Poor Oral Health Linked with Higher Risk of Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. TriNetX Database
2.2. Cohorts
2.3. Propensity Score Matching
2.4. Statistical Analyses
3. Results
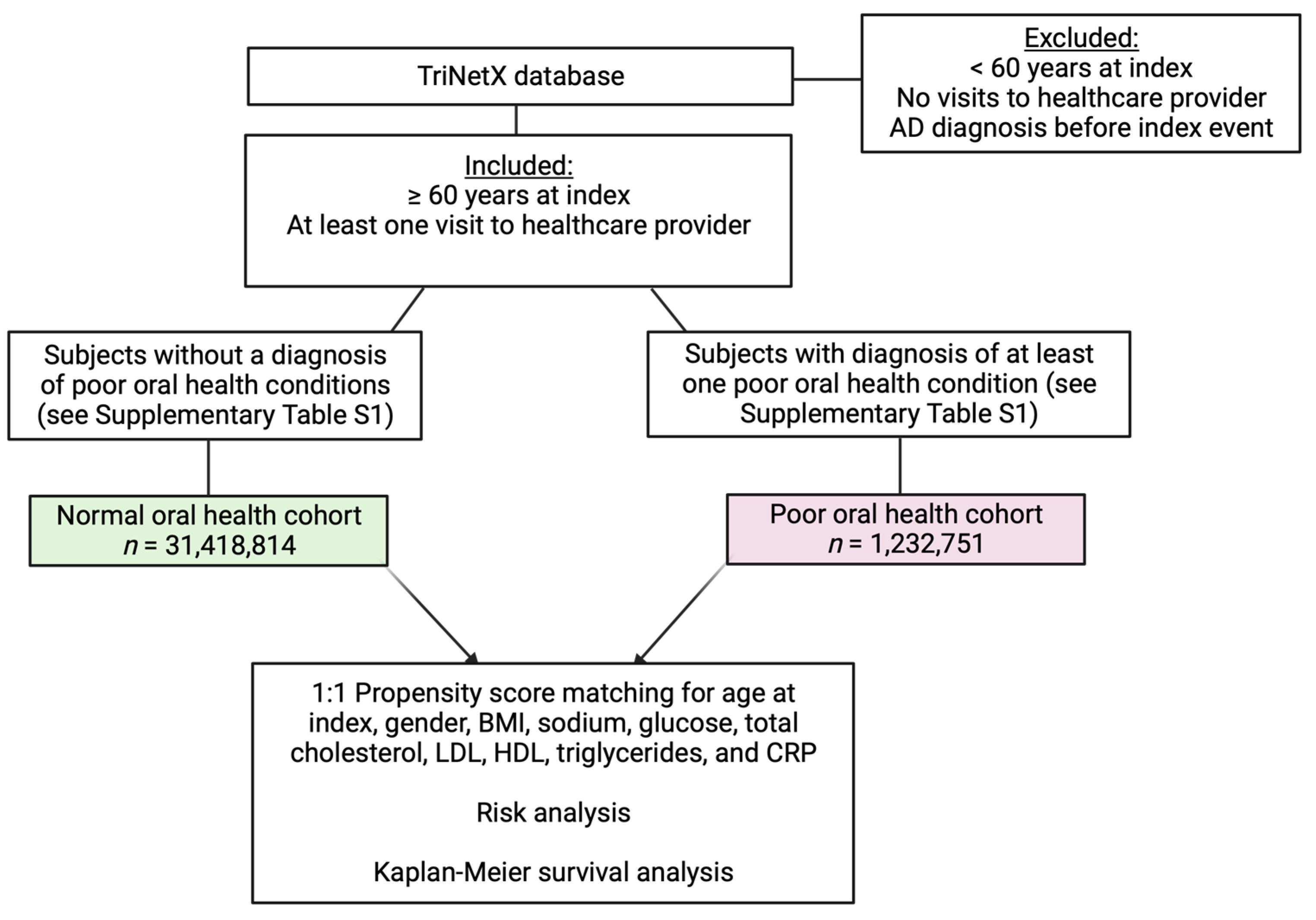
3.1. Generation of Normal Oral Health and Poor Oral Health Cohorts
3.2. Poor Oral Health Is Associated with an Increased Risk of AD and Decreased Survival Independent of Age, Gender, and Common Laboratory Measures
3.3. Different Oral Diseases Are Associated with Varying Risk of AD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W.; Doerr, C. Alzheimer Disease (Nursing); StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Breijyeh, Z.; Karaman, R. Comprehensive review on Alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Hamza, S.A.; Asif, S.; Bokhari, S.A.H. Oral health of individuals with dementia and Alzheimer’s disease: A review. J. Indian Soc. Periodontol. 2021, 25, 96–101. [Google Scholar] [PubMed]
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 83, 661–669. [Google Scholar] [PubMed]
- Gao, S.S.; Chen, K.J.; Duangthip, D.; Lo, E.C.M.; Chu, C.H. The oral health status of Chinese elderly people with and without dementia: A cross-sectional study. Int. J. Environ. Res. Public Health 2020, 17, 1913. [Google Scholar] [CrossRef] [PubMed]
- Linda, S.K.; Tri, B.R.; Dinni, A.; Chaidar, M.; Sri, L.; Eef, H. Oral hygiene status and cognitive function in Indonesian elderly. Int. J. Clin. Prev. Dent. 2015, 11, 261–264. [Google Scholar]
- Saito, S.; Ohi, T.; Murakami, T.; Komiyama, T.; Miyoshi, Y.; Endo, K.; Satoh, M.; Asayama, K.; Inoue, R.; Kikuya, M. Association between tooth loss and cognitive impairment in community-dwelling older Japanese adults: A 4-year prospective cohort study from the Ohasama study. BMC Oral Health 2018, 18, 142. [Google Scholar] [CrossRef]
- Ranjan, R.; Rout, M.; Mishra, M.; Kore, S.A. Tooth loss and dementia: An oro-neural connection. A cross-sectional study. J. Indian Soc. Periodontol. 2019, 23, 158–162. [Google Scholar] [CrossRef]
- Martande, S.S.; Pradeep, A.; Singh, S.P.; Kumari, M.; Suke, D.K.; Raju, A.P.; Naik, S.B.; Singh, P.; Guruprasad, C.; Chatterji, A. Periodontal health condition in patients with Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 2014, 29, 498–502. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Costi, T.; Alkhamis, N.; Bagattoni, S.; Sadotti, A.; Piana, G. Oral Health Status in Alzheimer’s Disease Patients: A Descriptive Study in an Italian Population. J. Contemp. Dent. Pract. 2018, 19, 483–489. [Google Scholar] [CrossRef]
- Holmer, J.; Eriksdotter, M.; Schultzberg, M.; Pussinen, P.J.; Buhlin, K. Association between periodontitis and risk of Alzheimer’s disease, mild cognitive impairment and subjective cognitive decline: A case–control study. J. Clin. Periodontol. 2018, 45, 1287–1298. [Google Scholar] [CrossRef]
