Neck Pain. Psychol. Sci. 2015, 26, 385–392. [Google Scholar] [CrossRef]
- Matamala-Gomez, M.; Slater, M.; Sanchez-Vives, M.V. Impact of virtual embodiment and exercises on functional ability and range of motion in orthopedic rehabilitation. Sci. Rep. 2022, 12, 5046. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.P.; Coppi, S.; Chancel, M.; Ehrsson, H.H. Combination of visuo-tactile and visuo-vestibular correlations in illusory body ownership and self-motion sensations. PLoS ONE 2022, 17, e0277080. [Google Scholar] [CrossRef] [PubMed]
- Alemanno, F.; Houdayer, E.; Emedoli, D.; Locatelli, M.; Mortini, P.; Mandelli, C.; Raggi, A.; Iannaccone, S. Efficacy of virtual reality to reduce chronic low back pain: Proof-of-concept of a nonpharmacological approach on pain, quality of life, neuropsychological and functional outcome. PLoS ONE 2019, 14, e0216858. [Google Scholar] [CrossRef] [PubMed]
- Limanowski, J.; Friston, K. Attentional Modulation of Vision Versus Proprioception During Action. Cereb. Cortex 2020, 30, 1637–1648. [Google Scholar] [CrossRef]
- Scandola, M.; Aglioti, S.M.; Lazzeri, G.; Avesani, R.; Ionta, S.; Moro, V. Visuo-motor and interoceptive influences on peripersonal space representation following spinal cord injury. Sci. Rep. 2020, 10, 5162. [Google Scholar] [CrossRef] [PubMed]
- Schindler, A.; Bartels, A. Integration of visual and non-visual self-motion cues during voluntary head movements in the human brain. NeuroImage 2018, 172, 597–607. [Google Scholar] [CrossRef]
- Pyasik, M.; Tieri, G.; Pia, L. Visual appearance of the virtual hand affects embodiment in the virtual hand illusion. Sci. Rep. 2020, 10, 5412. [Google Scholar] [CrossRef] [PubMed]
- Buetler, K.A.; Penalver-Andres, J.; Özen, Ö.; Ferriroli, L.; Müri, R.M.; Cazzoli, D.; Marchal-Crespo, L. “Tricking the Brain” Using Immersive Virtual Reality: Modifying the Self-Perception Over Embodied Avatar Influences Motor Cortical Excitability and Action Initiation. Front. Hum. Neurosci. 2022, 15, 787487. [Google Scholar] [CrossRef]
- Barton, G.J.; De Asha, A.R.; van Loon, E.C.; Geijtenbeek, T.; Robinson, M.A. Manipulation of visual biofeedback during gait with a time delayed adaptive Virtual Mirror Box. J. Neuroeng. Rehabil. 2014, 11, 101. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Kuo, L.-C.; Lin, Y.-C.; Su, F.-C.; Yang, T.-H.; Lin, C.-W. Effects of a Virtual Reality–Based Mirror Therapy Program on Improving Sensorimotor Function of Hands in Chronic Stroke Patients: A Randomized Controlled Trial. Neurorehabilit. Neural Repair 2022, 36, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, P. The body in the brain: Towards a representational neurobiology of somatoform disorders. Acta Neuropsychiatr. 2003, 15, 157–160. [Google Scholar] [CrossRef]
- Lin, C.-W.; Kuo, L.-C.; Lin, Y.-C.; Su, F.-C.; Hsu, H.-Y. Development and Testing of a Virtual Reality Mirror Therapy System for the Sensorimotor Performance of Upper Extremity: A Pilot Randomized Controlled Trial. IEEE Access 2021, 9, 14725–14734. [Google Scholar] [CrossRef]
- Murray, C.D.; Pettifer, S.; Howard, T.; Patchick, E.L.; Caillette, F.; Kulkarni, J.; Bamford, C. The treatment of phantom limb pain using immersive virtual reality: Three case studies. Disabil. Rehabil. 2007, 29, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.M.; Nilsen, D.M.; Gillen, G.; Yoon, J.; Stein, J. Immersive Virtual Reality Mirror Therapy for Upper Limb Recovery after Stroke: A Pilot Study. Am. J. Phys. Med. Rehabil. 2019, 98, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, S.; Prado, A.; Agrawal, S.K. An upper limb mirror therapy environment with hand tracking in virtual reality. In Proceedings of the 2020 8th IEEE RAS/EMBS International Conference for Biomedical Robotics and Biomechatronics (BioRob), New York, NY, USA, 29 November–1 December 2020; pp. 752–758. [Google Scholar]
- Berger, C.C.; Lin, B.; Lenggenhager, B.; Lanier, J.; Gonzalez-Franco, M. Follow Your Nose: Extended Arm Reach After Pinocchio Illusion in Virtual Reality. Front. Virtual Real. 2022, 3, 712375. [Google Scholar] [CrossRef]
- Gordon, C.; Barbullushi, A.; Tombolini, S.; Margiotta, F.; Ciacci, A.; Yosef, L.S.; Barker, L.; Martini, M. Visuo–tactile stimulation, but not type of movement, modulates pain during the vision of a moving virtual limb. Pain Manag. 2019, 9, 449–460. [Google Scholar] [CrossRef]
- Bourdin, P.; Martini, M.; Sanchez-Vives, M.V. Altered visual feedback from an embodied avatar unconsciously influences movement amplitude and muscle activity. Sci. Rep. 2019, 9, 19747. [Google Scholar] [CrossRef]
- Lanillos, P.; Franklin, S.; Maselli, A.; Franklin, D.W. Active strategies for multisensory conflict suppression in the virtual hand illusion. Sci. Rep. 2021, 11, 22844. [Google Scholar] [CrossRef]
