Walk Locomotion Kinematic Changes in a Model of Penetrating Hippocampal Injury in Male/Female Mice and Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Study Groups
2.2. Brain Injury Model
2.3. Tunnel Walk Recordings
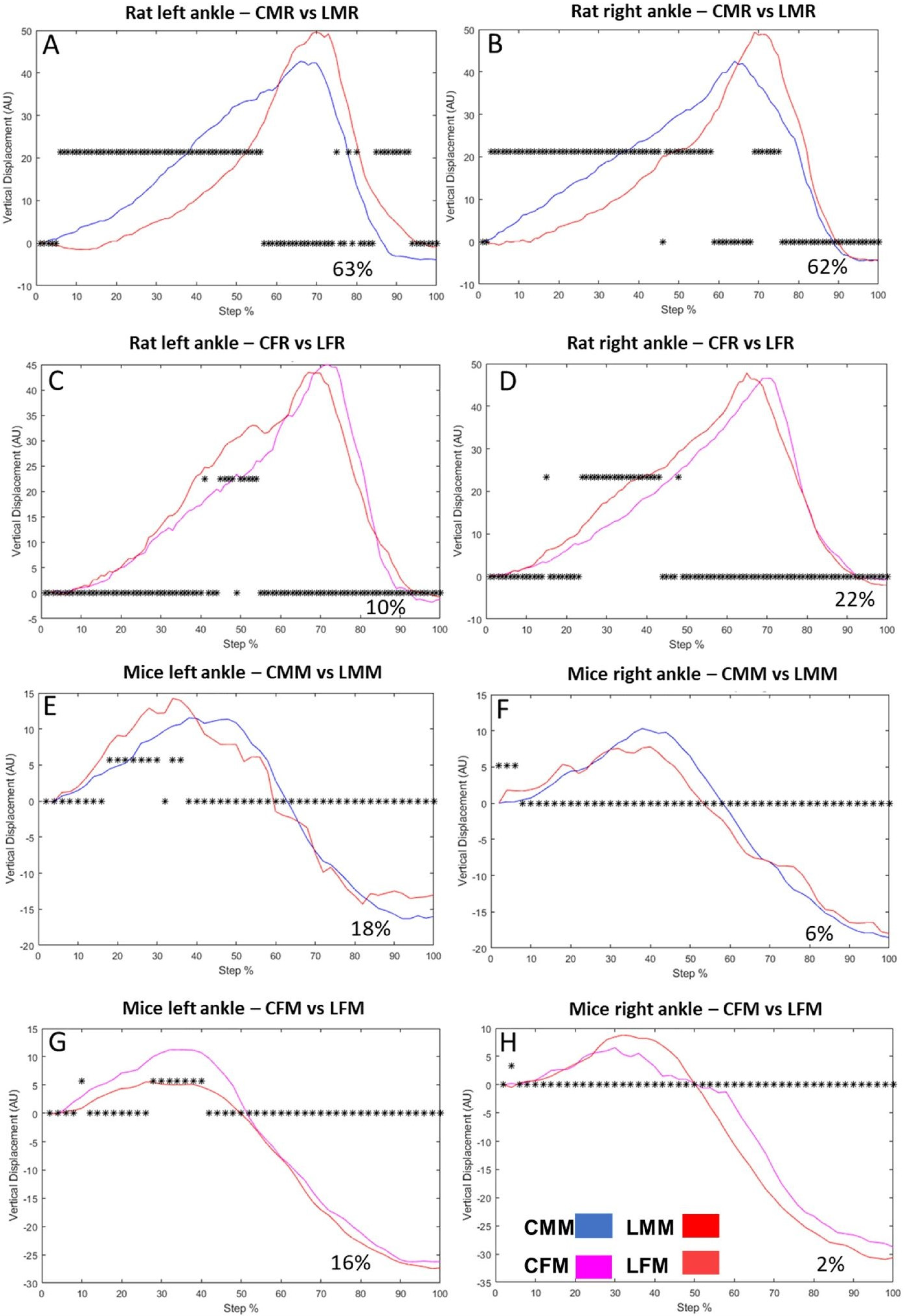
2.4. Dissimilarity Factor and Vertical Displacement Analysis
2.5. Statistical Analysis
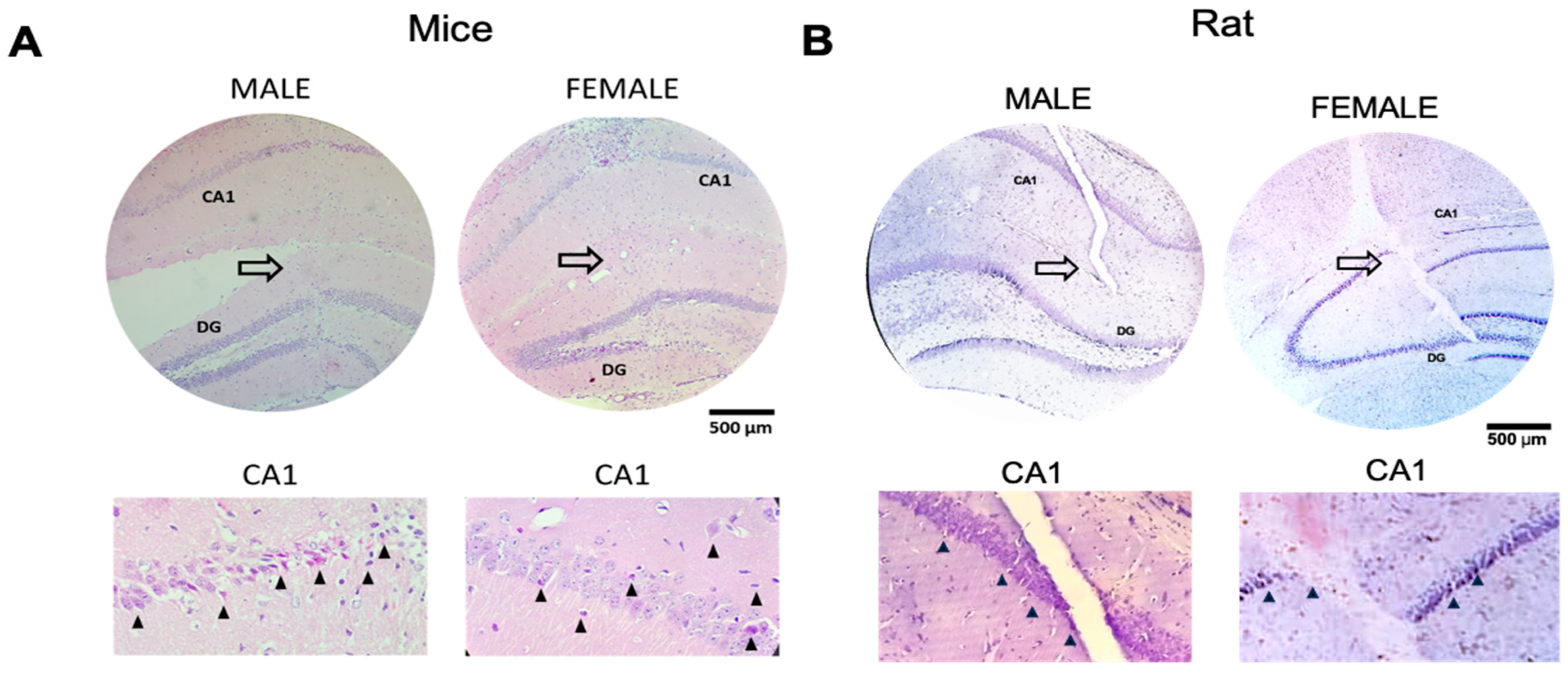
2.6. Histological Analysis
3. Results
3.1. Vertical Displacement in Hindlimbs Metatarsus, Ankle, and Knee in Rats and Mice
3.2. The Ankle VD in Intact Rats Differs from That in Intact Mice
3.3. The Knee VD Differs between Intact Rats versus Intact Mice
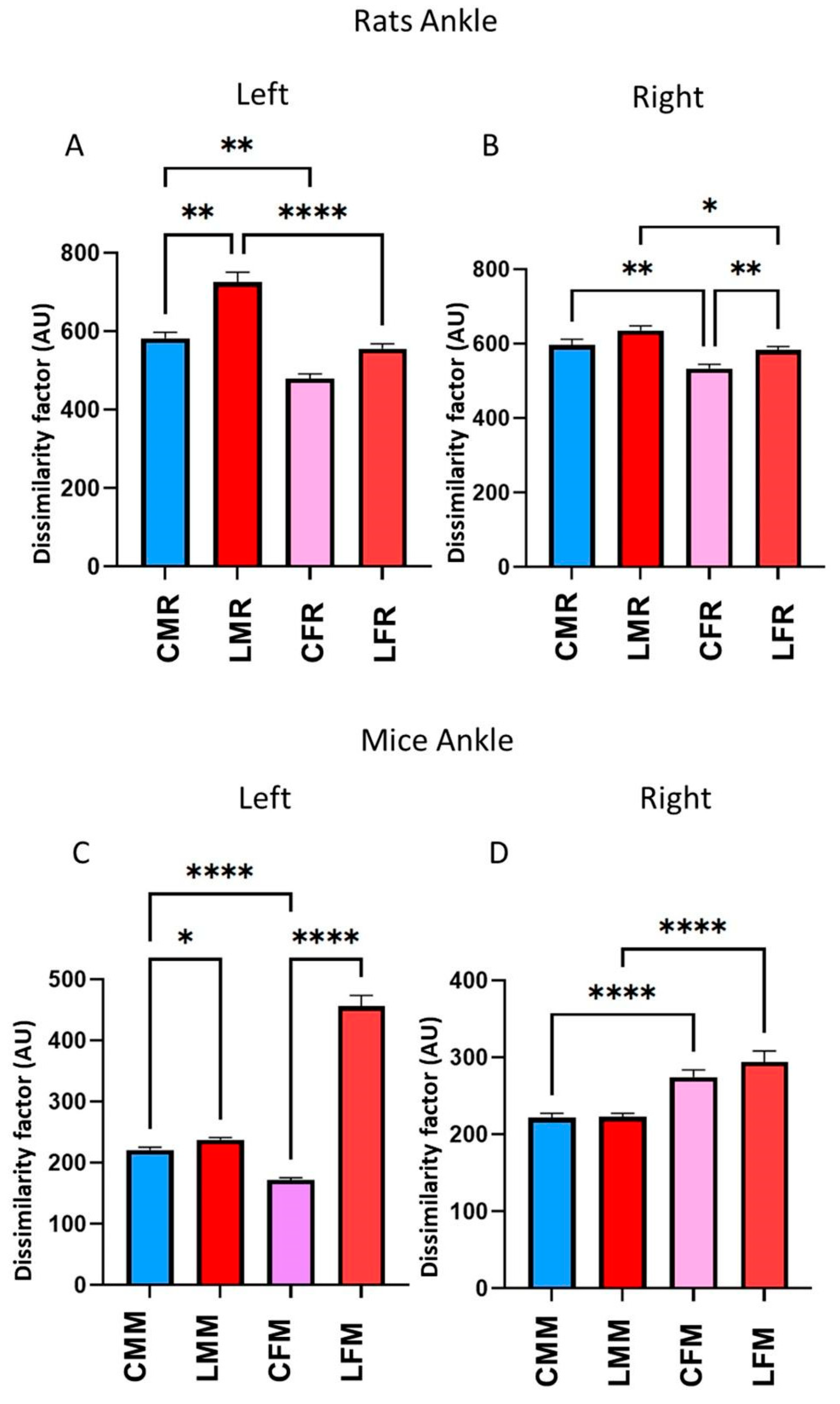
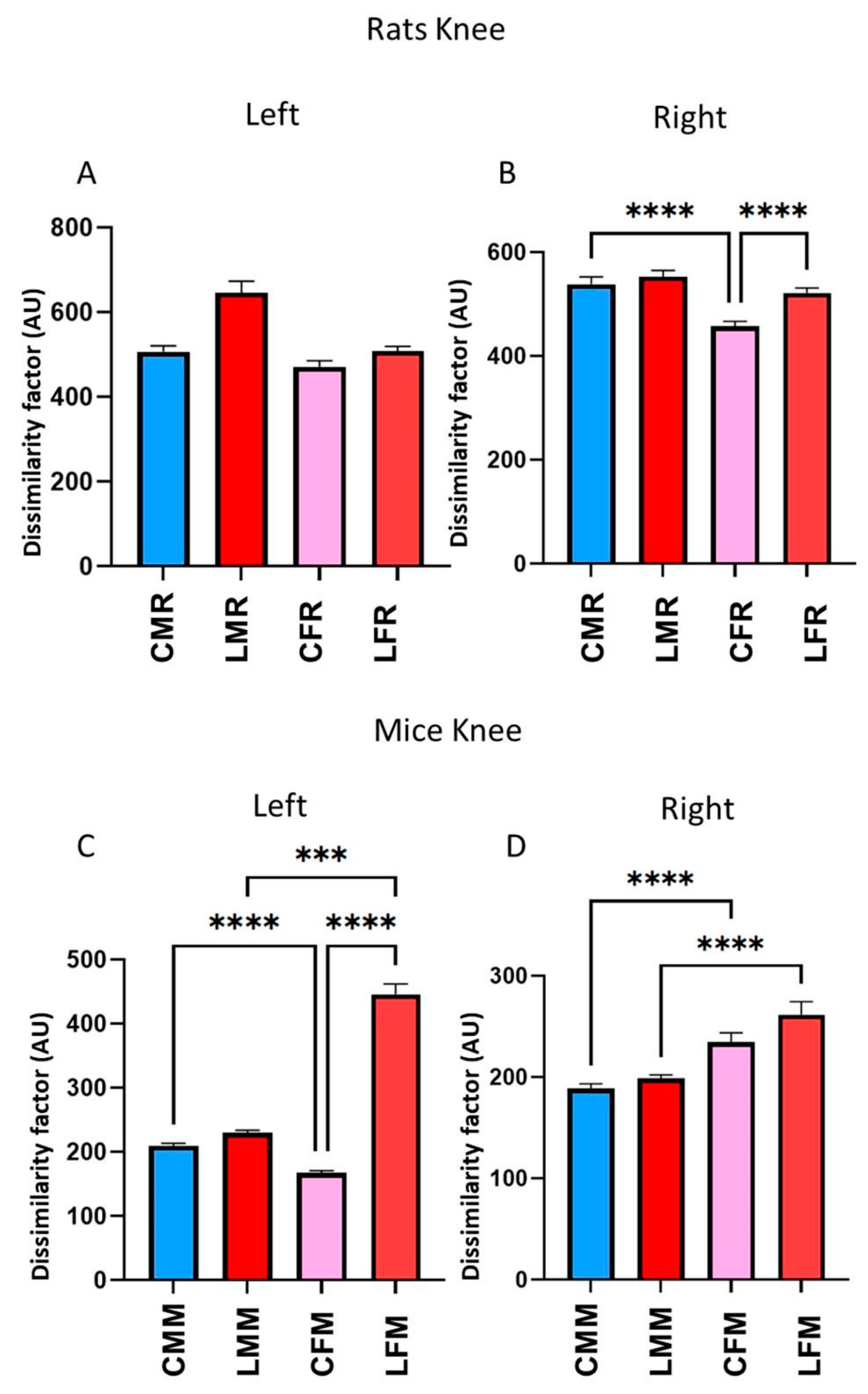
3.4. DF in Rats and Mice’s Metatarsus, Ankle, and Knee Joints
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, P.; Morris, R.; Amaral, D.; Bliss, T.; O’Keefe, J. The Hippocampus Book; Oxford University Press: New York, NY, USA, 2007; ISBN 9780199864133. [Google Scholar]
- Jeltsch, H.; Bertrand, F.; Lazarus, C.; Cassel, J.-C. Cognitive Performances and Locomotor Activity Following Dentate Granule Cell Damage in Rats: Role of Lesion Extent and Type of Memory Tested. Neurobiol. Learn. Mem. 2001, 76, 81–105. [Google Scholar] [CrossRef] [PubMed]
- Beauchet, O.; Launay, C.P.; Sekhon, H.; Montembeault, M.; Allali, G. Association of Hippocampal Volume with Gait Variability in Pre-Dementia and Dementia Stages of Alzheimer Disease: Results from a Cross-Sectional Study. Exp. Gerontol. 