- Aragón, F.; Zea-Sevilla, M.; Montero, J.; Sancho, P.; Corral, R.; Tejedor, C.; Frades-Payo, B.; Paredes-Gallardo, V.; Albaladejo, A. Oral health in Alzheimer’s disease: A multicenter case-control study. Clin. Oral Investig. 2018, 22, 3061–3070. [Google Scholar] [CrossRef]
- Ide, M.; Harris, M.; Stevens, A.; Sussams, R.; Hopkins, V.; Culliford, D.; Fuller, J.; Ibbett, P.; Raybould, R.; Thomas, R. Periodontitis and cognitive decline in Alzheimer’s disease. PLoS ONE 2016, 11, e0151081. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Choi, Y.Y. Association between oral health and dementia in the elderly: A population-based study in Korea. Sci. Rep. 2019, 9, 14407. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-K.; Wu, Y.-T.; Chang, Y.-C. Association between chronic periodontitis and the risk of Alzheimer’s disease: A retrospective, population-based, matched-cohort study. Alzheimers Res. Ther. 2017, 9, 56. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, F.; Wang, Z.; Qian, X.; Ji, Y.; Gong, L.; Ge, S.; Yan, F. Poor oral health conditions and cognitive decline: Studies in humans and rats. PLoS ONE 2020, 15, e0234659. [Google Scholar] [CrossRef]
- Riviere, G.R.; Riviere, K.H.; Smith, K.S. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol. Immunol. 2002, 17, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kamer, A.R.; Craig, R.G.; Pirraglia, E.; Dasanayake, A.P.; Norman, R.G.; Boylan, R.J.; Nehorayoff, A.; Glodzik, L.; Brys, M.; de Leon, M.J. TNF-α and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J. Neuroimmunol. 2009, 216, 92–97. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Weiss, J.; Hossain, S.; El-Hajj, Z.W.; Zonderman, A.B. Helicobacter pylori, periodontal pathogens, and their interactive association with incident all-cause and Alzheimer’s disease dementia in a large national survey. Mol. Psychiatry 2021, 26, 6038–6053. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.; Eribe, E.R.K.; Singhrao, S.K.; Olsen, I. High throughput sequencing detects gingivitis and periodontal oral bacteria in Alzheimer’s disease autopsy brains. J. Neurosci. Res. 2019, 1, 3. [Google Scholar] [CrossRef]
- Miklossy, J. Historic evidence to support a causal relationship between spirochetal infections and Alzheimer’s disease. Front. Aging Neurosci. 2015, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Ishida, N.; Ishihara, Y.; Ishida, K.; Tada, H.; Funaki-Kato, Y.; Hagiwara, M.; Ferdous, T.; Abdullah, M.; Mitani, A.; Michikawa, M. Periodontitis induced by bacterial infection exacerbates features of Alzheimer’s disease in transgenic mice. NPJ Aging Mech. Dis. 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- ISO/IEC 27001:2013; Information Technology—Security Techniques—Information Security Management Systems—Requirements. ISO: Geneva, Switzerland, 2013.
