Chronotype-Dependent Sleep Loss Is Associated with a Lower Amplitude in Circadian Rhythm and a Higher Fragmentation of REM Sleep in Young Healthy Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics, Study Design and Sample Characteristics
2.2. Assessment of Objective Measures of Sleep Timing, Sleep Architecture and Circadian Rhythms
2.3. Statistical Analyses
3. Results
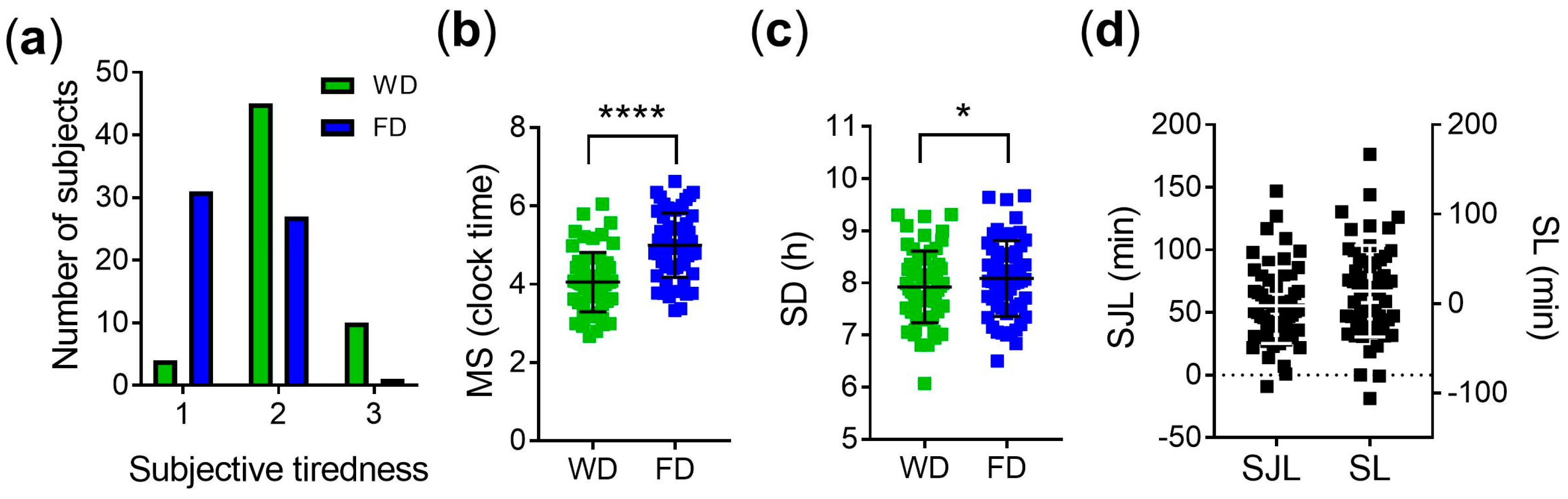
3.1. Subjective Measures of Sleep Quality and Objective Measures of Sleep Timing
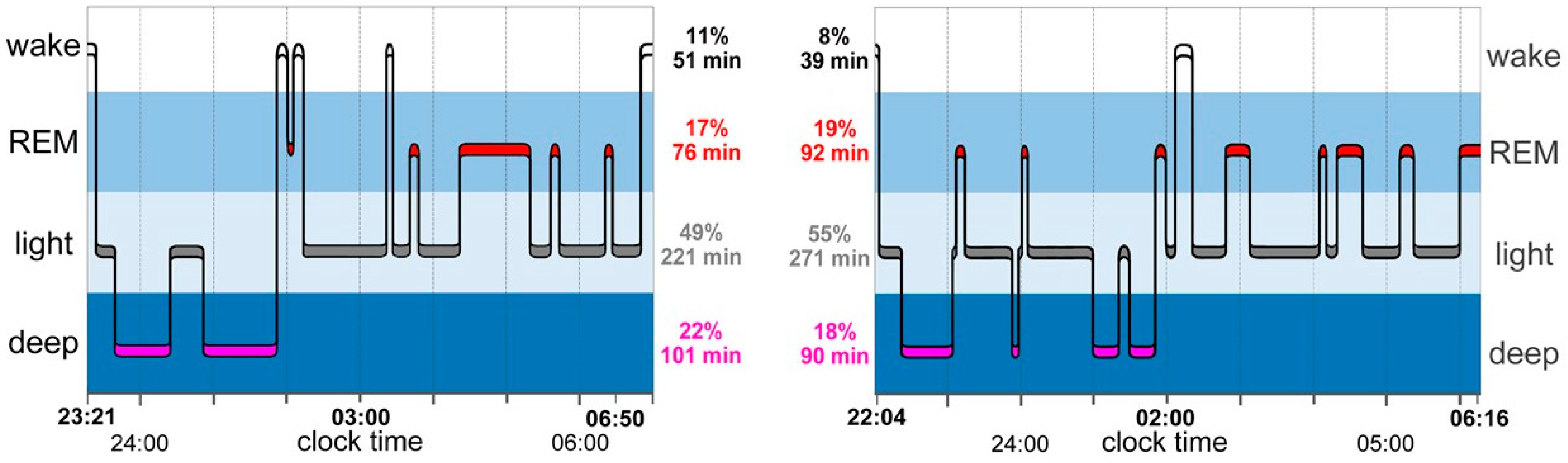
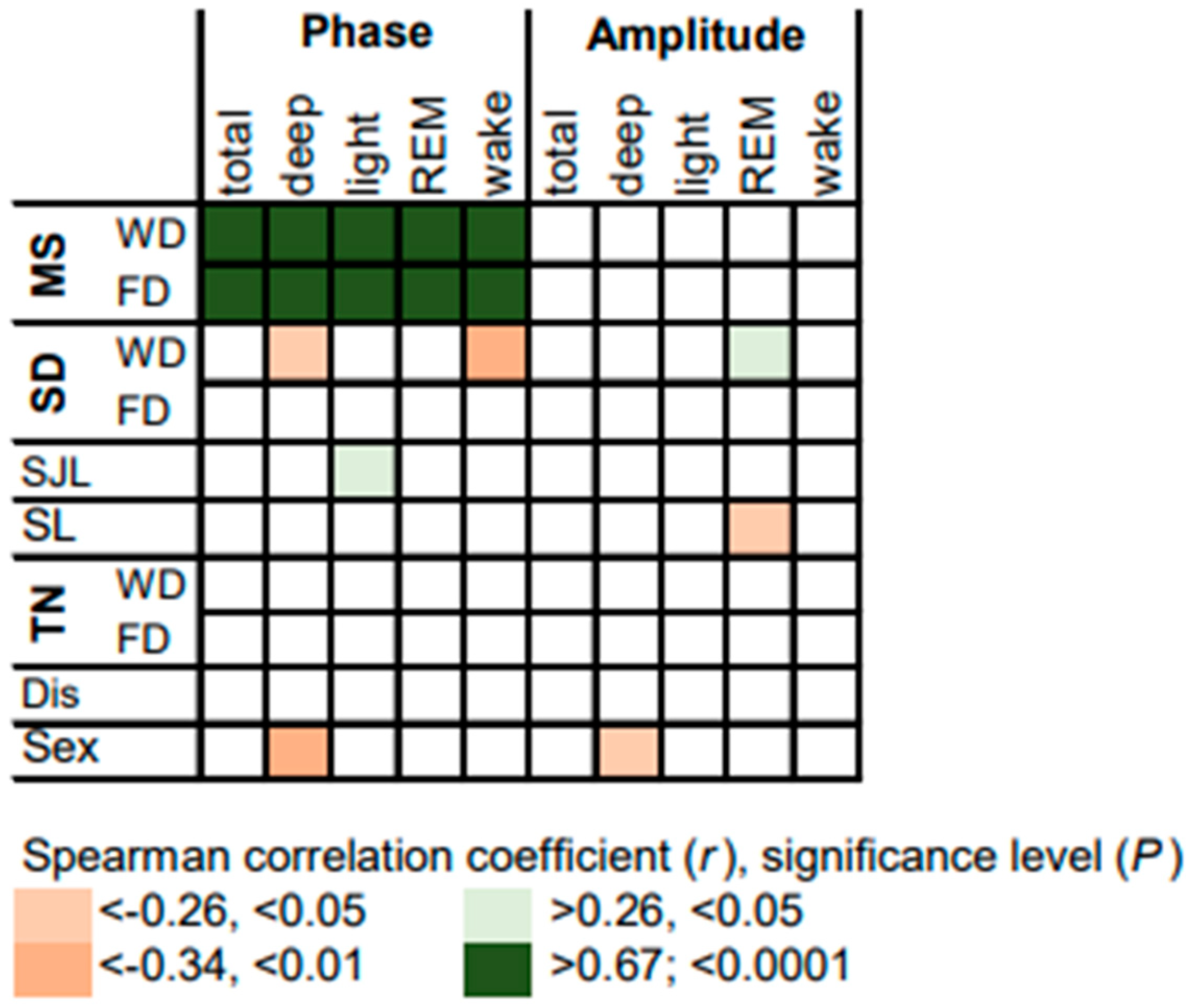
3.2. Circadian Rhythms of Sleep Stages
3.3. Sleep Architecture
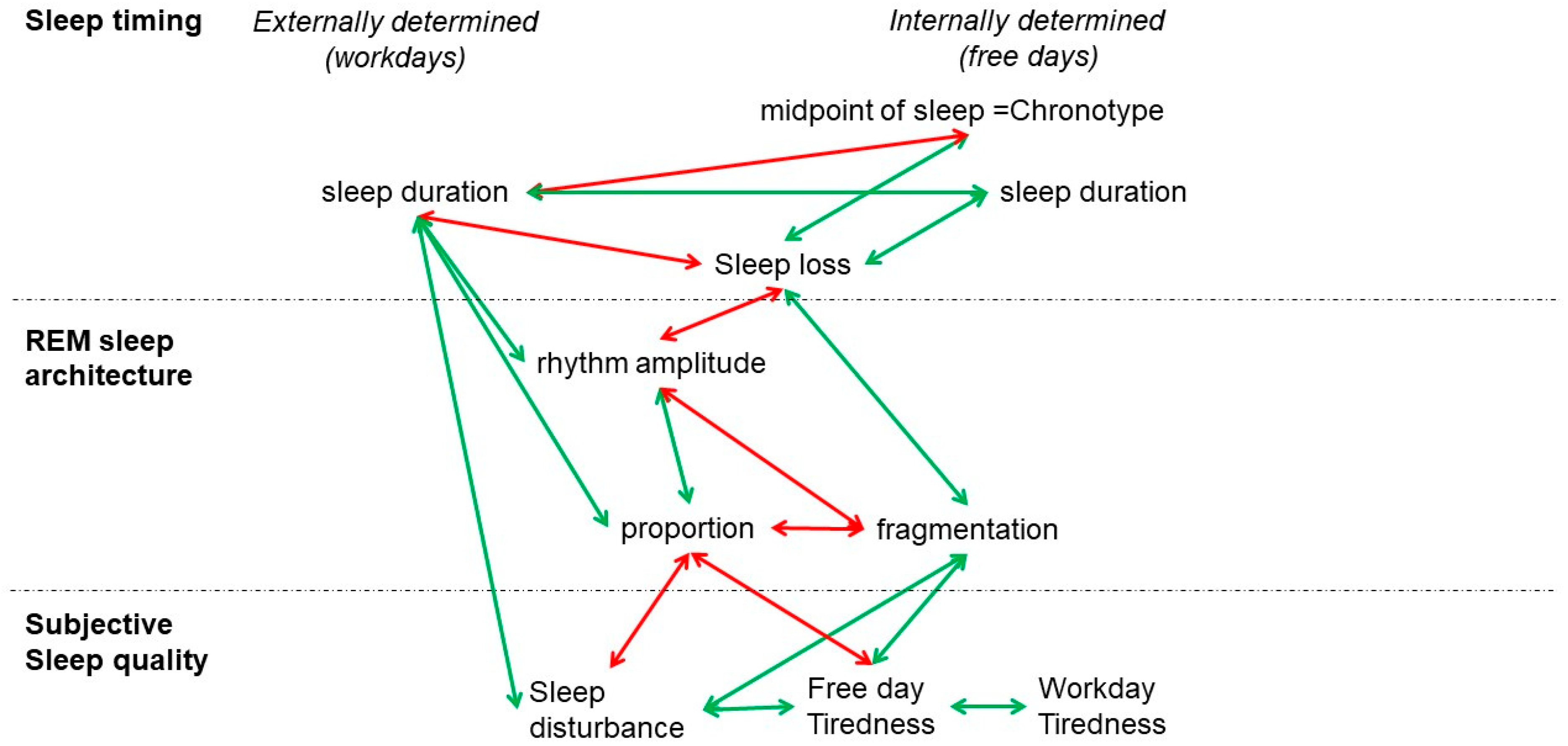
4. Discussion
5. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zager, A.; Andersen, M.L.; Ruiz, F.S.; Antunes, I.B.; Tufik, S. Effects of acute and chronic sleep loss on immune modulation of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R504–R509. [Google Scholar] [CrossRef]
- Killgore, W.D. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010, 185, 105–129. [Google Scholar] [CrossRef]