2019, 115, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Bender, F.; Gorbati, M.; Cadavieco, M.C.; Denisova, N.; Gao, X.; Holman, C.; Korotkova, T.; Ponomarenko, A. Theta Oscillations Regulate the Speed of Locomotion via a Hippocampus to Lateral Septum Pathway. Nat. Commun. 2015, 6, 8521. [Google Scholar] [CrossRef] [PubMed]
- López Ruiz, J.R.; Osuna Carrasco, L.P.; López Valenzuela, C.L.; Franco Rodríguez, N.E.; de la Torre Valdovinos, B.; Jiménez Estrada, I.; Dueñas Jiménez, J.M.; Dueñas Jiménez, S.H. The Hippocampus Participates in the Control of Locomotion Speed. Neuroscience 2015, 311, 207–215. [Google Scholar] [CrossRef]
- Kim, S.M.; Frank, L.M. Hippocampal Lesions Impair Rapid Learning of a Continuous Spatial Alternation Task. PLoS ONE 2009, 4, e5494. [Google Scholar] [CrossRef]
- Fuhrmann, F.; Justus, D.; Sosulina, L.; Kaneko, H.; Beutel, T.; Friedrichs, D.; Schoch, S.; Schwarz, M.K.; Fuhrmann, M.; Remy, S. Locomotion, Theta Oscillations, and the Speed-Correlated Firing of Hippocampal Neurons Are Controlled by a Medial Septal Glutamatergic Circuit. Neuron 2015, 86, 1253–1264. [Google Scholar] [CrossRef]
- Lu, L.; Ren, Y.; Yu, T.; Liu, Z.; Wang, S.; Tan, L.; Zeng, J.; Feng, Q.; Lin, R.; Liu, Y.; et al. Control of Locomotor Speed, Arousal, and Hippocampal Theta Rhythms by the Nucleus Incertus. Nat. Commun. 2020, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- León-Moreno, L.C.; Castañeda-Arellano, R.; Aguilar-García, I.G.; Desentis-Desentis, M.F.; Torres-Anguiano, E.; Gutiérrez-Almeida, C.E.; Najar-Acosta, L.J.; Mendizabal-Ruiz, G.; Ascencio-Piña, C.R.; Dueñas-Jiménez, J.M.; et