- Silva, M.V.F.; Loures, C.D.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M.d.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef]
- Andersen, K.; Launer, L.J.; Dewey, M.E.; Letenneur, L.; Ott, A.; Copeland, J.; Dartigues, J.-F.; Kragh–Sorensen, P.; Baldereschi, M.; Brayne, C. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. Neurology 1999, 53, 1992–1997. [Google Scholar] [CrossRef]
- Lobo, A.; Launer, L.J.; Fratiglioni, L.; Andersen, K.; Di Carlo, A.; Breteler, M.; Copeland, J.; Dartigues, J.; Jagger, C.; Martinez-Lage, J. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurology 2000, 54, S4–S9. [Google Scholar]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Kuller, L.H.; Lopez, O.L.; Diehr, P.; O’Meara, E.S.; Longstreth, W.; Luchsinger, J.A. Midlife and late-life obesity and the risk of dementia: Cardiovascular health study. Arch. Neurol. 2009, 66, 336–342. [Google Scholar] [CrossRef]
- Anstey, K.; Cherbuin, N.; Budge, M.; Young, J. Body mass index in midlife and late-life as a risk factor for dementia: A meta-analysis of prospective studies. Obes. Rev. 2011, 12, e426–e437. [Google Scholar] [CrossRef] [PubMed]
- Qizilbash, N.; Gregson, J.; Johnson, M.E.; Pearce, N.; Douglas, I.; Wing, K.; Evans, S.J.; Pocock, S.J. BMI and risk of dementia in two million people over two decades: A retrospective cohort study. Lancet Diabetes Endocrinol. 2015, 3, 431–436. [Google Scholar] [CrossRef]
- Popp, J.; Meichsner, S.; Kölsch, H.; Lewczuk, P.; Maier, W.; Kornhuber, J.; Jessen, F.; Lütjohann, D. Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer’s disease. Biochem. Pharmacol. 2013, 86, 37–42. [Google Scholar] [PubMed]
- Ullrich, C.; Pirchl, M.; Humpel, C. Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Mol. Cell. Neurosci. 2010, 45, 408–417. [Google Scholar] [PubMed]
- Huang, J.; Tao, Q.; Ang, T.F.A.; Farrell, J.; Zhu, C.; Wang, Y.; Stein, T.D.; Lunetta, K.L.; Massaro, J.; Mez, J. The impact of increasing levels of blood C-reactive protein on the inflammatory loci SPI1 and CD33 in Alzheimer’s disease. Transl. Psychiatry 2022, 12, 523. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [PubMed]
- Skoog, I.; Lernfelt, B.; Landahl, S.; Palmertz, B.; Andreasson, L.A.; Nilsson, L.; Persson, G.; Oden, A.; Svanborg, A. 15-year longitudinal study of blood pressure and dementia. Lancet 1996, 347, 1141–1145. [Google Scholar]
- Kimura, N. Diabetes mellitus induces Alzheimer’s disease pathology: Histopathological evidence from animal models. Int. J. Mol. Sci. 2016, 17, 503. [Google Scholar] [CrossRef]
- Laouali, N.; Fatouhi, D.E.; Aguayo, G.; Balkau, B.; Boutron-Ruault, M.-C.; Bonnet, F.; Fagherazzi, G. Type 2 diabetes and its characteristics are associated with poor oral health: Findings from 60,590 senior women from the E3N study. BMC Oral Health 2021, 21, 315. [Google Scholar]
- Han, S.-J.; Son, Y.-J.; Kim, B.-H. Association between diabetes mellitus and oral health status in patients with cardiovascular diseases: A nationwide population-based study. Int. J. Environ. Res. Public Health 2021, 18, 4889. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Yang, D.; Yang, S.; Zhao, Y.; Jiang, M.; Wang, X.; Zhao, L.; Liu, Q.; Lu, Z.; et al. Tooth loss and the risk of cognitive decline and dementia: A meta-analysis of cohort studies. Front. Neurol. 2023, 14, 1103052. [Google Scholar]
