Exploring Neurophysiological Mechanisms and Treatment Efficacies in Laryngeal Dystonia: A Transcranial Magnetic Stimulation Approach
Abstract
:1. Introduction
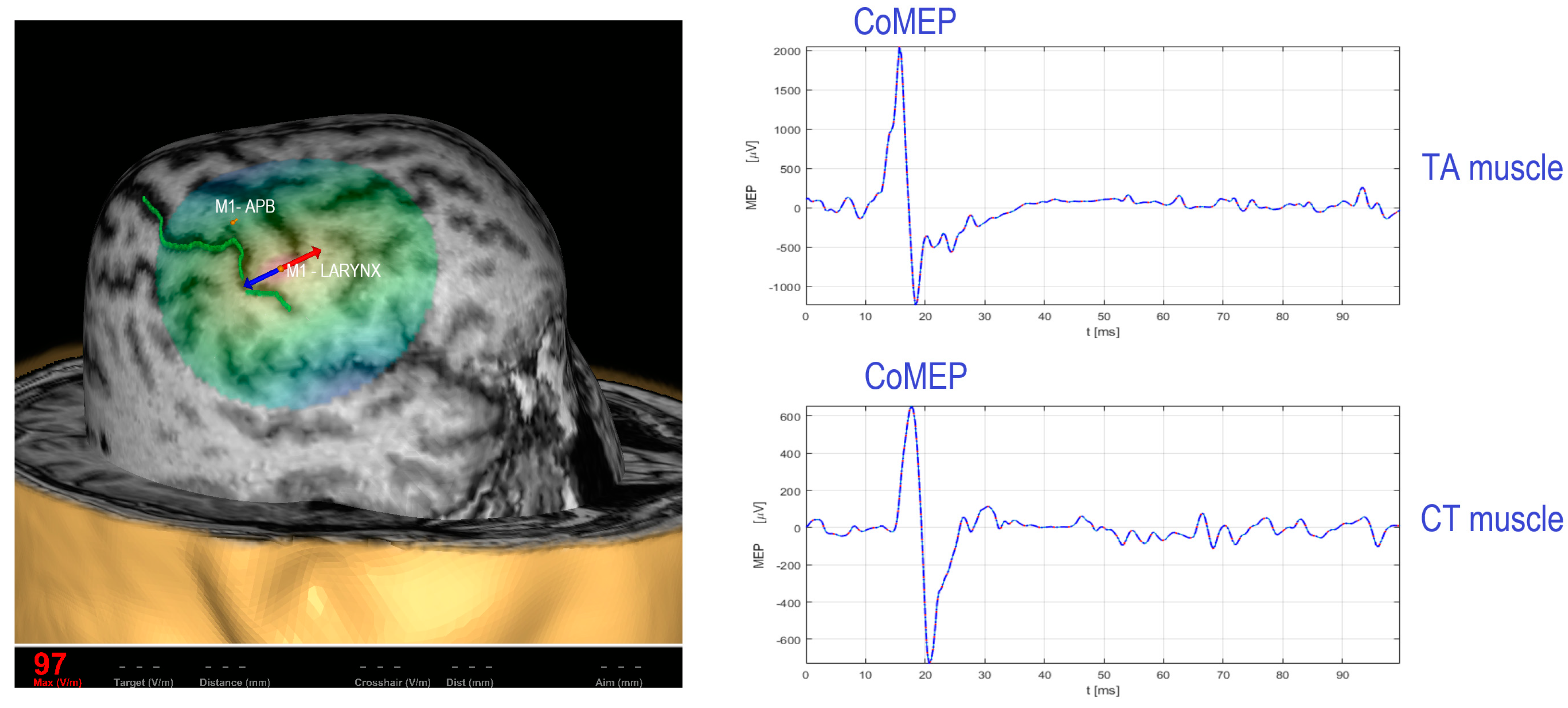
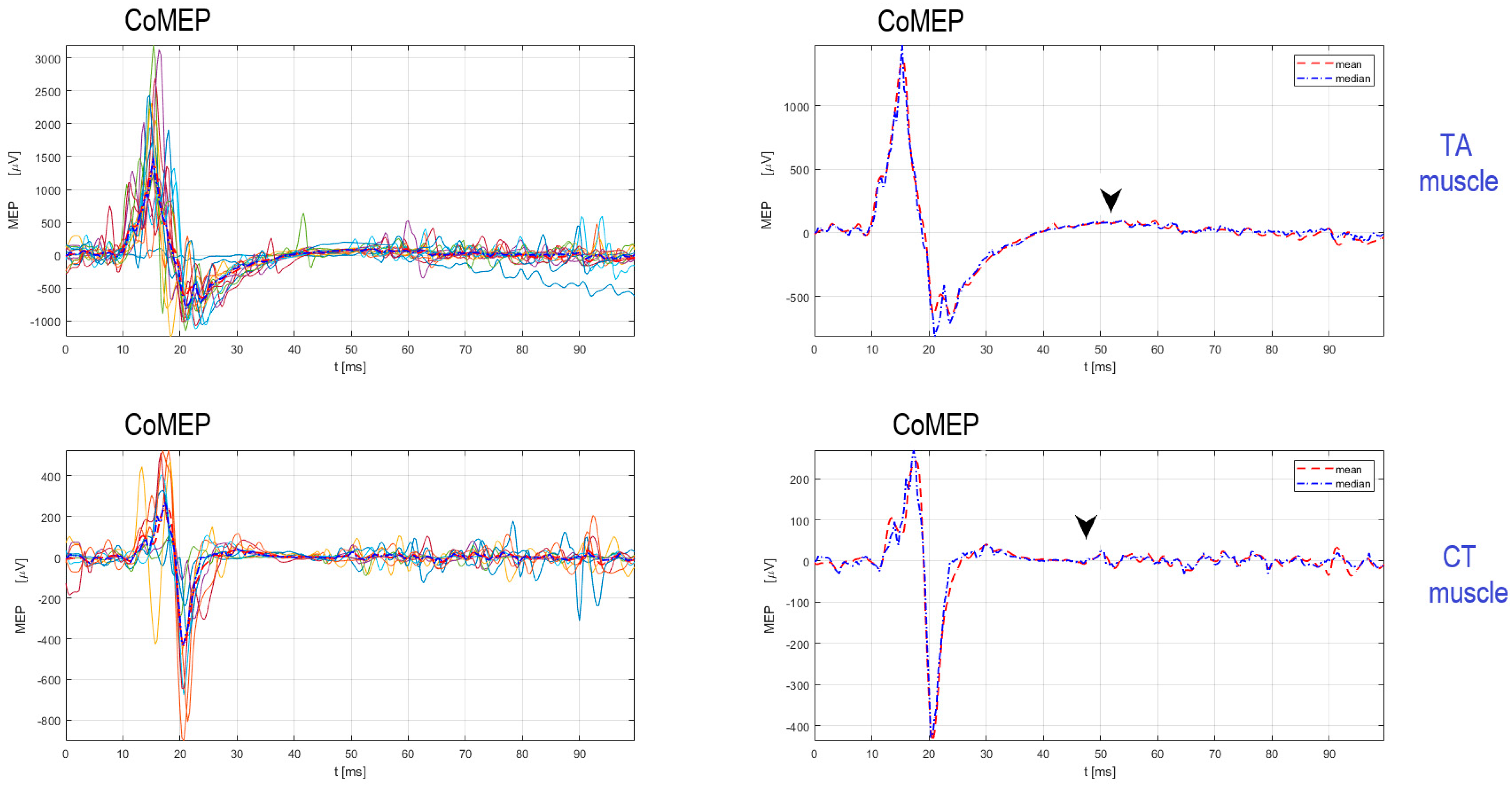
2. Existing TMS Methodology for Recording the Corticobulbar Motor Evoked Potentials from Cricothyroid and Thyroarytenoid Muscles
Procedure for Stimulation and Recording of CoMEPs from Laryngeal Muscles
3. Evaluation of Corticobulbar Motor Evoked Potentials and Cortical Silent Period in Thyroarytenoid and Cricothyroid Muscles in LD
