Abnormalities of Hippocampal Subfield and Amygdalar Nuclei Volumes and Clinical Correlates in Behavioral Variant Frontotemporal Dementia with Obsessive–Compulsive Behavior—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Neuropsychiatric Assessment
2.4. MRI Acquisition
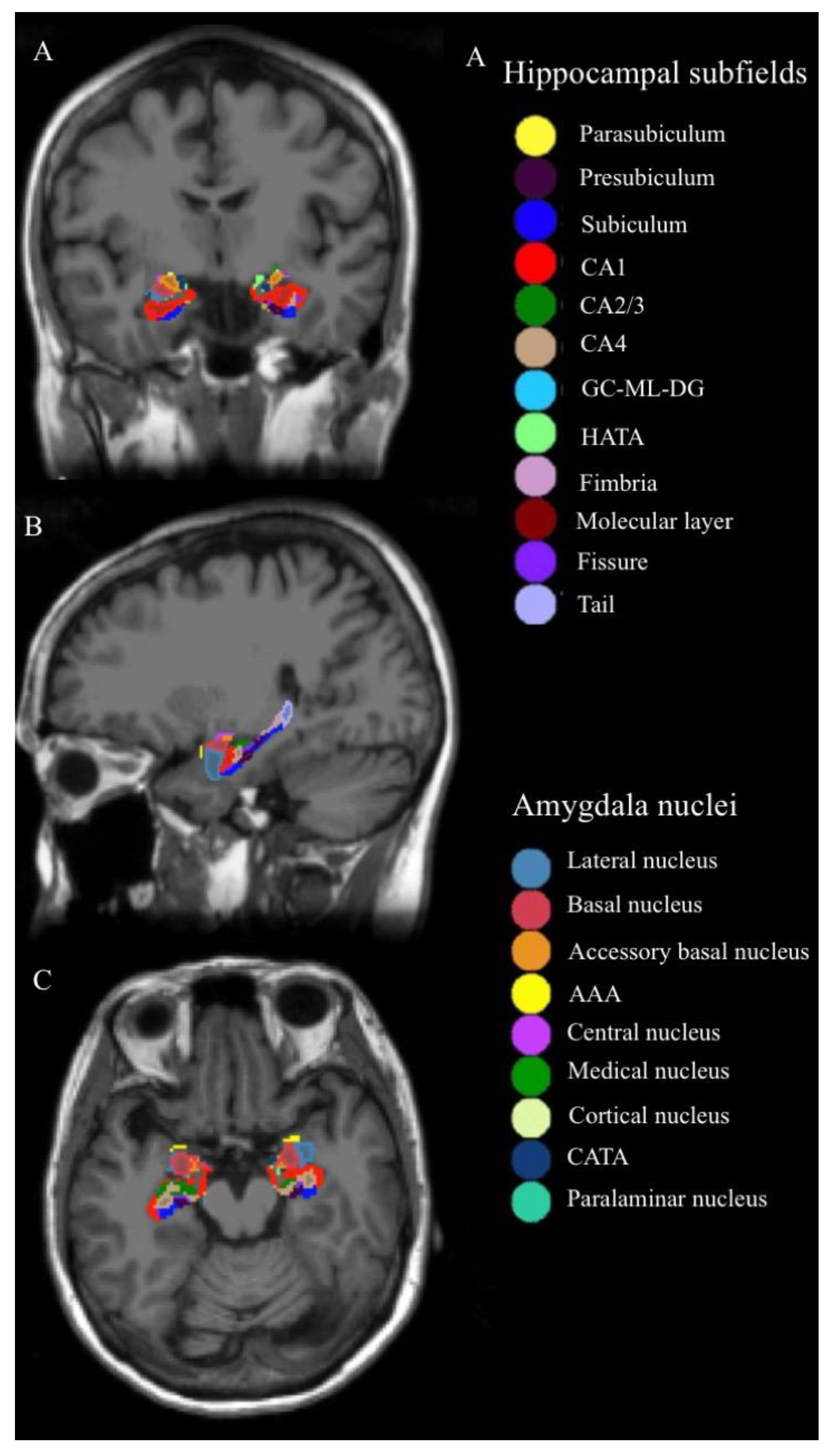
2.5. Analysis of Hippocampal Subfields and Amygdalar Nuclei Volumes
2.6. Mediation Analysis
2.7. Statistical Analysis
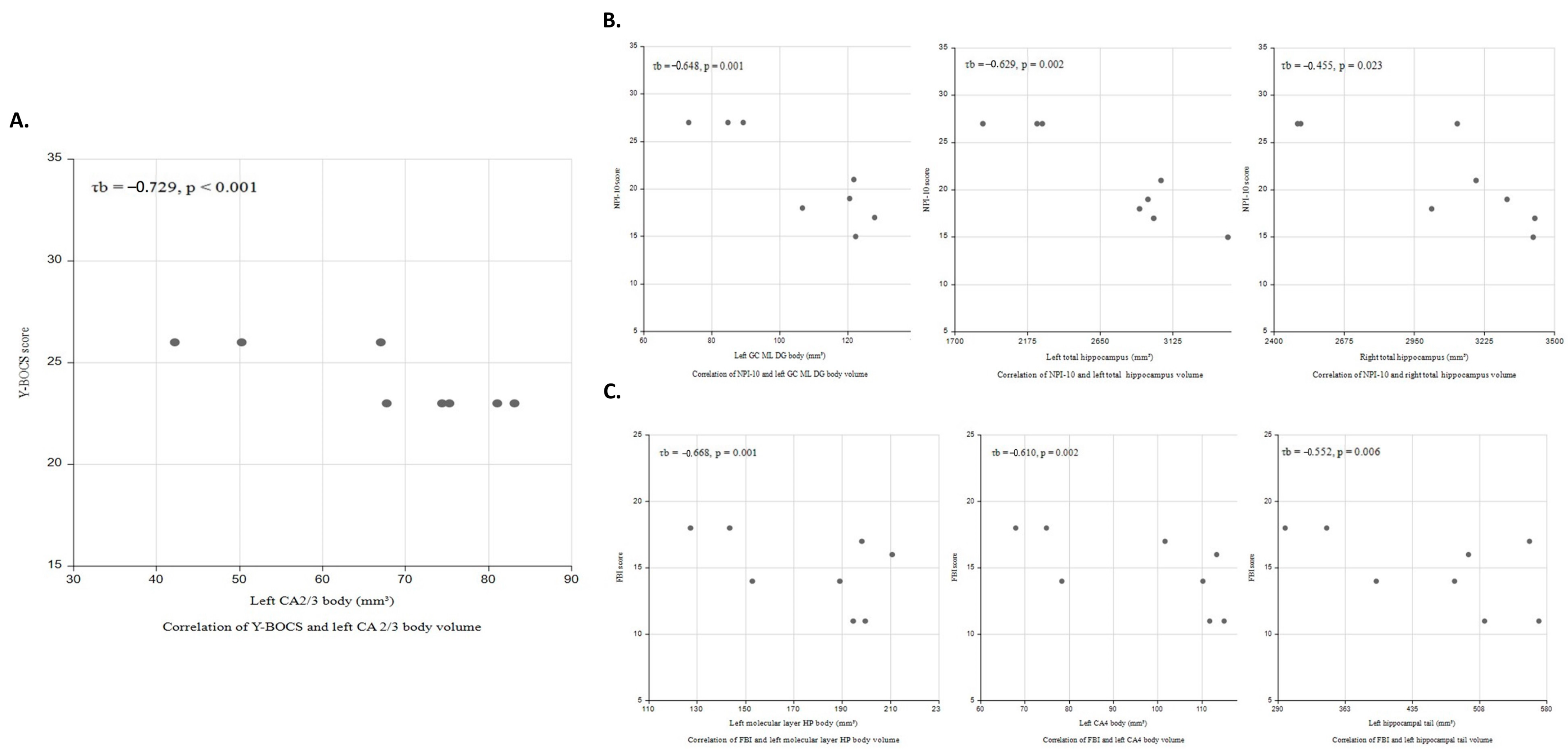
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, M.N.; Lau, C.I.; Lin, C.P. Precision Medicine for Frontotemporal Dementia. Front. Psychiatry 2019, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Castro-Suarez, S.; Guevara-Silva, E.; Caparó-Zamalloa, C.; Osorio-Marcatinco, V.; Meza-Vega, M.; Miller, B.; Cornejo-Olivas, M. Knowledge and Attitudes for the Management of Behavioral Variant of Frontotemporal Dementia. Front. Neurol. 2022, 12, 786448. [Google Scholar] [CrossRef] [PubMed]
- Katisko, K.; Cajanus, A.; Korhonen, T.; Remes, A.M.; Haapasalo, A.; Solje, E. Prodromal and Early bvFTD: Evaluating Clinical Features and Current Biomarkers. Front. Neurosci. 2019, 13, 658. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.L.; Darby, A.L.; Swartz, J.R.; Yener, G.G.; Mena, I. Dietary changes, compulsions and sexual behavior in frontotemporal degeneration. Dementia 1995, 6, 195–199. [Google Scholar] [CrossRef]
- Bertoux, M.; O’Callaghan, C.; Flanagan, E.; Hodges, J.R.; Hornberger, M. Fronto-Striatal Atrophy in Behavioral Variant Frontotemporal Dementia and Alzheimer’s Disease. Front. Neurol. 2015, 6, 147. [Google Scholar] [CrossRef]
- Flanagan, E.C.; Wong, S.; Dutt, A.; Tu, S.; Bertoux, M.; Irish, M.; Piguet, O.; Rao, S.; Hodges, J.R.; Ghosh, A.; et al. False Recognition in Behavioral Variant Frontotemporal Dementia and Alzheimer’s Disease—Disinhibition or Amnesia? Front. Aging Neurosci. 2016, 8, 177. [Google Scholar] [CrossRef]
- Li, P.; Quan, W.; Wang, Z.; Liu, Y.; Cai, H.; Chen, Y.; Wang, Y.; Zhang, M.; Tian, Z.; Zhang, H.; et al. Early-stage differentiation between Alzheimer’s disease and frontotemporal lobe degeneration: Clinical, neuropsychology, and neuroimaging features. Front. Aging Neurosci. 2022, 14, 981451. [Google Scholar] [CrossRef]
- Mackenzie, I.R.; Neumann, M. Molecular neuropathology of frontotemporal dementia: Insights into disease mechanisms from postmortem studies. J. Neurochem. 2016, 138 (Suppl. 1), 54–70. [Google Scholar] [CrossRef]
- Rosen, H.J.; Gorno-Tempini, M.L.; Goldman, W.P.; Perry, R.J.; Schuff, N.; Weiner, M.; Feiwell, R.; Kramer, J.H.; Miller, B.L. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 2002, 58, 198–208. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134 Pt 9, 2456–2477. [Google Scholar] [CrossRef]