- Pietropaoli, D.; Pinto, R.D.; Ferri, C.; Wright, J.T., Jr.; Giannoni, M.; Ortu, E.; Monaco, A. Poor oral health and blood pressure control among US hypertensive adults: Results from the national health and nutrition examination survey 2009 to 2014. Hypertension 2018, 72, 1365–1373. [Google Scholar]
- Athanasaki, A.; Melanis, K.; Tsantzali, I.; Stefanou, M.I.; Ntymenou, S.; Paraskevas, S.G.; Kalamatianos, T.; Boutati, E.; Lambadiari, V.; Voumvourakis, K.I.; et al. Type 2 diabetes mellitus as a risk factor for Alzheimer’s disease: Review and meta-analysis. Biomedicines 2022, 10, 778. [Google Scholar]
- Leszek, J.; Mikhaylenko, E.V.; Belousov, D.M.; Koutsouraki, E.; Szczechowiak, K.; Kobusiak-Prokopowicz, M.; Mysiak, A.; Diniz, B.S.; Somasundaram, S.G.; Kirkland, C.E.; et al. The links between cardiovascular diseases and Alzheimer’s disease. Curr. Neuropharmacol. 2021, 19, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, D.R.; Divaris, K.; Mazul, A.L.; Weissler, M.C.; Zevallos, J.P.; Olshan, A.F. Poor oral health affects survival in head and neck cancer. Oral Oncol. 2017, 73, 111–117. [Google Scholar] [PubMed]
- Kotronia, E.; Brown, H.; Papacosta, A.O.; Lennon, L.T.; Weyant, R.J.; Whincup, P.H.; Wannamethee, S.G.; Ramsay, S.E. Oral health and all-cause, cardiovascular disease, and respiratory mortality in older people in the UK and USA. Sci. Rep. 2021, 11, 16452. [Google Scholar] [PubMed]
- Demmer, R.T.; Norby, F.L.; Lakshminarayan, K.; Walker, K.A.; Pankow, J.S.; Folsom, A.R.; Mosley, T.; Beck, J.; Lutsey, P.L. Periodontal disease and incident dementia: The Atherosclerosis Risk in Communities Study (ARIC). Neurology 2020, 95, e1660–e1671. [Google Scholar]
- Xu, W.; Tan, L.; Wang, H.-F.; Tan, M.-S.; Tan, L.; Li, J.-Q.; Zhao, Q.-F.; Yu, J.-T. Education and risk of dementia: Dose-response meta-analysis of prospective cohort studies. Mol. Neurobiol. 2016, 53, 3113–3123. [Google Scholar]
- Batista, M.J.; Lawrence, H.P.; Sousa, M.D.L.R.D. Oral health literacy and oral health outcomes in an adult population in Brazil. BMC Public Health 2018, 18, 60. [Google Scholar]
- Cepova, E.; Cicvakova, M.; Kolarcik, P.; Markovska, N.; Geckova, A.M. Associations of multidimensional health literacy with reported oral health promoting behaviour among Slovak adults: A cross-sectional study. BMC Oral Health 2018, 18, 44. [Google Scholar]
- Márquez-Arrico, C.-F.; Almerich-Silla, J.-M.; Montiel-Company, J.-M. Oral health knowledge in relation to educational level in an adult population in Spain. J. Clin. Exp. Dent. 2019, 11, e1143–e1150. [Google Scholar] [CrossRef] [PubMed]
- Weatherspoon, D.; Chattopadhyay, A. International classification of diseases codes and their use in dentistry. J. Dent. Oral Craniofac. Epidemiol. 2013, 1, 20–26. [Google Scholar] [PubMed]
- Fang, W.-L.; Jiang, M.-J.; Gu, B.-B.; Wei, Y.-M.; Fan, S.-N.; Liao, W.; Zheng, Y.-Q.; Liao, S.-W.; Xiong, Y.; Xiao, S.-H.; et al. Tooth loss as a risk factor for dementia: Systematic review and meta-analysis of 21 observational studies. BMC Psychiatry 2018, 18, 345. [Google Scholar] [CrossRef]
- Asher, S.; Stephen, R.; Mantyla, P.; Suominen, A.L.; Solomon, A. Periodontal health, cognitive decline, and dementia: A systematic review and meta-analysis of longitudinal studies. J. Am. Geriatr. Soc. 2022, 70, 2695–2709. [Google Scholar] [CrossRef]