4. New Approaches in the Treatment of LD: Repetitive Transcranial Magnetic Stimulation (rTMS) and Laryngeal Vibration
5. Invasive Treatments of LD: Where Do We Stand?
5.1. Deep Brain Stimulation (DBS) in Dystonia: Neurosurgical Procedure
5.2. ENT Surgical Procedures in LD Treatment
6. Outcome Measures in the Treatment of LD
7. Genetic Studies in LD: The Missing Puzzles and Future Perspective
8. Discussion and Future Directions
New Approaches for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Simonyan, K.; Barkmeier-Kraemer, J.; Blitzer, A.; Hallett, M.; Houde, J.F.; Jacobson Kimberley, T.; Ozelius, L.J.; Pitman, M.J.; Richardson, R.M.; Sharma, N.; et al. Laryngeal Dystonia: Multidisciplinary Update on Terminology, Pathophysiology, and Research Priorities. Neurology 2021, 96, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Battistella, G.; Simonyan, K. Clinical Implications of Dystonia as a Neural Network Disorder. Adv. Neurobiol. 2023, 31, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Khosravani, S.; Chen, G.; Ozelius, L.J.; Simonyan, K. Neural endophenotypes and predictors of laryngeal dystonia penetrance and manifestation. Neurobiol. Dis. 2021, 148, 105223. [Google Scholar] [CrossRef] [PubMed]
- Simonyan, K.; Cho, H.; Hamzehei Sichani, A.; Rubien-Thomas, E.; Hallett, M. The Direct Basal Ganglia Pathway Is Hyperfunctional in Focal Dystonia. Brain 2017, 140, 3179–3190. [Google Scholar] [CrossRef]
- Blitzer, A.; Brin, M.F.; Simonyan, K.; Ozelius, L.J.; Frucht, S.J. Phenomenology, genetics, and CNS network abnormalities in laryngeal dystonia: A 30-year experience. Laryngoscope 2018, 128, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- de Lima Xavier, L.; Simonyan, K. The extrinsic risk and its association with neural alterations in spasmodic dysphonia. Park. Relat. Disord. 2019, 65, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Barkmeier, J.M.; Case, J.L.; Ludlow, C.L. Identification of symptoms for spasmodic dysphonia and vocal tremor: A comparison of expert and nonexpert judges. J. Commun. Disord. 2001, 34, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Woo, P.; Murry, T. Vibratory Onset of Adductor Spasmodic Dysphonia and Muscle Tension Dysphonia: A High-Speed Video Study. J. Voice 2020, 34, 598–603. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O.; Müller, A.H.; Sittel, C. Neurolaryngology. In Textbook of Surgery of Larynx and Trachea, 2nd ed.; Remacle, M., Eckel, H.E., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 301–333. [Google Scholar] [CrossRef]
- Faham, M.; Ahmadi, A.; Silverman, E.; Harouni, G.G.; Dabirmoghaddam, P. Quality of Life After Botulinum Toxin Injection in Patients with Adductor Spasmodic Dysphonia; a Systematic Review and Meta-analysis. J. Voice 2021, 35, 71–283. [Google Scholar] [CrossRef]
- Prudente, C.N.; Mo Chen, M.; Stipancic, K.L.; Marks, K.L.; Samargia-Grivette, S.; Goding, G.S.; Green, J.R.; Kimberley, T.J. Effects of low-frequency repetitive transcranial magnetic stimulation in adductor laryngeal dystonia: A safety, feasibility, and pilot study. Exp. Brain Res. 2022, 240, 561–574. [Google Scholar] [CrossRef]
- Khosravani, S.; Mahnan, A.; Yeh, I.L.; Aman, J.E.; Watson, P.J.; Zhang, Y.; Goding, G.; Konczak, J. Laryngeal vibration as a non-invasive neuromodulation therapy for spasmodic dysphonia. Sci. Rep. 2019, 9, 17955. [Google Scholar] [CrossRef] [PubMed]