al. Kinematic Changes in a Mouse Model of Penetrating Hippocampal Injury and Their Recovery After Intranasal Administration of Endometrial Mesenchymal Stem Cell-Derived Extracellular Vesicles. Front. Cell Neurosci. 2020, 14, 579162. [Google Scholar] [CrossRef]
- Ghosh, M.; Shanahan, B.E.; Furtak, S.C.; Mashour, G.A.; Burwell, R.D.; Ahmed, O.J. Instantaneous Amplitude and Shape of Postrhinal Theta Oscillations Differentially Encode Running Speed. Behav. Neurosci. 2020, 134, 516–528. [Google Scholar] [CrossRef]
- León-Moreno, L.C.; Castañeda-Arellano, R.; Rivas-Carrillo, J.D.; Dueñas-Jiménez, S.H. Challenges and Improvements of Developing an Ischemia Mouse Model Through Bilateral Common Carotid Artery Occlusion. J. Stroke Cerebrovasc. Dis. 2020, 29, 104773. [Google Scholar] [CrossRef]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal Replacement from Endogenous Precursors in the Adult Brain after Stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Bershteyn, M.; Bröer, S.; Parekh, M.; Maury, Y.; Havlicek, S.; Kriks, S.; Fuentealba, L.; Lee, S.; Zhou, R.; Subramanyam, G.; et al. Human Pallial MGE-Type GABAergic Interneuron Cell Therapy for Chronic Focal Epilepsy. Cell Stem Cell 2023, 30, 1331–1350.e11. [Google Scholar] [CrossRef] [PubMed]
- Ellenbroek, B.; Youn, J. Rodent Models in Neuroscience Research: Is It a Rat Race? Dis. Model. Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Field, E.F.; Pellis, S.M. The Brain as the Engine of Sex Differences in the Organization of Movement in Rats. Arch. Sex. Behav. 2008, 37, 30–42. [Google Scholar] [CrossRef]