- Ju, Y.E.; McLeland, J.S.; Toedebusch, C.D.; Xiong, C.; Fagan, A.M.; Duntley, S.P.; Morris, J.C.; Holtzman, D.M. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013, 70, 587–593. [Google Scholar] [CrossRef]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythm. 2003, 18, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Lack, L.; Bailey, M.; Lovato, N.; Wright, H. Chronotype differences in circadian rhythms of temperature, melatonin, and sleepiness as measured in a modified constant routine protocol. Nat. Sci. Sleep 2009, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.N.; Robillard, D.L.; Skene, D.J.; Smits, M.; Williams, A.; Arendt, J.; von Schantz, M. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep 2003, 26, 413–415. [Google Scholar] [CrossRef]
- Viola, A.U.; Archer, S.N.; James, L.M.; Groeger, J.A.; Lo, J.C.; Skene, D.J.; von Schantz, M.; Dijk, D.J. PER3 polymorphism predicts sleep structure and waking performance. Curr. Biol. 2007, 17, 613–618. [Google Scholar] [CrossRef]
- Wittmann, M.; Dinich, J.; Merrow, M.; Roenneberg, T. Social jetlag: Misalignment of biological and social time. Chronobiol. Int. 2006, 23, 497–509. [Google Scholar] [CrossRef]
- Van den Berg, J.F.; Kivela, L.; Antypa, N. Chronotype and depressive symptoms in students: An investigation of possible mechanisms. Chronobiol. Int. 2018, 35, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Sudy, A.R.; Ella, K.; Bodizs, R.; Kaldi, K. Association of Social Jetlag With Sleep Quality and Autonomic Cardiac Control During Sleep in Young Healthy Men. Front. Neurosci. 2019, 13, 950. [Google Scholar] [CrossRef]
- Pilz, L.K.; Keller, L.K.; Lenssen, D.; Roenneberg, T. Time to rethink sleep quality: PSQI scores reflect sleep quality on workdays. Sleep 2018, 41, zsy029. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.L.; Agnew, H.W., Jr.; Webb, W.B. Sleep Patterns in Young Adults: An Eeg Study. Electroencephalogr. Clin. Neurophysiol. 1964, 17, 376–381. [Google Scholar] [CrossRef]