- Espadaler, J.; Rogić, M.; Deletis, V.; Leon, A.; Quijada, C.; Conesa, G. Representation of cricothyroid muscles at the primary motor cortex (M1) in healthy subjects, mapped by navigated transcranial magnetic stimulation (nTMS). Clin. Neurophysiol. 2012, 123, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Deletis, V.; Rogić, M.; Fernandez-Conejero, I.; Gabarrós, A.; Jerončić, A. Neurophysiologic markers in laryngeal muscles indicate functional anatomy of laryngeal primary motor cortex and premotor cortex in the caudal opercular part of inferior frontal gyrus. Clin. Neurophysiol. 2014, 125, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Rogić Vidaković, M.; Zmajević Schönwald, M.; Rotim, K.; Jurić, T.; Vulević, Z.; Tafra, R.; Banožić, A.; Hamata, Ž.; Đogaš, Z. Excitability of contralateral and ipsilateral projections of corticobulbar pathways recorded as corticobulbar motor evoked potentials of the cricothyroid muscles. Clin. Neurophysiol. 2015, 126, 1570–1577. [Google Scholar] [CrossRef]
- Deletis, V.; Fernandez-Conejero, I.; Ulkatan, S.; Costantino, P. Methodology for intraoperatively eliciting motor evoked potentials in the vocal muscles by electrical stimulation of the corticobulbar tract. Clin. Neurophysiol. 2009, 120, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Deletis, V.; Fernández-Conejero, I.; Ulkatan, S.; Rogić, M.; Carbó, E.L.; Hiltzik, D. Methodology for intra-operative recording of the corticobulbar motor evoked potentials from cricothyroid muscles. Clin. Neurophysiol. 2011, 122, 1883–1889. [Google Scholar] [CrossRef]
- Chen, M.; Summers, R.L.S.; Goding, G.S.; Samargia, S.; Ludlow, C.L.; Prudente, C.N.; Kimberley, T.J. Evaluation of the Cortical Silent Period of the Laryngeal Motor Cortex in Healthy Individuals. Front. Neurosci. 2017, 11, 88. [Google Scholar] [CrossRef]
- Chen, M.; Summers, R.L.S.; Prudente, C.N.; Prudente, C.N.; Goding, G.S.; Samargia-Grivette, S.; Ludlow, C.L.; Kimberley, T.J. Transcranial magnetic stimulation and functional magnet resonance imaging evaluation of adductor spasmodic dysphonia during phonation. Brain Stimul. 2020, 13, 908–915. [Google Scholar] [CrossRef]
- Hirano, M.; Ohala, J. Use of hooked-wire electrodes for electromyography of the intrinsic laryngeal muscles. J. Speech Hear. Res. 1969, 12, 362–373. [Google Scholar] [CrossRef]
- Konstantinović, I.; Bošković, B.; Šoda, J.; Dolić, K.; Đogaš, Z.; Lapčić, M.; Ledenko, V.; Vrgoč, T.; Rogić Vidaković, M. The Cortical Silent Period in the Cricothyroid Muscle as a Neurophysiologic Feature for Dystonia Observation: E-Field-Navigated Transcranial Magnetic (TMS) Study. Biomedicines 2023, 11, 1373. [Google Scholar] [CrossRef]
- Rogić Vidaković, M.; Konstantinović, I.; Bošković, B.; Šoda, J. Corticobulbar excitability in abductor laryngeal dystonia disease: The diagnostic role of transcranial magnetic stimulation. QJM 2023, 116, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Rogić Vidaković, M.; Gunjača, I.; Bukić, I.; Košta, V.; Šoda, J.; Konstantinović, I.; Bošković, B.; Bilić, I.; Režić Mužinić, N. The Patho-Neurophysiological Basis and Treatment of Focal Laryngeal Dystonia: A Narrative Review and Two Case Reports Applying TMS over the Laryngeal Motor Cortex. J. Clin. Med. 2022, 11, 3453. [Google Scholar] [CrossRef]