- Tsuneishi, M.; Yamamoto, T.; Yamaguchi, T.; Kodama, T.; Sato, T. Association between number of teeth and Alzheimer’s disease using the National Database of Health Insurance Claims and Specific Health Checkups of Japan. PLoS ONE 2021, 16, e0251056. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, M.; Gioia, G.D.; Caloro, G.A.; Capocasal, G.; Zhurakivska, K.; Troiano, G.; Russo, L.L.; Muzio, L.L. The association between tooth loss and Alzheimer’s disease: A systematic review with meta-analysis of case control studies. Dent. J. 2019, 7, 49. [Google Scholar]
- Yoo, J.-J.; Yoon, J.-H.; Kang, M.-J.; Oh, N. The effect of missing teeth on dementia in older people: A nationwide population-based cohort study in South Korea. BMC Oral Health 2019, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Nadim, R.; Tang, J.; Dilmohamed, A.; Yuan, S.; Wu, C.; Bakre, A.T.; Partridge, M.; Ni, J.; Copeland, J.R.; Antsey, K.J.; et al. Influence of periodontal disease on risk of dementia: A systematic literature review and a meta-analysis. Eur. J. Epidemiol. 2020, 35, 821–833. [Google Scholar] [PubMed]
- Delaby, C.; Hirtz, C.; Lehmann, S. Overview of the blood biomarkers in Alzheimer’s disease: Promises and challenges. Rev. Neurol. 2023, 179, 161–172. [Google Scholar]
- Sadrameli, M.; Bathini, P.; Alberi, L. Linking mechanisms of periodontitis to Alzheimer’s disease. Curr. Opin. Neurol. 2020, 33, 230–238. [Google Scholar] [CrossRef]
- Cestari, J.A.F.; Fabri, G.M.C.; Kalil, J.; Nitrini, R.; Jacob-Filho, W.; de Siqueira, J.T.T.; Siquiera, S.R.D.T. Oral infections and cytokine levels in patients with Alzheimer’s disease and mild cognitive impairment compared with controls. J. Alzheimers Dis. 2016, 52, 1479–1485. [Google Scholar] [CrossRef]
- Liu, S.; Dashper, S.G.; Zhao, R. Association between oral bacteria and Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2023, 91, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, B.; Yao, H.; Liu, D.; Chen, R.; Zhou, S.; Ji, Y.; Zeng, L.; Du, M. Profiling the oral microbiomes in patients with Alzheimer’s disease. Oral Dis. 2023, 29, 1341–1355. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, D.S.; Jain, S.; Yata, V.K.; Mishra, S.P.; Kumar, A.; Fraser, A.; Kociolek, J.; Dangiolo, M.; Smith, A.; Golden, A.; et al. Unique trans-kingdom microbiome structural and functional signatures predict cognitive decline in older adults. Geroscience 2023. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef] [PubMed]


| Demographic or Laboratory Measure | Cohort 1 (Normal Oral Health) | Cohort 2 (Poor Oral Health) |
|---|---|---|
| Number of patients in cohort | 31,418,814 | 1,232,751 |
| Female (%) | 16,675,337 (53.1%) | 671,281 (54.5%) |
| Male (%) | 14,420,798 (45.9%) | 544,851 (44.2%) |
| Unknown gender or other (%) | 322,679 (1.0%) | 16,619 (1.3%) |
| Age (years) | 73.9 ± 9.5 | 72.4 ± 8.9 |
| Race (%) | ||
| White | 18,438,027 (58.7%) | 792,863 (64.2%) |
| Black or African American | 2,817,690 (9.0%) | 176,132 (14.1%) |
| Asian | 815,954 (2.6%) | 38,015 (3.7%) |
| American Indian or Alaska Native | 82,909 (0.3%) | 3736 (0.3%) |
| Native Hawaiian/Other Pacific Islander | 79,977 (0.3%) | 1162 (0.3%) |
| Unknown race or unreported | 9,142,875 (29.1%) | 214,499 (17.4%) |
| BMI (kg/m2) | 29.1 ± 6.7 | 28.6 ± 6.5 |
| Blood sodium (mol/vol) | 139.0 ± 3.3 | 139.0 ± 3.5 |
| Blood glucose (mass/vol) | 118.0 ± 50.3 | 123.0 ± 61.2 |