- Josephs, K.A.; Whitwell, J.L.; Knopman, D.S.; Boeve, B.F.; Vemuri, P.; Senjem, M.L.; Parisi, J.E.; Ivnik, R.J.; Dickson, D.W.; Petersen, R.C.; et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology 2009, 73, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Rosso, S.M.; Roks, G.; Stevens, M.; de Koning, I.; Tanghe, H.L.J.; Kamphorst, W.; Ravid, R.; Niermeijer, M.F.; van Swieten, J.C. Complex compulsive behaviour in the temporal variant of frontotemporal dementia. J. Neurol. 2001, 248, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Ames, D.; Cummings, J.L.; Wirshing, W.C.; Quinn, B.; Mahler, M. Repetitive and compulsive behavior in frontal lobe degenerations. J. Neuropsychiatry Clin. Neurosci. 1994, 6, 100–113. [Google Scholar]
- Mendez, M.F.; Perryman, K.M.; Miller, B.L.; Swartz, J.R.; Cummings, J.L. Compulsive behaviors as presenting symptoms of frontotemporal dementia. J. Geriatr. Psychiatry Neurol. 1997, 10, 154–157. [Google Scholar] [CrossRef]
- Mourik, J.C.; Rosso, S.M.; Niermeijer, M.F.; Duivenvoorden, H.J.; Van Swieten, J.C.; Tibben, A. Frontotemporal dementia: Behavioral symptoms and caregiver distress. Dement. Geriatr. Cogn. Disord. 2004, 18, 299–306. [Google Scholar] [CrossRef]
- Cagnin, A.; Di Lorenzo, R.; Marra, C.; Bonanni, L.; Cupidi, C.; Lagana, V.; Rubino, E.; Vacca, A.; Provero, P.; Isella, V.; et al. Behavioral and Psychological Effects of Coronavirus Disease-19 Quarantine in Patients with Dementia. Front. Psychiatry 2020, 11, 578015. [Google Scholar] [CrossRef]
- Lu, P.H.; Mendez, M.F.; Lee, G.J.; Leow, A.D.; Lee, H.W.; Shapira, J.; Jimenez, E.; Boeve, B.B.; Caselli, R.J.; Graff-Radford, N.R.; et al. Patterns of brain atrophy in clinical variants of frontotemporal lobar degeneration. Dement. Geriatr. Cogn. Disord. 2013, 35, 34–50. [Google Scholar] [CrossRef]
- Maia da Silva, M.N.; Porto, F.H.G.; Lopes, P.M.G.; Sodre de Castro Prado, C.; Frota, N.A.F.; Alves, C.H.L.; Alves, G.S. Frontotemporal Dementia and Late-Onset Bipolar Disorder: The Many Directions of a Busy Road. Front. Psychiatry 2021, 12, 768722. [Google Scholar] [CrossRef]
- Kamalian, A.; Khodadadifar, T.; Saberi, A.; Masoudi, M.; Camilleri, J.A.; Eickhoff, C.R.; Zarei, M.; Pasquini, L.; Laird, A.R.; Fox, P.T.; et al. Convergent regional brain abnormalities in behavioral variant frontotemporal dementia: A neuroimaging meta-analysis of 73 studies. Alzheimers Dement. 2022, 14, e12318. [Google Scholar] [CrossRef]
- Henriquez, F.; Cabello, V.; Baez, S.; de Souza, L.C.; Lillo, P.; Martinez-Pernia, D.; Olavarria, L.; Torralva, T.; Slachevsky, A. Multidimensional Clinical Assessment in Frontotemporal Dementia and Its Spectrum in Latin America and the Caribbean: A Narrative Review and a Glance at Future Challenges. Front. Neurol. 2021, 12, 768591. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Qi, T.; Xu, J.; Jiang, L.; Zhang, F.; Dai, N.; Cheng, Y.; Xu, X. Reduced Interhemispheric Functional Connectivity in Obsessive-Compulsive Disorder Patients. Front. Psychiatry 2019, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Bota, R.G.; Brody, A.L. Brain-behavior relationships in obsessive-compulsive disorder. Semin. Clin. Neuropsychiatry 2001, 6, 82–101. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Li, Q.; Zhang, X.; Liu, L.; Yan, J.; Zhang, D.; Yan, H.; Yue, W. Altered Resting-State Brain Activity in Schizophrenia and Obsessive-Compulsive Disorder Compared with Non-Psychiatric Controls: Commonalities and Distinctions across Disorders. Front. Psychiatry 2021, 12, 681701. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, M.; Yildirim, H.; Ozdemir, H.; Ozler, S.; Kara, B.; Ozler, Z.; Kanmaz, E.; Mermi, O.; Tezcan, E. Hippocampus and amygdalar volumes in patients with refractory obsessive-compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Segi-Nishida, E. The Effect of Serotonin-Targeting Antidepressants on Neurogenesis and Neuronal Maturation of the Hippocampus Mediated via 5-HT1A and 5-HT4 Receptors. Front. Cell. Neurosci. 2017, 11, 142. [Google Scholar] [CrossRef]
- Castro, J.E.; Varea, E.; Marquez, C.; Cordero, M.I.; Poirier, G.; Sandi, C. Role of the amygdala in antidepressant effects on hippocampal cell proliferation and survival and on depression-like behavior in the rat. PLoS ONE 2010, 5, e8618. [Google Scholar] [CrossRef] [PubMed]
- Piguet, O.; Hodges, J.R. Behavioural-variant frontotemporal dementia: An update. Dement. Neuropsychol. 2013, 7, 10–18. [Google Scholar] [CrossRef]
- Goodman, W.K.; Price, L.H.; Rasmussen, S.A.; Mazure, C.; Fleischmann, R.L.; Hill, C.L.; Heninger, G.R.; Charney, D.S. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch. Gen. Psychiatry 1989, 46, 1006–1011. [Google Scholar] [CrossRef]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Zhang, S.; Qiu, Q.; Qian, S.; Lin, X.; Yan, F.; Sun, L.; Xiao, S.; Wang, J.; Fang, Y.; Li, X. Determining Appropriate Screening Tools and Cutoffs for Cognitive Impairment in the Chinese Elderly. Front. Psychiatry 2021, 12, 773281. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Nagaraja, A.V.; Ratnavalli, E. Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison in Alzheimer’s disease, vascular dementia and frontotemporal dementia. J. Neurol. Sci. 2005, 236, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, A.; Davidson, W.; Fox, H. Frontal behavioral inventory: Diagnostic criteria for frontal lobe dementia. Can. J. Neurol. Sci. 1997, 24, 29–36. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.J.; Lee, B.H.; Lee, P.; Park, J.H.; Seo, S.W.; Jeong, Y. Behavioral Reserve in Behavioral Variant Frontotemporal Dementia. Front. Aging Neurosci. 2022, 14, 875589. [Google Scholar] [CrossRef]
- Iglesias, J.E.; Augustinack, J.C.; Nguyen, K.; Player, C.M.; Player, A.; Wright, M.; Roy, N.; Frosch, M.P.; McKee, A.C.; Wald, L.L.; et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage 2015, 115, 117–137. [Google Scholar] [CrossRef]
- Saygin, Z.M.; Kliemann, D.; Iglesias, J.E.; van der Kouwe, A.J.W.; Boyd, E.; Reuter, M.; Stevens, A.; Van Leemput, K.; McKee, A.; Frosch, M.P.; et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: Manual segmentation to automatic atlas. Neuroimage 2017, 155, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Preacher, K.J.; Hayes, A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 2008, 40, 879–891. [Google Scholar] [CrossRef]