- Bütefisch, C.M.; Boroojerdi, B.; Chen, R.; Battaglia, F.; Hallett, M. Task-dependent intracortical inhibition is impaired in focal hand dystonia. Mov. Disord. 2005, 20, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Ganos, C.; Ferrè, E.R.; Marotta, A.; Kassavetis, P.; Rothwell, J.; Bhatia, K.P.; Haggard, P. Cortical inhibitory function in cervical dystonia. Clin. Neurophysiol. 2018, 129, 466–472. [Google Scholar] [CrossRef]
- Samargia, S.; Schmidt, R.; Kimberley, T.J. Shortened cortical silent period in adductor spasmodic dysphonia: Evidence for widespread cortical excitability. Neurosci. Lett. 2014, 560, 12–15. [Google Scholar] [CrossRef]
- McCambridge, A.B.; Bradnam, L.V. Cortical neurophysiology of primary isolated dystonia and non-dystonic adults: A meta-analysis. Eur. J. Neurosci. 2021, 53, 1300–1323. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.L.; Chen, M.; Kimberley, T.J. Probing the inhibitory motor circuits in adductor laryngeal dystonia during a dystonia-unrelated task. Park. Relat. Disord. 2023, 115, 105812. [Google Scholar] [CrossRef]
- Evidente, V.G.H.; Ponce, F.A.; Evidente, M.H.; Lambert, M.; Garrett, R.; Sugumaran, M.; Lott, D.G. Adductor Spasmodic Dysphonia Improves with Bilateral Thalamic Deep Brain Stimulation: Report of 3 Cases Done Asleep and Review of Literature. Tremor Other Hyperkinetic Mov. 2020, 10, 60. [Google Scholar] [CrossRef]
- Krüger, M.T.; Hu, A.; Honey, C.R. Deep Brain Stimulation for Spasmodic Dysphonia: A Blinded Comparison of Unilateral and Bilateral Stimulation in Two Patients. Ster. Funct. Neurosurg. 2020, 98, 200–205. [Google Scholar] [CrossRef]
- Poologaindran, A.; Ivanishvili, Z.; Morrison, M.D.; Rammage, L.A.; Sandhu, M.K.; Polyhronopoulos, N.E.; Honey, C.R. The effect of unilateral thalamic deep brain stimulation on the vocal dysfunction in a patient with spasmodic dysphonia: Interrogating cerebellar and pallidal neural circuits. J. Neurosurg. 2018, 128, 575–582. [Google Scholar] [CrossRef]
- Shoffel-Havakuk, H.; Marks, K.L.; Morton, M.; Johns, M.M., 3rd; Hapner, E.R. Validation of the OMNI vocal effort scale in the treatment of adductor spasmodic dysphonia. Laryngoscope 2019, 129, 448–453. [Google Scholar] [CrossRef]
- Stewart, C.F.; Allen, E.L.; Tureen, P.; Diamond, B.E.; Blitzer, A.; Brin, M.F. Adductor Spasmodic Dysphonia: Standard Evaluation of Symptoms and Severity. J. Voice 1997, 11, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Berke, G.S.; Blackwell, K.E.; Gerratt, B.R.; Verneil, A.; Jackson, K.S.; Sercarz, J.A. Selective laryngeal adductor denervation-reinnervation: A new surgical treatment for adductor spasmodic dysphonia. Ann. Otol. Rhinol. Laryngol. 1999, 108, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, D.K.; Mendelsohn, A.H.; Blumin, J.H.; Berke, G.S. Long-term follow-up results of selective laryngeal adductor denervation-reinnervation surgery for adductor spasmodic dysphonia. Laryngoscope 2006, 116, 635–642. [Google Scholar] [CrossRef] [PubMed]
- DeConde, A.S.; Long, J.L.; Armin, B.B.; Berke, G.S. Functional reinnervation of vocal folds after selective laryngeal adductor denervation-reinnervation surgery for spasmodic dysphonia. J. Voice 2012, 26, 602–603. [Google Scholar] [CrossRef]
- Long, J.L.; Berke, G.S. Selective laryngeal adductor denervation-reinnervation surgery for spasmodic dysphonia. Oper. Tech. Otolaryngol.-Head Neck Surg. 2012, 23, 183–187. [Google Scholar] [CrossRef]
- Dewan, K.; Gerald S Berke, G.S. Bilateral Vocal Fold Medialization: A Treatment for Abductor Spasmodic Dysphonia. J. Voice 2019, 33, 45–48. [Google Scholar] [CrossRef]