- Field, E.F.; Whishaw, I.Q.; Pellis, S.M. A Kinematic Analysis of Evasive Dodging Movements Used during Food Protection in the Rat (Rattus Norvegicus): Evidence for Sex Differences in Movement. J. Comp. Psychol. 1996, 110, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Rekart, J.L.; Sandoval, C.J.; Routtenberg, A. Learning-Induced Axonal Remodeling: Evolutionary Divergence and Conservation of Two Components of the Mossy Fiber System within Rodentia. Neurobiol. Learn. Mem. 2007, 87, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Moran, A.L.; Warren, G.L.; Lowe, D.A. Soleus and EDL Muscle Contractility across the Lifespan of Female C57BL/6 Mice. Exp. Gerontol. 2005, 40, 966–975. [Google Scholar] [CrossRef]
- Bojados, M.; Herbin, M.; Jamon, M. Kinematics of Treadmill Locomotion in Mice Raised in Hypergravity. Behav. Brain Res. 2013, 244, 48–57. [Google Scholar] [CrossRef]
- Drzymała-Celichowska, H.; Raikova, R.; Krutki, P. Decomposition of Motor Unit Tetanic Contractions of Rat Soleus Muscle: Differences between Males and Females. J. Biomech. 2015, 48, 3097–3102. [Google Scholar] [CrossRef]
- Gaulden, A.D.; Burson, N.; Sadik, N.; Ghosh, I.; Khan, S.J.; Brummelte, S.; Kallakuri, S.; Perrine, S.A. Effects of Fentanyl on Acute Locomotor Activity, Behavioral Sensitization, and Contextual Reward in Female and Male Rats. Drug Alcohol. Depend. 2021, 229, 109101. [Google Scholar] [CrossRef]