- Szeszko, P.R.; Robinson, D.; Alvir, J.M.; Bilder, R.M.; Lencz, T.; Ashtari, M.; Wu, H.; Bogerts, B. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch. Gen. Psychiatry 1999, 56, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.B.; Shin, Y.W.; Kim, S.H.; Yoo, S.Y.; Lee, J.M.; Kim, I.Y.; Kim, S.I.; Kwon, J.S. Hippocampal shape deformity analysis in obsessive-compulsive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2007, 257, 185–190. [Google Scholar] [CrossRef] [PubMed]
- McMurtray, A.M.; Chen, A.K.; Shapira, J.S.; Chow, T.W.; Mishkin, F.; Miller, B.L.; Mendez, M.F. Variations in regional SPECT hypoperfusion and clinical features in frontotemporal dementia. Neurology 2006, 66, 517–522. [Google Scholar] [CrossRef]
- Irwin, D.J.; Cairns, N.J.; Grossman, M.; McMillan, C.T.; Lee, E.B.; Van Deerlin, V.M.; Lee, V.M.; Trojanowski, J.Q. Frontotemporal lobar degeneration: Defining phenotypic diversity through personalized medicine. Acta Neuropathol. 2015, 129, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.; Tavares, T.P.; Palaniyappan, L.; Finger, E.C. Hoarding and obsessive-compulsive behaviours in frontotemporal dementia: Clinical and neuroanatomic associations. Cortex 2019, 121, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, J.D.; Ridgway, G.R.; Modat, M.; Ourselin, S.; Mead, S.; Fox, N.C.; Rossor, M.N.; Warren, J.D. Distinct profiles of brain atrophy in frontotemporal lobar degeneration caused by progranulin and tau mutations. Neuroimage 2010, 53, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, M.; Iglesias, J.E.; Scelsi, M.A.; Cash, D.M.; Cardoso, M.J.; Modat, M.; Altmann, A.; Ourselin, S.; Warren, J.D.; Rohrer, J.D. Hippocampal Subfield Volumetry: Differential Pattern of Atrophy in Different Forms of Genetic Frontotemporal Dementia. J. Alzheimers Dis. 2018, 64, 497–504. [Google Scholar] [CrossRef]
- La Joie, R.; Perrotin, A.; de La Sayette, V.; Egret, S.; Doeuvre, L.; Belliard, S.; Eustache, F.; Desgranges, B.; Chetelat, G. Hippocampal subfield volumetry in mild cognitive impairment, Alzheimer’s disease and semantic dementia. Neuroimage Clin. 2013, 3, 155–162. [Google Scholar] [CrossRef]
- Cavada, C.; Company, T.; Tejedor, J.; Cruz-Rizzolo, R.J.; Reinoso-Suarez, F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb. Cortex 2000, 10, 220–242. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, L.A.; Leung, B.K.; Boldt, S.; Liang, S.; Balleine, B.W. Goal-directed actions transiently depend on dorsal hippocampus. Nat. Neurosci. 2020, 23, 1194–1197. [Google Scholar] [CrossRef]
- Kashyap, H.; Abramovitch, A. Neuropsychological Research in Obsessive-Compulsive Disorder: Current Status and Future Directions. Front. Psychiatry 2021, 12, 721601. [Google Scholar] [CrossRef]
- Gillan, C.M.; Papmeyer, M.; Morein-Zamir, S.; Sahakian, B.J.; Fineberg, N.A.; Robbins, T.W.; de Wit, S. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am. J. Psychiatry 2011, 168, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Chuah, L.Y.; Sim, S.K.; Chee, M.W. Hippocampal region-specific contributions to memory performance in normal elderly. Brain Cogn. 2010, 72, 400–407. [Google Scholar] [CrossRef]