- Benito, D.A.; Ferster, A.P.O.; Sataloff, R.T. Bilateral Posterior Cricoarytenoid Myoneurectomy for Abductor Spasmodic Dysphonia. J. Voice 2020, 34, 127–129. [Google Scholar] [CrossRef]
- Nomoto, M.; Tokashiki, R.; Hiramatsu, H.; Konomi, U.; Motohashi, R.; Sakurai, E.; Toyomura, F.; Ueda, Y.; Inoue, S.; Tsukahara, K.; et al. The Comparison of Thyroarytenoid Muscle Myectomy and Type II Thyroplasty for Spasmodic Dysphonia. J. Voice 2015, 29, 501–506. [Google Scholar] [CrossRef]
- Gandhi, S.; Remacle, M.; Mishra, P.; Desai, V. Vocal outcome after endoscopic thyroarytenoid myoneurectomy in patients with adductor spasmodic dysphonia. Eur. Arch. Otorhinolaryngol. 2014, 271, 3249–3254. [Google Scholar] [CrossRef]
- Tsuji, D.H.; Takahashi, M.T.; Imamura, R.; Hachiya, A.; Sennes, L.U. Endoscopic laser thyroarytenoid myoneurectomy in patients with adductor spasmodic dysphonia: A pilot study on long-term outcome on voice quality. J. Voice 2012, 26, 666.e7–666.e12. [Google Scholar] [CrossRef]
- Nakamura, K.; Muta, H.; Watanabe, Y.; Mochizuki, R.; Yoshida, T.; Suzuki, M. Surgical treatment for adductor spasmodic dysphonia-efficacy of bilateral thyroarytenoid myectomy under microlaryngoscopy. Acta Otolaryngol. 2008, 128, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Sanuki, T.; Yumoto, E. Long-term Evaluation of Type 2 Thyroplasty with Titanium Bridges for Adductor Spasmodic Dysphonia. Otolaryngol. Head Neck Surg. 2017, 157, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Sanuki, T.; Eiji Yumoto, E.; Yutaka Toya, Y.; Kumai, Y. Voice tuning with new instruments for type II thyroplasty in the treatment of adductor spasmodic dysphonia. Auris Nasus Larynx 2016, 43, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, N.; Tsuji, D.H.; Yamamoto, Y.; Iizuka, Y. Midline lateralization thyroplasty for adductor spasmodic dysphonia. Ann. Otol. Rhinol. Laryngol. 2000, 109, 187–193. [Google Scholar] [CrossRef]
- Rosen, C.A.; Lee, A.S.; Osborne, J.; Zullo, T.; Murry, T. Development and validation of the voice handicap index-10. Laryngoscope 2004, 114, 1549–1556. [Google Scholar] [CrossRef]
- Hogikyan, N.D.; G Sethuraman, G. Validation of an instrument to measure voice-related quality of life (V-RQOL). J. Voice 1999, 13, 557–569. [Google Scholar] [CrossRef]
- Kempster, G.B.; Gerratt, B.R.; Verdolini Abbott, K.; Barkmeier-Kraemer, J.; Hillman, R.E. Consensus auditory-perceptual evaluation of voice: Development of a standardized clinical protocol. Am. J. Speech Lang Pathol. 2009, 18, 124–132. [Google Scholar] [CrossRef]
- Barsties, V.; Latoszek, B.; Mayer, J.; Watts, C.R.; Lehnert, B. Advances in Clinical Voice Quality Analysis with VOXplot. J. Clin. Med. 2023, 12, 4644. [Google Scholar] [CrossRef]
- Barsties, V.; Latoszek, B.; Lehnert, B.; Janotte, B. Validation of the Acoustic Voice Quality Index Version 03.01 and Acoustic Breathiness Index in German. J. Voice 2020, 34, 157.e17–157.e25. [Google Scholar] [CrossRef]
- Barsties, V.; Latoszek, B.; Mathmann, P.; Neumann, K. The cepstral spectral index of dysphonia, the acoustic voice quality index and the acoustic breathiness index as novel multiparametric indices for acoustic assessment of voice quality. Curr. Opin. Otolaryngol. Head Neck Surg. 2021, 29, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Boersma, P.; Weenink, D. Praat: Doing Phonetics by Computer [Computer Program]. Version 6.2.06. Available online: https://www.praat.org (accessed on 1 September 2023).