- Melnikova, T.; Park, D.; Becker, L.; Lee, D.; Cho, E.; Sayyida, N.; Tian, J.; Bandeen-Roche, K.; Borchelt, D.R.; Savonenko, A. V Sex-Related Dimorphism in Dentate Gyrus Atrophy and Behavioral Phenotypes in an Inducible TTa:APPsi Transgenic Model of Alzheimer’s Disease. Neurobiol. Dis. 2016, 96, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Lotan, A.; Lifschytz, T.; Wolf, G.; Keller, S.; Ben-Ari, H.; Tatarsky, P.; Pillar, N.; Oved, K.; Sharabany, J.; Merzel, T.K.; et al. Differential Effects of Chronic Stress in Young-Adult and Old Female Mice: Cognitive-Behavioral Manifestations and Neurobiological Correlates. Mol. Psychiatry 2018, 23, 1432–1445. [Google Scholar] [CrossRef] [PubMed]
- Pairojana, T.; Phasuk, S.; Suresh, P.; Huang, S.P.; Pakaprot, N.; Chompoopong, S.; Hsieh, T.C.; Liu, I.Y. Age and Gender Differences for the Behavioral Phenotypes of 3xTg Alzheimer’s Disease Mice. Brain Res. 2021, 1762, 147437. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, M.E.; Monteleone, M.C.; Pastor, V.; Grillo Balboa, J.; Alzamendi, A.; Brocco, M.A.; Antonelli, M.C. Early-Life Stress Reprograms Stress-Coping Abilities in Male and Female Juvenile Rats. Mol. Neurobiol. 2021, 58, 5837–5856. [Google Scholar] [CrossRef]
- Riljak, V.; Laštůvka, Z.; Mysliveček, J.; Borbélyová, V.; Otáhal, J. Early Postnatal Hypoxia Induces Behavioral Deficits but Not Morphological Damage in the Hippocampus in Adolescent Rats. Physiol. Res. 2020, 69, 165–179. [Google Scholar] [CrossRef]