- Blum, S.; Habeck, C.; Steffener, J.; Razlighi, Q.; Stern, Y. Functional connectivity of the posterior hippocampus is more dominant as we age. Cogn. Neurosci. 2014, 5, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Dragoi, A.M.; Pecie, L.G.; Patrichi, B.E.; Ladea, M. Morphopathological changes in obsessive-compulsive disorder. Rom. J. Morphol. Embryol. 2020, 61, 51–60. [Google Scholar] [CrossRef]
- Barnes, J.; Whitwell, J.L.; Frost, C.; Josephs, K.A.; Rossor, M.; Fox, N.C. Measurements of the amygdala and hippocampus in pathologically confirmed Alzheimer disease and frontotemporal lobar degeneration. Arch. Neurol. 2006, 63, 1434–1439. [Google Scholar] [CrossRef]
- Jenike, M.A.; Breiter, H.C.; Baer, L.; Kennedy, D.N.; Savage, C.R.; Olivares, M.J.; O’Sullivan, R.L.; Shera, D.M.; Rauch, S.L.; Keuthen, N.; et al. Cerebral structural abnormalities in obsessive-compulsive disorder. A quantitative morphometric magnetic resonance imaging study. Arch. Gen. Psychiatry 1996, 53, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Miller, B.L.; Kramer, J.H.; Rankin, K.; Wyss-Coray, C.; Gearhart, R.; Phengrasamy, L.; Weiner, M.; Rosen, H.J. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology 2004, 62, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, M.; Iglesias, J.E.; Cash, D.M.; Warren, J.D.; Rohrer, J.D. Amygdala subnuclei are differentially affected in the different genetic and pathological forms of frontotemporal dementia. Alzheimers Dement. 2019, 11, 136–141. [Google Scholar] [CrossRef]
- Perry, D.C.; Whitwell, J.L.; Boeve, B.F.; Pankratz, V.S.; Knopman, D.S.; Petersen, R.C.; Jack, C.R., Jr.; Josephs, K.A. Voxel-based morphometry in patients with obsessive-compulsive behaviors in behavioral variant frontotemporal dementia. Eur. J. Neurol. 2012, 19, 911–917. [Google Scholar] [CrossRef]
- Peterson, B.S.; Choi, H.A.; Hao, X.; Amat, J.A.; Zhu, H.; Whiteman, R.; Liu, J.; Xu, D.; Bansal, R. Morphologic features of the amygdala and hippocampus in children and adults with Tourette syndrome. Arch. Gen. Psychiatry 2007, 64, 1281–1291. [Google Scholar] [CrossRef]
- Cherubini, E.; Miles, R. The CA3 region of the hippocampus: How is it? What is it for? How does it do it? Front. Cell. Neurosci. 2015, 9, 19. [Google Scholar] [CrossRef]
- Pennington, C.; Hodges, J.R.; Hornberger, M. Neural correlates of episodic memory in behavioral variant frontotemporal dementia. J. Alzheimers Dis. 2011, 24, 261–268. [Google Scholar] [CrossRef]
- Tu, S.; Spiers, H.J.; Hodges, J.R.; Piguet, O.; Hornberger, M. Egocentric versus Allocentric Spatial Memory in Behavioral Variant Frontotemporal Dementia and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 59, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Savage, C.R.; Deckersbach, T.; Wilhelm, S.; Rauch, S.L.; Baer, L.; Reid, T.; Jenike, M.A. Strategic processing and episodic memory impairment in obsessive compulsive disorder. Neuropsychology 2000, 14, 141–151. [Google Scholar] [CrossRef]
- Milan, G.; Lamenza, F.; Iavarone, A.; Galeone, F.; Lore, E.; de Falco, C.; Sorrentino, P.; Postiglione, A. Frontal Behavioural Inventory in the differential diagnosis of dementia. Acta Neurol. Scand. 2008, 117, 260–265. [Google Scholar] [CrossRef]