| Triglycerides (mass/vol) | 136.0 ± 95.4 | 142.0 ± 258 |
| Total cholesterol (mass/vol) | 177.0 ± 45.1 | 185.0 ± 46.7 |
| LDL cholesterol (mass/vol) | 99.6 ± 37.2 | 106.0 ± 39.1 |
| HDL cholesterol (mass/vol) | 52.5 ± 18.3 | 52.2 ± 17.6 |
| CRP (mass/vol) | 24.0 ± 47.7 | 30.6 ± 61.0 |
| Variable(s) Matched | n | Patients with AD | Risk | Risk Difference (p-Value) | Risk Ratio (95% CI) | Kaplan–Meier Survivability (%) | p-Value (Log-Rank Test) | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| None | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 31,418,814 | 179,136 | 0.006 | 0.008 | 2.363 | 98.89% | 1.868 | ||
| Cohort 2 | 1,232,751 | 16,609 | 0.013 | (p < 0.001 *) | (2.326, 2.401) | 98.00% | (1.838, 1.898) | ||
| Age only | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 1,233,771 | 6864 | 0.006 | 0.008 | 2.489 | 98.92% | 1.975 | ||
| Cohort 2 | 1,233,771 | 17,083 | 0.014 | (p < 0.001 *) | (2.420, 2.559) | 97.95% | (1.921, 2.032) | ||
| Gender only | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 1,221,975 | 7235 | 0.006 | 0.008 | 2.318 | 98.85% | 1.834 | ||
| Cohort 2 | 1,221,975 | 16,774 | 0.014 | (p < 0.001 *) | (2.256, 2.383) | 97.96% | (1.784, 1.885) | ||
| Age and gender | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 1,231,244 | 6784 | 0.006 | 0.008 | 2.508 | 98.94% | 1.995 | ||
| Cohort 2 | 1,231,244 | 17,017 | 0.014 | (p < 0.001 *) | (2.439, 2.580) | 97.96% | (1.939, 2.052) |
| Measure Matched with Age | n | Patients with AD | Risk | Risk Difference (p-Value) | Risk Ratio (95% CI) | Kaplan–Meier Survivability (%) | p-Value (Log-Rank Test) | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 919,405 | 5161 | 0.006 | 0.007 | 2.289 | 98.93% | 1.860 | ||
| Cohort 2 | 919,405 | 11,813 | 0.013 | (p < 0.001 *) | (2.216, 2.365) | 98.06% | (1.800, 1922) | ||
| Sodium (mmol/L) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 951,651 | 4551 | 0.005 | 0.007 | 2.564 | 99.15% | 2.241 | ||
| Cohort 2 | 951,651 | 11,670 | 0.012 | (p < 0.001 *) | (2.478, 2.653) | 98.16% | (2.166, 2.319) | ||
| Glucose (mg/dL) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 951,822 | 4632 | 0.005 | 0.008 | 2.556 | 99.14% | 2.241 | ||
| Cohort 2 | 951,822 | 11,838 | 0.012 | (p < 0.001 *) | (2.471, 2.644) | 98.13% | (2.166, 2.318) | ||
| Triglycerides (mg/dL) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 979,884 | 5085 | 0.005 | 0.007 | 2.422 | 99.07% | 2.118 | ||
| Cohort 2 | 979,884 | 12,315 | 0.013 | (p < 0.001 *) | (2.344, 2.502) | 98.10% | (2.050, 2.189) | ||
| Cholesterol (mg/dL) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 974,220 | 5078 | 0.005 | 0.007 | 2.413 | 99.06% | 2.121 | ||
| Cohort 2 | 974,220 | 12,254 | 0.013 | (p < 0.001 *) | (2.336, 2.493) | 98.09% | (2.053, 2.192) | ||
| LDL (mg/dL) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 973,827 | 5089 | 0.005 | 0.007 | 2.390 | 99.06% | 2.100 | ||
| Cohort 2 | 973,827 | 12,165 | 0.012 | (p < 0.001 *) | (2.314, 2.470) | 98.10% | (2.032, 2.170) | ||
| HDL (mg/dL) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 952,564 | 4941 | 0.005 | 0.007 | 2.387 | 99.07% | 2.105 | ||
| Cohort 2 | 952,564 | 11,794 | 0.012 | (p < 0.001 *) | (2.309, 2.467) | 98.12% | (2.036, 2.176) | ||
| CRP (mg/dL) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 1,116,404 | 6001 | 0.005 | 0.008 | 2.505 | 98.96% | 2.000 | ||