- Williams, R.L.; Agnew, H.W., Jr.; Webb, W.B. Sleep patterns in the young adult female: An EEG study. Electroencephalogr. Clin. Neurophysiol. 1966, 20, 264–266. [Google Scholar] [CrossRef]
- Walther, B.W.; Schulz, H. Recurrent isolated sleep paralysis: Polysomnographic and clinical findings. Somnologie-Schlafforschung Schlafmed. 2004, 8, 53–60. [Google Scholar] [CrossRef]
- Cesari, M.; Christensen, J.A.E.; Muntean, M.L.; Mollenhauer, B.; Sixel-Doring, F.; Sorensen, H.B.D.; Trenkwalder, C.; Jennum, P. A data-driven system to identify REM sleep behavior disorder and to predict its progression from the prodromal stage in Parkinson’s disease. Sleep Med. 2021, 77, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Rechichi, I.; Iadarola, A.; Zibetti, M.; Cicolin, A.; Olmo, G. Assessing REM Sleep Behaviour Disorder: From Machine Learning Classification to the Definition of a Continuous Dissociation Index. Int. J. Env. Res. Public Health 2021, 19, 248. [Google Scholar] [CrossRef]
- Fitbit. Available online: https://help.fitbit.com/articles/en_US/Help_article/2163.htm (accessed on 7 July 2023).