- Odermatt, I.A.; Buetler, K.A.; Wenk, N.; Özen, Ö.; Penalver-Andres, J.; Nef, T.; Mast, F.W.; Marchal-Crespo, L. Congruency of Information Rather Than Body Ownership Enhances Motor Performance in Highly Embodied Virtual Reality. Front. Neurosci. 2021, 15, 678909. [Google Scholar] [CrossRef]
- Harvie, D.S.; Smith, R.T.; Hunter, E.V.; Davis, M.G.; Sterling, M.; Moseley, G.L. Using visuo-kinetic virtual reality to induce illusory spinal movement: The MoOVi Illusion. PeerJ 2017, 5, e3023. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamada, M.; Kuznetsov, N.A.; Diekfuss, J.A.; Raisbeck, L.D. The effect of attentional focus on movement accuracy in an immersive and interactive virtual reality environment. Neurosci. Lett. 2021, 752, 135814. [Google Scholar] [CrossRef]
- Aoyagi, K.; Wen, W.; An, Q.; Hamasaki, S.; Yamakawa, H.; Tamura, Y.; Yamashita, A.; Asama, H. Modified sensory feedback enhances the sense of agency during continuous body movements in virtual reality. Sci. Rep. 2021, 11, 2553. [Google Scholar] [CrossRef]
- Osumi, M.; Nobusako, S.; Zama, T.; Taniguchi, M.; Shimada, S.; Morioka, S. Sensorimotor incongruence alters limb perception and movement. Hum. Mov. Sci. 2018, 57, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Seinfeld, S.; Müller, J. Impact of visuomotor feedback on the embodiment of virtual hands detached from the body. Sci. Rep. 2020, 10, 19747. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.; McVeigh, J.G.; McCreesh, K.; Rio, E.; Dekkers, T.; Lewis, J.S. Exploring the effectiveness of immersive Virtual Reality interventions in the management of musculoskeletal pain: A state-of-the-art review. Phys. Ther. Rev. 2021, 26, 262–275. [Google Scholar] [CrossRef]
- Donegan, T.; Ryan, B.E.; Sanchez-Vives, M.V.; Świdrak, J. Altered bodily perceptions in chronic neuropathic pain conditions and implications for treatment using immersive virtual reality. Front. Hum. Neurosci. 2022, 16, 1024910. [Google Scholar] [CrossRef]
- Austin, P.D.; Siddall, P.J. Virtual reality for the treatment of neuropathic pain in people with spinal cord injuries: A scoping review. J. Spinal Cord Med. 2021, 44, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.; Chau, B.; Yeo, E.; Ta, P. Virtual reality for spinal cord injury-associated neuropathic pain: Systematic review. Ann. Phys. Rehabil. Med. 2019, 62, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Leemhuis, E.; Giuffrida, V.; Giannini, A.M.; Pazzaglia, M. A Therapeutic Matrix: Virtual Reality as a Clinical Tool for Spinal Cord Injury-Induced Neuropathic Pain. Brain Sci. 2021, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Medina, J. Influence of the Body Schema on Multisensory Integration: Evidence from the Mirror Box Illusion. Sci. Rep. 2017, 7, 5060. [Google Scholar] [CrossRef] [PubMed]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Randomised clinical trials, secondary analysis of randomised clinical trials, and pilot randomised clinical trials | Studies that included other types of non-immersive VR intervention, and/or non-virtual intervention(s) Studies about children |
| Immersive virtual reality interventions compared to [i] no intervention; [ii] sham control; [iii] usual care control; or [iv] active control. | Full text not available Protocol for randomised clinical trials |
| Published in peer reviewed journal or conference proceedings | |
| Published since year 2018 | |
| Written in English language |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Cortes, M.; Guerra-Armas, J.; Pineda-Galan, C.; La Touche, R.; Luque-Suarez, A. Sensorimotor Uncertainty of Immersive Virtual Reality Environments for People in Pain: Scoping Review. Brain Sci. 2023, 13, 1461. https://doi.org/10.3390/brainsci13101461
Flores-Cortes M, Guerra-Armas J, Pineda-Galan C, La Touche R, Luque-Suarez A. Sensorimotor Uncertainty of Immersive Virtual Reality Environments for People in Pain: Scoping Review. Brain Sciences. 2023; 13(10):1461. https://doi.org/10.3390/brainsci13101461
Chicago/Turabian StyleFlores-Cortes, Mar, Javier Guerra-Armas, Consolacion Pineda-Galan, Roy La Touche, and Alejandro Luque-Suarez. 2023. "Sensorimotor Uncertainty of Immersive Virtual Reality Environments for People in Pain: Scoping Review" Brain Sciences 13, no. 10: 1461. https://doi.org/10.3390/brainsci13101461
APA StyleFlores-Cortes, M., Guerra-Armas, J., Pineda-Galan, C., La Touche, R., & Luque-Suarez, A. (2023). Sensorimotor Uncertainty of Immersive Virtual Reality Environments for People in Pain: Scoping Review. Brain Sciences, 13(10), 1461. https://doi.org/10.3390/brainsci13101461