| Cohort 2 | 1,116,404 | 15,030 | 0.013 | (p < 0.001 *) | (2.431, 2.580) | 98.01% | (1.941, 2.060) |
| Measure Matched with Gender | n | Patients with AD | Risk | Risk Difference (p-Value) | Risk Ratio (95% CI) | Kaplan–Meier Survivability (%) | p-Value (Log-Rank Test) | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 921,644 | 5919 | 0.006 | 0.006 | 2.011 | 98.76% | 1.617 | ||
| Cohort 2 | 921,644 | 11,904 | 0.013 | (p < 0.001 *) | (1.950, 2.075) | 98.05% | (1.567, 1.668) | ||
| Sodium (mmol/L) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 952,388 | 5384 | 0.006 | 0.007 | 2.255 | 98.98% | 1.942 | ||
| Cohort 2 | 952,388 | 12,142 | 0.013 | (p < 0.001 *) | (2.184, 2.328) | 98.08% | (1.880, 2.005) | ||
| Glucose (mg/dL) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 952,489 | 5399 | 0.006 | 0.007 | 2.272 | 98.98% | 1.966 | ||
| Cohort 2 | 952,489 | 12,267 | 0.013 | (p < 0.001 *) | (2.201, 2.346) | 98.06% | (1.904, 2.030) | ||
| Triglycerides (mg/dL) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 958,532 | 5649 | 0.006 | 0.007 | 2.145 | 98.94% | 1.860 | ||
| Cohort 2 | 958,532 | 12,116 | 0.013 | (p < 0.001 *) | (2.078, 2.213) | 98.09% | (1.802, 1.920) | ||
| Cholesterol (mg/dL) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 955,709 | 5636 | 0.005 | 0.007 | 2.138 | 98.94% | 1.866 | ||
| Cohort 2 | 955,709 | 12,050 | 0.013 | (p < 0.001 *) | (2.072, 2.206) | 98.08% | (1.808, 1.926) | ||
| LDL (mg/dL) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 953,687 | 5645 | 0.006 | 0.007 | 2.116 | 98.93% | 1.845 | ||
| Cohort 2 | 953,687 | 11,945 | 0.013 | (p < 0.001 *) | (2.050, 2.184) | 98.10% | (1.787, 1.904) | ||
| HDL (mg/dL) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 953,537 | 5622 | 0.006 | 0.007 | 2.136 | 98.94% | 1.861 | ||
| Cohort 2 | 953,537 | 12,006 | 0.013 | (p < 0.001 *) | (2.069, 2.204) | 98.09% | (1.803, 1.921) | ||
| CRP (mg/dL) | p < 0.001 * | p < 0.001 * | |||||||
| Cohort 1 | 1,118,368 | 6819 | 0.006 | 0.007 | 2.214 | 98.82% | 1.753 | ||
| Cohort 2 | 1,118,368 | 15,099 | 0.014 | (p < 0.001 *) | (2.152, 2.278) | 98.00% | (1.704, 1.804) |
| Oral Disease or Condition | n | Patients with AD | Risk | Risk Difference (p-Value) | Risk Ratio (95% CI) | Kaplan–Meier Survivability (%) | p-Value (Log-Rank Test) | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| UMLS:ICD10CM:K01 | p = 0.215 | p = 0.215 | |||||||
| Embedded and impacted teeth | |||||||||
| Cohort 1 | 13,922 | 58 | 0.004 | 0.002 | 1.517 | 99.25% | 1.233 | ||
| Cohort 2 | 13,922 | 88 | 0.006 | (p = 0.013 *) | (1.090, 2.112) | 99.12% | (0.885, 1.718) | ||
| UMLS:ICD10CM:K02 | p < 0.001 * | p < 0.001 * | |||||||
| Dental caries | |||||||||
| Cohort 1 | 270,176 | 1134 | 0.004 | 0.008 | 2.918 | 99.23% | 2.358 | ||
| Cohort 2 | 270,176 | 3309 | 0.012 | (p < 0.001 *) | (2.728, 3.121) | 98.25% | (2.204, 2.523) | ||
| UMLS:ICD10CM:K03 | p < 0.001 * | p = 0.313 | |||||||
| Other diseases of hard tissues of teeth | |||||||||
| Cohort 1 | 43,765 | 190 | 0.004 | 0.008 | 2.684 | 99.20% | 2.056 | ||
| Cohort 2 | 43,765 | 510 | 0.012 | (p < 0.001 *) | (2.274, 3.169) | 98.39% | (1.740, 2.428) | ||
| UMLS:ICD10CM:K04 | p < 0.001 * | p < 0.001 * | |||||||
| Diseases of pulp and periapical tissues | |||||||||
| Cohort 1 | 177,872 | 787 | 0.004 | 0.007 | 2.593 | 99.17% | 2.132 | ||