- Hlavnička, J.; Tykalová, T.; Ulmanová, O.; Dušek, P.; Horáková, D.; Růžička, E.; Klempíř, J.; Rusz, J. Characterizing vocal tremor in progressive neurological diseases via automated acoustic analyses. Clin. Neurophysiol. 2020, 131, 1155–1165. [Google Scholar] [CrossRef]
- Charlesworth, G.; Bhatia, K.P.; Wood, N.W. The genetics of dystonia: New twists in an old tale. Brain 2013, 136 Pt 7, 2017–2037. [Google Scholar] [CrossRef] [PubMed]
- Hersheson, J.; Mencacci, N.E.; Davis, M.; MacDonald, N.; Trabzuni, D.; Ryten, M.; Pittman, A.; Paudel, R.; Kara, E.; Fawcett, K.; et al. Mutations in the autoregulatory domain of β-tubulin 4a cause hereditary dystonia. Ann. Neurol. 2013, 73, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Putzel, G.G.; Fuchs, T.; Battistella, G.; Rubien-Thomas, E.; Frucht, S.J.; Blitzer, A.; Ozelius, L.J.; Simonyan, K. GNAL mutation in isolated laryngeal dystonia. Mov. Disord. 2016, 31, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.R.; Davis, R.L.; Tchan, M.C.; Wali, G.M.; Mahant, N.; Ng, K.; Kotschet, K.; Siow, S.-F.; Gu, J.; Walls, Z.; et al. Whole genome sequencing for the genetic diagnosis of heterogenous dystonia phenotypes. Park. Relat. Disord. 2019, 69, 111–118. [Google Scholar] [CrossRef]
- Putzel, G.G.; Battistella, G.; Rumbach, A.F.; Ozelius, L.J.; Sabuncu, M.R.; Simonyan, K. Polygenic risk of spasmodic dysphonia is associated with vulnerable sensorimotor connectivity. Cereb. Cortex 2018, 28, 158–166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogić Vidaković, M.; Šoda, J.; Kuluva, J.E.; Bošković, B.; Dolić, K.; Gunjača, I. Exploring Neurophysiological Mechanisms and Treatment Efficacies in Laryngeal Dystonia: A Transcranial Magnetic Stimulation Approach. Brain Sci. 2023, 13, 1591. https://doi.org/10.3390/brainsci13111591
Rogić Vidaković M, Šoda J, Kuluva JE, Bošković B, Dolić K, Gunjača I. Exploring Neurophysiological Mechanisms and Treatment Efficacies in Laryngeal Dystonia: A Transcranial Magnetic Stimulation Approach. Brain Sciences. 2023; 13(11):1591. https://doi.org/10.3390/brainsci13111591
Chicago/Turabian StyleRogić Vidaković, Maja, Joško Šoda, Joshua Elan Kuluva, Braco Bošković, Krešimir Dolić, and Ivana Gunjača. 2023. "Exploring Neurophysiological Mechanisms and Treatment Efficacies in Laryngeal Dystonia: A Transcranial Magnetic Stimulation Approach" Brain Sciences 13, no. 11: 1591. https://doi.org/10.3390/brainsci13111591
APA StyleRogić Vidaković, M., Šoda, J., Kuluva, J. E., Bošković, B., Dolić, K., & Gunjača, I. (2023). Exploring Neurophysiological Mechanisms and Treatment Efficacies in Laryngeal Dystonia: A Transcranial Magnetic Stimulation Approach. Brain Sciences, 13(11), 1591. https://doi.org/10.3390/brainsci13111591