- de Flores, R.; Mutlu, J.; Bejanin, A.; Gonneaud, J.; Landeau, B.; Tomadesso, C.; Mezenge, F.; de La Sayette, V.; Eustache, F.; Chetelat, G. Intrinsic connectivity of hippocampal subfields in normal elderly and mild cognitive impairment patients. Hum. Brain Mapp. 2017, 38, 4922–4932. [Google Scholar] [CrossRef]
- Romero-Torres, B.M.; Alvarado-Ramirez, Y.A.; Duran-Alonzo, S.R.; Ruiz-Contreras, A.E.; Herrera-Solis, A.; Amancio-Belmont, O.; Prospero-Garcia, O.E.; Mendez-Diaz, M. A potential role of hippocampus on impulsivity and alcohol consumption through CB1R. Pharmacol. Biochem. Behav. 2023, 225, 173558. [Google Scholar] [CrossRef]
- Blair, R.J. The Neurobiology of Impulsive Aggression. J. Child Adolesc. Psychopharmacol. 2016, 26, 4–9. [Google Scholar] [CrossRef]
- Banwinkler, M.; Theis, H.; Prange, S.; van Eimeren, T. Imaging the Limbic System in Parkinson’s Disease—A Review of Limbic Pathology and Clinical Symptoms. Brain Sci. 2022, 12, 1248. [Google Scholar] [CrossRef] [PubMed]
- Noble, E.E.; Wang, Z.; Liu, C.M.; Davis, E.A.; Suarez, A.N.; Stein, L.M.; Tsan, L.; Terrill, S.J.; Hsu, T.M.; Jung, A.H.; et al. Hypothalamus-hippocampus circuitry regulates impulsivity via melanin-concentrating hormone. Nat. Commun. 2019, 10, 4923. [Google Scholar] [CrossRef]
- Garcia-Fuster, M.J.; Rhodes, J.S.; Mandyam, C.D. The role of dentate gyrus neurogenesis in neuropsychiatric disorders. Neural Plast. 2013, 2013, 584382. [Google Scholar] [CrossRef] [PubMed]
- Engin, E.; Smith, K.S.; Gao, Y.; Nagy, D.; Foster, R.A.; Tsvetkov, E.; Keist, R.; Crestani, F.; Fritschy, J.M.; Bolshakov, V.Y.; et al. Modulation of anxiety and fear via distinct intrahippocampal circuits. Elife 2016, 5, e14120. [Google Scholar] [CrossRef] [PubMed]
- Haukvik, U.K.; Tamnes, C.K.; Soderman, E.; Agartz, I. Neuroimaging hippocampal subfields in schizophrenia and bipolar disorder: A systematic review and meta-analysis. J. Psychiatr. Res. 2018, 104, 217–226. [Google Scholar] [CrossRef]
- Forman, M.S.; Farmer, J.; Johnson, J.K.; Clark, C.M.; Arnold, S.E.; Coslett, H.B.; Chatterjee, A.; Hurtig, H.I.; Karlawish, J.H.; Rosen, H.J.; et al. Frontotemporal dementia: Clinicopathological correlations. Ann. Neurol. 2006, 59, 952–962. [Google Scholar] [CrossRef]
- Yang, C.; Tang, J.; Liu, N.; Yao, L.; Xu, M.; Sun, H.; Tao, B.; Gong, Q.; Cao, H.; Zhang, W.; et al. The Effects of Antipsychotic Treatment on the Brain of Patients with First-Episode Schizophrenia: A Selective Review of Longitudinal MRI Studies. Front. Psychiatry 2021, 12, 593703. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.H.; Cha, J.; Vedaei, F.; Dunlop, B.W.; Craighead, W.E.; Mayberg, H.S.; Choi, K.S. Treatment-Specific Hippocampal Subfield Volume Changes with Antidepressant Medication or Cognitive-Behavior Therapy in Treatment-Naive Depression. Front. Psychiatry 2021, 12, 718539. [Google Scholar] [CrossRef] [PubMed]