| Cohort 2 | 177,872 | 2041 | 0.011 | (p < 0.001 *) | (2.389, 2.815) | 98.31% | (1.963, 2.315) | ||
| UMLS:ICD10CM:K05 | p < 0.001 * | p = 0.001 * | |||||||
| Gingivitis and periodontal diseases | |||||||||
| Cohort 1 | 138,077 | 621 | 0.004 | 0.009 | 2.823 | 99.14% | 2.195 | ||
| Cohort 2 | 138,077 | 1753 | 0.013 | (p < 0.001 *) | (2.577, 3.092) | 98.21% | (2.003, 2.405) | ||
| UMLS:ICD10CM:K06 | p < 0.001 * | p = 0.376 | |||||||
| Other diseases of gingiva and edentulous alveolar ridge | |||||||||
| Cohort 1 | 32,500 | 204 | 0.006 | 0.010 | 2.637 | 98.76% | 2.066 | ||
| Cohort 2 | 32,500 | 538 | 0.017 | (p < 0.001 *) | (2.246, 3.096) | 97.53% | (1.758, 2.428) | ||
| UMLS:ICD10CM:K08 | p < 0.001 * | p < 0.001 * | |||||||
| Other disorders of teeth and supporting structures | |||||||||
| Cohort 1 | 300,038 | 1493 | 0.005 | 0.011 | 3.186 | 99.07% | 2.529 | ||
| Cohort 2 | 300,038 | 4757 | 0.016 | (p < 0.001 *) | (3.007, 3.376) | 97.72% | (2.386, 2.681) | ||
| UMLS:ICD10CM:K09 | p = 0.061 | p = 0.465 | |||||||
| Cysts of oral region, not elsewhere classified | |||||||||
| Cohort 1 | 9954 | 50 | 0.005 | 0.004 | 1.760 | 99.05% | 1.392 | ||
| Cohort 2 | 9954 | 88 | 0.009 | (p = 0.001 *) | (1.245, 2.488) | 98.65% | (0.984, 1.970) | ||
| UMLS:ICD10CM:K11 | p < 0.001 * | p = 0.347 | |||||||
| Diseases of salivary glands | |||||||||
| Cohort 1 | 236,672 | 1596 | 0.007 | 0.008 | 2.283 | 98.66% | 1.772 | ||
| Cohort 2 | 236,672 | 3644 | 0.015 | (p < 0.001 *) | (2.153, 2.421) | 97.67% | (1.671, 1.879) | ||
| UMLS:ICD10CM:K12 | p < 0.001 * | p = 0.096 | |||||||
| Stomatitis and related lesions | |||||||||
| Cohort 1 | 215,892 | 1168 | 0.005 | 0.006 | 2.115 | 98.95% | 1.684 | ||
| Cohort 2 | 215,892 | 2470 | 0.011 | (p < 0.001 *) | (1.973, 2.267) | 98.26% | (1.571, 1.806) | ||
| UMLS:ICD10CM:K13 | p < 0.001 * | p = 0.004 * | |||||||
| Other diseases of lip and oral mucosa | |||||||||
| Cohort 1 | 267,285 | 1628 | 0.006 | 0.008 | 2.295 | 98.93% | 1.752 | ||
| Cohort 2 | 267,285 | 3737 | 0.014 | (p < 0.001 *) | (2.166, 2.432) | 97.93% | (1.653, 1.858) | ||
| UMLS:ICD10CM:K14 | p < 0.001 * | p = 0.726 | |||||||
| Diseases of tongue | |||||||||
| Cohort 1 | 119,952 | 733 | 0.006 | 0.007 | 2.097 | 98.78% | 1.617 | ||
| Cohort 2 | 119,952 | 1537 | 0.013 | (p < 0.001 *) | (1.921, 2.289) | 98.02% | (1.481, 1.766) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, M.S.; Miller, B.C.; Mahani, M.; Mhaskar, R.; Tsalatsanis, A.; Jain, S.; Yadav, H. Poor Oral Health Linked with Higher Risk of Alzheimer’s Disease. Brain Sci. 2023, 13, 1555. https://doi.org/10.3390/brainsci13111555
Kulkarni MS, Miller BC, Mahani M, Mhaskar R, Tsalatsanis A, Jain S, Yadav H. Poor Oral Health Linked with Higher Risk of Alzheimer’s Disease. Brain Sciences. 2023; 13(11):1555. https://doi.org/10.3390/brainsci13111555
Chicago/Turabian StyleKulkarni, Mihir S., Brandi C. Miller, Manan Mahani, Rahul Mhaskar, Athanasios Tsalatsanis, Shalini Jain, and Hariom Yadav. 2023. "Poor Oral Health Linked with Higher Risk of Alzheimer’s Disease" Brain Sciences 13, no. 11: 1555. https://doi.org/10.3390/brainsci13111555
APA StyleKulkarni, M. S., Miller, B. C., Mahani, M., Mhaskar, R., Tsalatsanis, A., Jain, S., & Yadav, H. (2023). Poor Oral Health Linked with Higher Risk of Alzheimer’s Disease. Brain Sciences, 13(11), 1555. https://doi.org/10.3390/brainsci13111555