- Cook, J.D.; Eftekari, S.C.; Dallmann, E.; Sippy, M.; Plante, D.T. Ability of the Fitbit Alta HR to quantify and classify sleep in patients with suspected central disorders of hypersomnolence: A comparison against polysomnography. J. Sleep Res. 2019, 28, e12789. [Google Scholar] [CrossRef]
- Moreno-Pino, F.; Porras-Segovia, A.; Lopez-Esteban, P.; Artes, A.; Baca-Garcia, E. Validation of Fitbit Charge 2 and Fitbit Alta HR Against Polysomnography for Assessing Sleep in Adults With Obstructive Sleep Apnea. J. Clin. Sleep Med. 2019, 15, 1645–1653. [Google Scholar] [CrossRef]
- Weiss, C.; Woods, K.; Filipowicz, A.; Ingram, K.K. Sleep Quality, Sleep Structure, and PER3 Genotype Mediate Chronotype Effects on Depressive Symptoms in Young Adults. Front. Psychol. 2020, 11, 2028. [Google Scholar] [CrossRef]
- von Gall, C.; Muth, T.; Angerer, P. Sleep Duration on Workdays Is Correlated with Subjective Workload and Subjective Impact of High Workload on Sleep in Young Healthy Adults. Brain Sci. 2023, 13, 818. [Google Scholar] [CrossRef]
- Juda, M.; Vetter, C.; Roenneberg, T. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J. Biol. Rhythm. 2013, 28, 141–151. [Google Scholar] [CrossRef]
- Leise, T.L. Wavelet-based analysis of circadian behavioral rhythms. Methods Enzym. 2015, 551, 95–119. [Google Scholar] [CrossRef]
- Roenneberg, T.; Kuehnle, T.; Pramstaller, P.P.; Ricken, J.; Havel, M.; Guth, A.; Merrow, M. A marker for the end of adolescence. Curr. Biol. 2004, 14, R1038–R1039. [Google Scholar] [CrossRef] [PubMed]
- Rea, E.M.; Nicholson, L.M.; Mead, M.P.; Egbert, A.H.; Bohnert, A.M. Daily relations between nap occurrence, duration, and timing and nocturnal sleep patterns in college students. Sleep Health 2022, 8, 356–363. [Google Scholar] [CrossRef]
- Mead, M.P.; Huynh, P.; Le, T.Q.; Irish, L.A. Temporal Associations Between Daytime Napping and Nocturnal Sleep: An Exploration of Random Slopes. Ann. Behav. Med. 2022, 56, 1101–1109. [Google Scholar] [CrossRef]
- Harvey, A.G.; Stinson, K.; Whitaker, K.L.; Moskovitz, D.; Virk, H. The subjective meaning of sleep quality: A comparison of individuals with and without insomnia. Sleep 2008, 31, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Winnebeck, E.C.; Fischer, D.; Leise, T.; Roenneberg, T. Dynamics and Ultradian Structure of Human Sleep in Real Life. Curr. Biol. 2018, 28, 49–59.e45. [Google Scholar] [CrossRef] [PubMed]
- Ekhart, D.; Wicht, H.; Kersken, T.; Ackermann, H.; Kaczmarczyk, M.; Pretzsch, G.; Alexander, H.; Korf, H.W. Dynamics of core body temperature cycles in long-term measurements under real life conditions in women. Chronobiol. Int. 2018, 35, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Mongrain, V.; Carrier, J.; Dumont, M. Chronotype and sex effects on sleep architecture and quantitative sleep EEG in healthy young adults. Sleep 2005, 28, 819–827. [Google Scholar] [CrossRef]