- Frodl, T.; Jager, M.; Smajstrlova, I.; Born, C.; Bottlender, R.; Palladino, T.; Reiser, M.; Moller, H.J.; Meisenzahl, E.M. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: A 3-year prospective magnetic resonance imaging study. J. Psychiatry Neurosci. 2008, 33, 423–430. [Google Scholar]
- Tae, W.S.; Kim, S.S.; Lee, K.U.; Nam, E.C.; Kim, K.W. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology 2008, 50, 569–581. [Google Scholar] [CrossRef]
- Reuter, M.; Schmansky, N.J.; Rosas, H.D.; Fischl, B. within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012, 61, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
| bvFTD with OCB (n = 8) | Controls (n = 8) | p | Effect Size | ||
|---|---|---|---|---|---|
| Age, year | 66.0 ± 5.7 | 64.5 ± 5.3 | 0.598 | 0.27 | |
| Sex (Female/Male) | 6/2 | 6/2 | 0.715 | ||
| Education, year | 6.0 ± 1.1 | 6.1 ± 1.0 | 0.798 | 0.08 | |
| Handedness (right) | 8 | 8 | |||
| MMSE | 20.1 ± 6.9 | 30 ± 0 | 0.002 | 0.81 | |
| CDR | 1 ± 0 | 0 | |||
| Y-BOCS | 0 | ||||
| Total | 24.1 ± 1.6 | 0 | |||
| Obsession | 8.8 ± 1.0 | 0 | |||
| Compulsion | 15.4 ± 0.5 | 0 | |||
| NPI-10 | |||||
| Total | 21.4 ± 5.0 | 0 | |||
| Severity | 9.5 ± 0.5 | 0 | |||
| Frequency | 11.4 ± 1.6 | 0 | |||
| FBI | |||||
| FBI-total | 14.9 ± 2.9 | 0 | |||
| Negative | 3.1 ± 0.8 | 0 | |||
| Disinhibition | 11.8 ± 2.2 | 0 | |||
| TIV | 1,495,629 ± 254,069 | 1,418,600 ± 199,993 | 0.511 | 0.34 | |
| Subfields (mm3) | bvFTD with OCB (n = 8) | Controls (n = 8) | GLM, Age, Sex, TIV | |
|---|---|---|---|---|
| Left | Parasubiculum | 52 ± 20 | 60 ± 10 | 0.142 |
| Presubiculum-head | 106 ± 32 | 139 ± 17 | 0.011 | |
| Presubiculum-body | 144 ± 38 | 156 ± 16 | 0.463 | |
| Subiculum-head | 154 ± 35 | 191 ± 25 | 0.008 | |
| Subiculum-body | 203 ± 47 | 242 ± 22 | 0.026 | |
| CA1-head | 411 ± 80 | 500 ± 68 | 0.005 | |
| CA1-body | 98 ± 26 | 140 ± 25 | 0.001 * | |
| CA2/3-head | 87 ± 26 | 112 ± 20 | 0.017 | |
| CA2/3-body | 68 ± 15 | 98 ± 16 | <0.001 * | |
| CA4-head | 97 ± 25 | 122 ± 15 | 0.009 | |
| CA4-body | 97 ± 20 | 123 ± 13 | 0.001 * | |
| GC-ML-DG-head | 114 ± 28 | 147 ± 21 | 0.006 | |
| GC-ML-DG-body | 106 ± 21 | 136 ± 13 | 0.001 * | |
| Molecular_layer_HP-head | 258 ± 57 | 322 ± 37 | 0.004 | |
| Molecular_layer_HP-body | 177 ± 31 | 232 ± 23 | <0.001 * | |
| HATA | 44 ± 10 | 52 ± 8 | 0.013 | |
| Fimbria | 51 ± 21 | 71 ± 14 | 0.027 | |
| Hippocampal tail | 457 ± 101 | 593 ± 79 | <0.001 * | |
| Hippocampal fissure | 139 ± 22 | 152 ± 32 | 0.053 | |
| Whole hippocampus | 2724 ± 535 | 3437 ± 336 | <0.001 * | |
| Right | Parasubiculum | 56 ± 14 | 55 ± 8 | 0.773 |
| Presubiculum-head | 116 ± 16 | 128 ± 16 | 0.026 | |
| Presubiculum-body | 140 ± 19 | 146 ± 14 | 0.420 | |
| Subiculum-head | 167 ± 25 | 186 ± 27 | 0.007 | |
| Subiculum-body | 210 ± 24 | 237 ± 35 | 0.015 | |
| CA1-head | 468 ± 66 | 526 ± 75 | 0.004 | |
| CA1-body | 110 ± 22 | 139 ± 15 | 0.001 * | |
| CA2/3-head | 107 ± 16 | 122 ± 19 | 0.024 | |
| CA2/3-body | 83 ± 17 | 100 ± 13 | 0.003 | |
| CA4-head | 119 ± 17 | 129 ± 18 | 0.055 | |
| CA4-body | 110 ± 19 | 127 ± 18 | 0.004 | |
| GC-ML-DG-head | 140 ± 22 | 154 ± 23 | 0.032 | |
| GC-ML-DG-body | 120 ± 222 | 137 ± 17 | 0.004 | |