- Zhao, L.; Mulligan, M.K.; Nowak, T.S. Substrain- and Sex-Dependent Differences in Stroke Vulnerability in C57BL/6 Mice. J. Cereb. Blood Flow Metab. 2019, 39, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.R.; Hunter, N.; Lin, S.; Robinson, E.M.; Gillis, W.; Conlin, E.B.; Anyoha, R.; Shansky, R.M.; Datta, S.R. Mouse Spontaneous Behavior Reflects Individual Variation Rather than Estrous State. Curr. Biol. 2023, 33, 1358–1364.e4. [Google Scholar] [CrossRef]
- Mickley, G.A.; Ferguson, J.L.; Nemeth, T.J.; Mulvihill, M.A.; Alderks, C.E. Spontaneous Perseverative Turning in Rats with Radiation-Induced Hippocampal Damage. Behav. Neurosci. 1989, 103, 722–911. [Google Scholar] [CrossRef]
- Sheppard, K.; Gardin, J.; Sabnis, G.S.; Peer, A.; Darrell, M.; Deats, S.; Geuther, B.; Lutz, C.M.; Kumar, V. Stride-Level Analysis of Mouse Open Field Behavior Using Deep-Learning-Based Pose Estimation. Cell Rep. 2022, 38, 110231. [Google Scholar] [CrossRef]
- Grillner, S.; Kozlov, A. The CPGs for Limbed Locomotion–Facts and Fiction. Int. J. Mol. Sci. 2021, 22, 5882. [Google Scholar] [CrossRef]
- Grillner, S.; El Manira, A. Current Principles of Motor Control, with Special Reference to Vertebrate Locomotion. Physiol. Rev. 2020, 100, 271–320. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-García, I.G.; Jiménez-Estrada, I.; Castañeda-Arellano, R.; Alpirez, J.; Mendizabal-Ruiz, G.; Dueñas-Jiménez, J.M.; Gutiérrez-Almeida, C.E.; Osuna-Carrasco, L.P.; Ramírez-Abundis, V.; Dueñas-Jiménez, S.H. Locomotion Outcome Improvement in Mice with Glioblastoma Multiforme after Treatment with Anastrozole. Brain Sci. 2023, 13, 496. [Google Scholar] [CrossRef] [PubMed]