- Tsai, L.L.; Li, S.P. Sleep patterns in college students: Gender and grade differences. J. Psychosom. Res. 2004, 56, 231–237. [Google Scholar] [CrossRef]
- Santhi, N.; Lazar, A.S.; McCabe, P.J.; Lo, J.C.; Groeger, J.A.; Dijk, D.J. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E2730–E2739. [Google Scholar] [CrossRef] [PubMed]
- Spitschan, M.; Santhi, N.; Ahluwalia, A.; Fischer, D.; Hunt, L.; Karp, N.A.; Levi, F.; Pineda-Torra, I.; Vidafar, P.; White, R. Sex differences and sex bias in human circadian and sleep physiology research. eLife 2022, 11, e65419. [Google Scholar] [CrossRef] [PubMed]
- Dijk, D.J.; Landolt, H.P. Sleep Physiology, Circadian Rhythms, Waking Performance and the Development of Sleep-Wake Therapeutics. Handb. Exp. Pharmacol. 2019, 253, 441–481. [Google Scholar] [CrossRef] [PubMed]
- Barbato, G. REM Sleep: An Unknown Indicator of Sleep Quality. Int. J. Env. Res. Public Health 2021, 18, 12976. [Google Scholar] [CrossRef]





| Variable | Score | Amount (Proportion) | ||
|---|---|---|---|---|
| Sex | ||||
| Female | 1 | 43 (73%) 1 | ||
| Male | 2 | 16 (27%) | ||
| Tiredness on waking | ||||
| Workdays | ||||
| Well rested | 1 | 4 (7%) | ||
| Tired | 2 | 45 (76%) | ||
| Very tired | 3 | 10 (17%) | ||
| Free days | ||||
| Well rested | 1 | 31 (52%) | ||
| Tired | 2 | 27 (46%) | ||
| Very tired | 3 | 1 (2%) | ||
| Sleep disturbance | ||||
| No | 1 | 55 (93%) | ||
| Yes | 2 | 4 (7%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Gall, C.; Holub, L.; Pfeffer, M.; Eickhoff, S. Chronotype-Dependent Sleep Loss Is Associated with a Lower Amplitude in Circadian Rhythm and a Higher Fragmentation of REM Sleep in Young Healthy Adults. Brain Sci. 2023, 13, 1482. https://doi.org/10.3390/brainsci13101482
von Gall C, Holub L, Pfeffer M, Eickhoff S. Chronotype-Dependent Sleep Loss Is Associated with a Lower Amplitude in Circadian Rhythm and a Higher Fragmentation of REM Sleep in Young Healthy Adults. Brain Sciences. 2023; 13(10):1482. https://doi.org/10.3390/brainsci13101482
Chicago/Turabian Stylevon Gall, Charlotte, Leon Holub, Martina Pfeffer, and Simon Eickhoff. 2023. "Chronotype-Dependent Sleep Loss Is Associated with a Lower Amplitude in Circadian Rhythm and a Higher Fragmentation of REM Sleep in Young Healthy Adults" Brain Sciences 13, no. 10: 1482. https://doi.org/10.3390/brainsci13101482
APA Stylevon Gall, C., Holub, L., Pfeffer, M., & Eickhoff, S. (2023). Chronotype-Dependent Sleep Loss Is Associated with a Lower Amplitude in Circadian Rhythm and a Higher Fragmentation of REM Sleep in Young Healthy Adults. Brain Sciences, 13(10), 1482. https://doi.org/10.3390/brainsci13101482