| Molecular_layer_HP-head | 292 ± 40 | 330 ± 42 | 0.003 | |
| Molecular_layer_HP-body | 195 ± 27 | 231 ± 22 | 0.001 * | |
| HATA | 47 ± 9 | 56 ± 8 | 0.019 | |
| Fimbria | 65 ± 12 | 66 ± 21 | 0.261 | |
| Hippocampal tail | 514 ± 75 | 599 ± 99 | 0.004 | |
| Hippocampal-fissure | 1622 ± 30 | 174 ± 41 | 0.156 | |
| Whole hippocampus | 3060 ± 374 | 3469 ± 398 | 0.001 * |
| Amygdala Nuclei (mm3) | bvFTD with OCB (n = 8) | Controls (n = 8) | GLM, Age, Sex, TIV | |
|---|---|---|---|---|
| Left | Lateral nucleus | 565 (130) | 654 (113) | 0.037 |
| Basal nucleus | 329 (81) | 403 (62) | 0.022 | |
| Accessory basal nucleus | 183 (46) | 235 (33) | 0.026 | |
| Anterior amygdaloid area | 41 (9) | 52 (9) | 0.011 | |
| Central nucleus | 30 (9) | 39 (7) | 0.055 | |
| Medial nucleus | 15 (4) | 19 (5) | 0.111 | |
| Cortical nucleus | 17 (5) | 24 (4) | 0.021 | |
| CATA | 128 (27) | 155 (26) | 0.042 | |
| Paralaminar nucleus | 39 (10) | 47 (8) | 0.033 | |
| Whole amygdala | 1346 (303) | 1627 (246) | 0.022 | |
| Right | Lateral nucleus | 589 (95) | 650 (81) | 0.073 |
| Basal nucleus | 378 (59) | 419 (59) | 0.033 | |
| Accessory basal nucleus | 216 (39) | 254 (38) | 0.023 | |
| Anterior amygdaloid area | 49 (8) | 56 (9) | 0.086 | |
| Central nucleus | 35 (8) | 43 (8) | 0.058 | |
| Medial nucleus | 20 (8) | 22 (7) | 0.347 | |
| Cortical nucleus | 21 (4) | 26 (5) | 0.032 | |
| CATA | 143 (27) | 164 (24) | 0.050 | |
| Paralaminar nucleus | 43 (7) | 47 (7) | 0.094 | |
| Whole amygdala | 1494 (235) | 1679 (226) | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.-N.; Hu, L.-Y.; Tsai, C.-F.; Hong, C.-J.; Chou, Y.-H.; Chang, C.-C.; Yang, K.-C.; You, Z.-H.; Lau, C.I. Abnormalities of Hippocampal Subfield and Amygdalar Nuclei Volumes and Clinical Correlates in Behavioral Variant Frontotemporal Dementia with Obsessive–Compulsive Behavior—A Pilot Study. Brain Sci. 2023, 13, 1582. https://doi.org/10.3390/brainsci13111582
Liu M-N, Hu L-Y, Tsai C-F, Hong C-J, Chou Y-H, Chang C-C, Yang K-C, You Z-H, Lau CI. Abnormalities of Hippocampal Subfield and Amygdalar Nuclei Volumes and Clinical Correlates in Behavioral Variant Frontotemporal Dementia with Obsessive–Compulsive Behavior—A Pilot Study. Brain Sciences. 2023; 13(11):1582. https://doi.org/10.3390/brainsci13111582
Chicago/Turabian StyleLiu, Mu-N, Li-Yu Hu, Chia-Fen Tsai, Chen-Jee Hong, Yuan-Hwa Chou, Chiung-Chih Chang, Kai-Chun Yang, Zi-Hong You, and Chi Ieong Lau. 2023. "Abnormalities of Hippocampal Subfield and Amygdalar Nuclei Volumes and Clinical Correlates in Behavioral Variant Frontotemporal Dementia with Obsessive–Compulsive Behavior—A Pilot Study" Brain Sciences 13, no. 11: 1582. https://doi.org/10.3390/brainsci13111582
APA StyleLiu, M.-N., Hu, L.-Y., Tsai, C.-F., Hong, C.-J., Chou, Y.-H., Chang, C.-C., Yang, K.-C., You, Z.-H., & Lau, C. I. (2023). Abnormalities of Hippocampal Subfield and Amygdalar Nuclei Volumes and Clinical Correlates in Behavioral Variant Frontotemporal Dementia with Obsessive–Compulsive Behavior—A Pilot Study. Brain Sciences, 13(11), 1582. https://doi.org/10.3390/brainsci13111582