- Green, E.J.; Barnes, C.A.; McNaughton, B.L. Behavioral State Dependence of Homo- and Hetero-Synaptic Modulation of Dentate Gyrus Excitability. Exp. Brain Res. 1993, 93, 55–65. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alpirez, J.; Leon-Moreno, L.C.; Aguilar-García, I.G.; Castañeda-Arellano, R.; Dueñas-Jiménez, J.M.; Asencio-Piña, C.R.; Dueñas-Jiménez, S.H. Walk Locomotion Kinematic Changes in a Model of Penetrating Hippocampal Injury in Male/Female Mice and Rats. Brain Sci. 2023, 13, 1545. https://doi.org/10.3390/brainsci13111545
Alpirez J, Leon-Moreno LC, Aguilar-García IG, Castañeda-Arellano R, Dueñas-Jiménez JM, Asencio-Piña CR, Dueñas-Jiménez SH. Walk Locomotion Kinematic Changes in a Model of Penetrating Hippocampal Injury in Male/Female Mice and Rats. Brain Sciences. 2023; 13(11):1545. https://doi.org/10.3390/brainsci13111545
Chicago/Turabian StyleAlpirez, Jonatan, Lilia Carolina Leon-Moreno, Irene Guadalupe Aguilar-García, Rolando Castañeda-Arellano, Judith Marcela Dueñas-Jiménez, Cesar Rodolfo Asencio-Piña, and Sergio Horacio Dueñas-Jiménez. 2023. "Walk Locomotion Kinematic Changes in a Model of Penetrating Hippocampal Injury in Male/Female Mice and Rats" Brain Sciences 13, no. 11: 1545. https://doi.org/10.3390/brainsci13111545
APA StyleAlpirez, J., Leon-Moreno, L. C., Aguilar-García, I. G., Castañeda-Arellano, R., Dueñas-Jiménez, J. M., Asencio-Piña, C. R., & Dueñas-Jiménez, S. H. (2023). Walk Locomotion Kinematic Changes in a Model of Penetrating Hippocampal Injury in Male/Female Mice and Rats. Brain Sciences, 13(11), 1545. https://doi.org/10.3390/brainsci13111545






