Evaluation of Antidepressant Activity of Capsaicin Nanoemulsion in Nicotine Withdrawal-Induced Depression in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Formulation of the Nanoemulsion of CAP
2.3. Evaluation of the Nanoemulsion
2.3.1. Determination of Droplet Size, Polydispersity Index (PDI), and Zeta Potential
2.3.2. Entrapment Efficiency (EE)
2.3.3. Production Yield
2.4. In Vivo Study
2.4.1. Experimental Animals
2.4.2. Induction of Depression in Mice
2.5. Behavioral Assessment
2.5.1. Tail Suspension Test (TST)
2.5.2. Force Swim Test (FST)
2.5.3. Open Field Test (OFT)
2.6. Biochemical Parameters
2.6.1. Evaluation of Superoxide Dismutase (SOD) and Reduced Glutathione (GSH)
2.6.2. Total Protein
2.6.3. Superoxide Dismutase (SOD)
Reagent Preparation
2.6.4. Reduced Glutathione (GSH)
Reagent Preparation
2.6.5. Lipid Peroxidation (MDA)
2.7. Statistical Analysis
3. Results
3.1. Characterization of CAP Nanoemulsion
3.1.1. Determination of Droplet Size and PDI
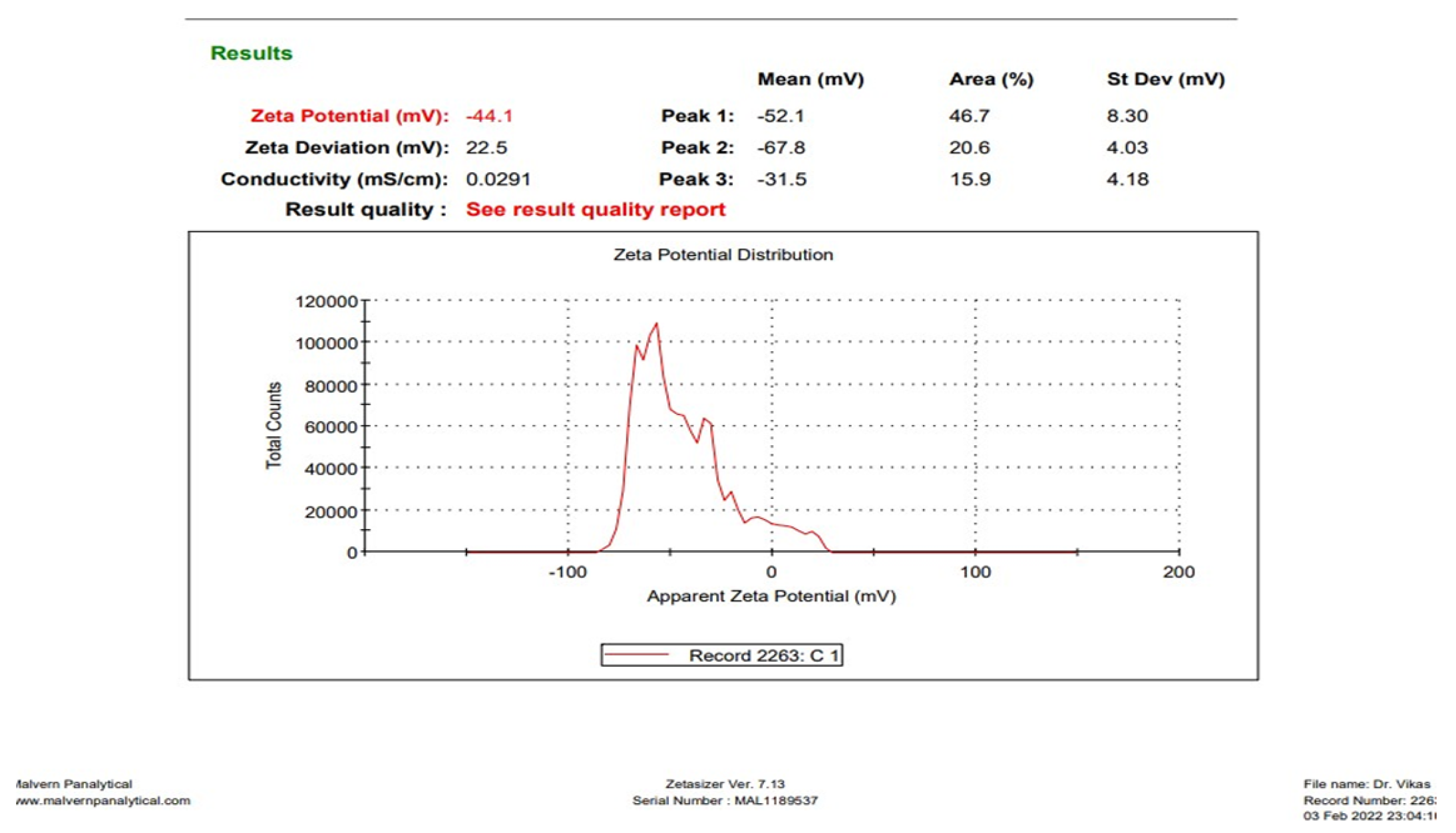
3.1.2. Determination of Zeta Potential
3.1.3. EE
3.1.4. Product Yield
3.2. Behavioral Assessments of NWM
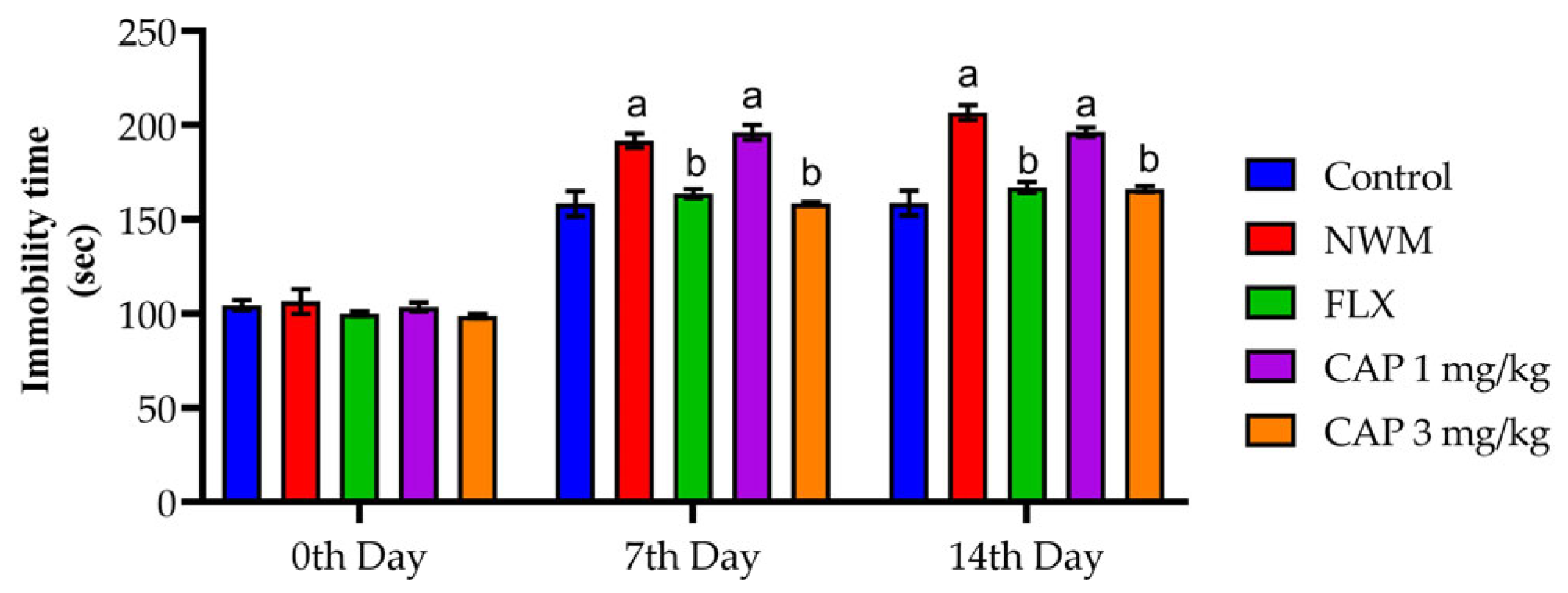
3.2.1. TST
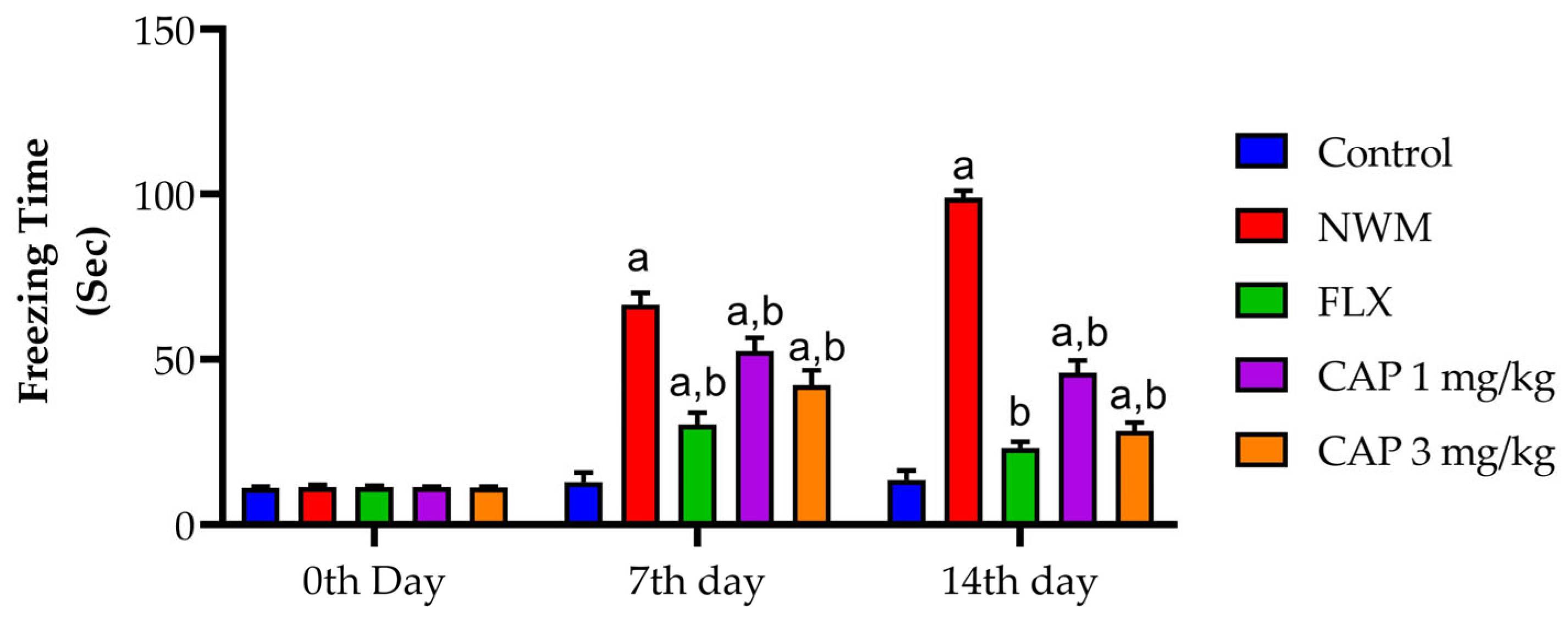
3.2.2. FST
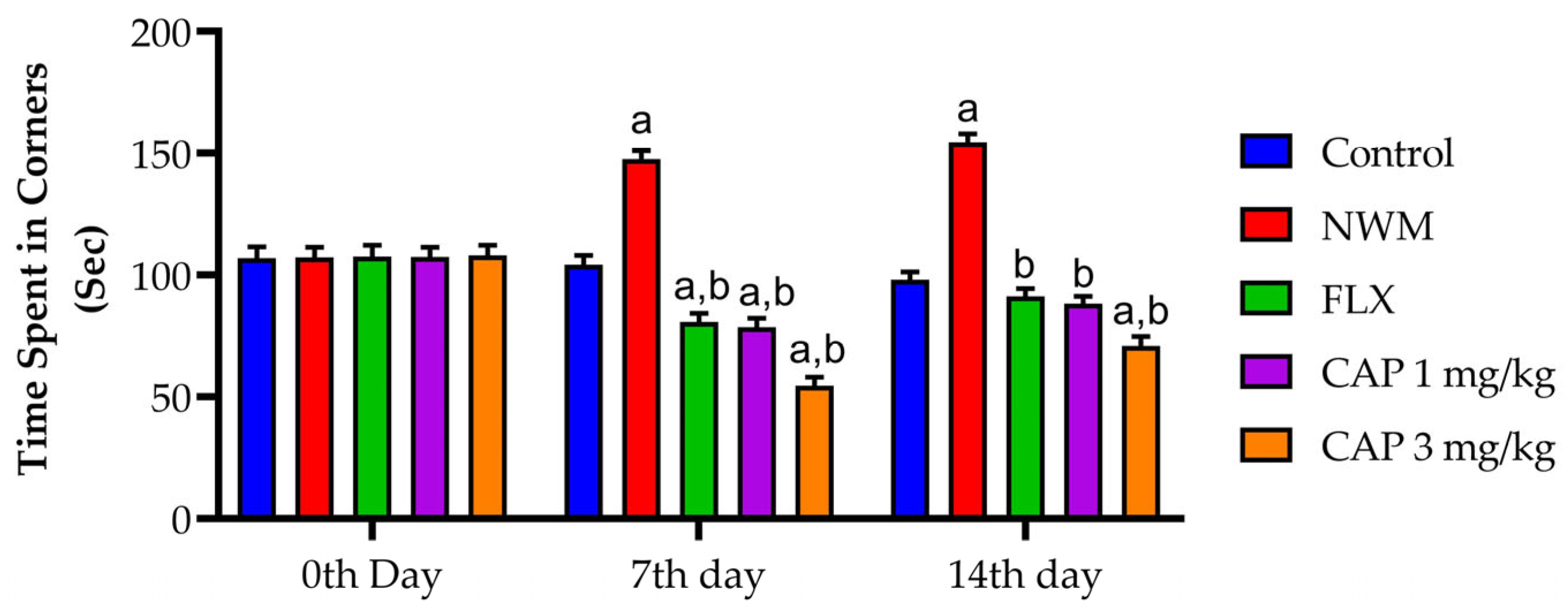
3.2.3. OFT
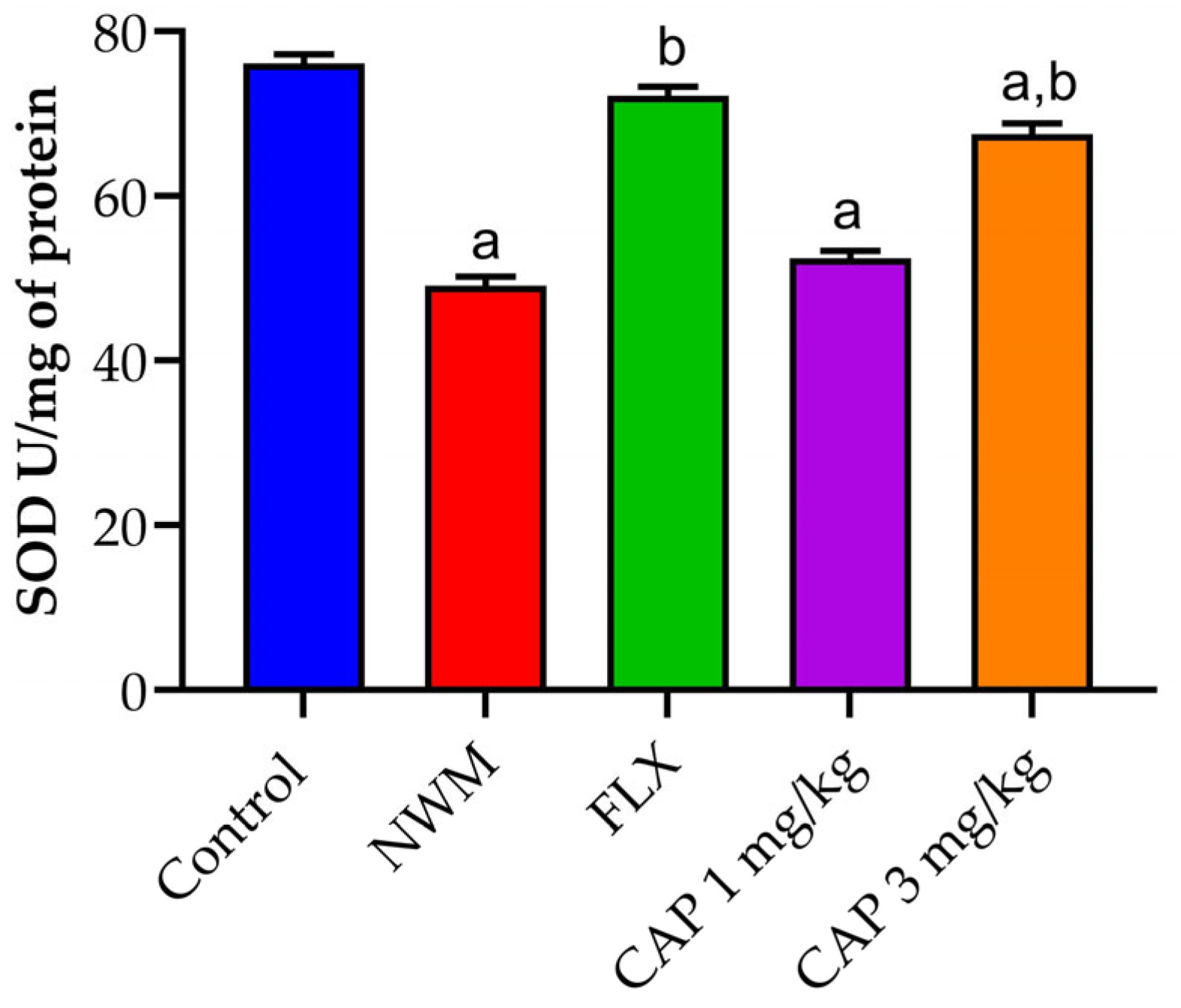
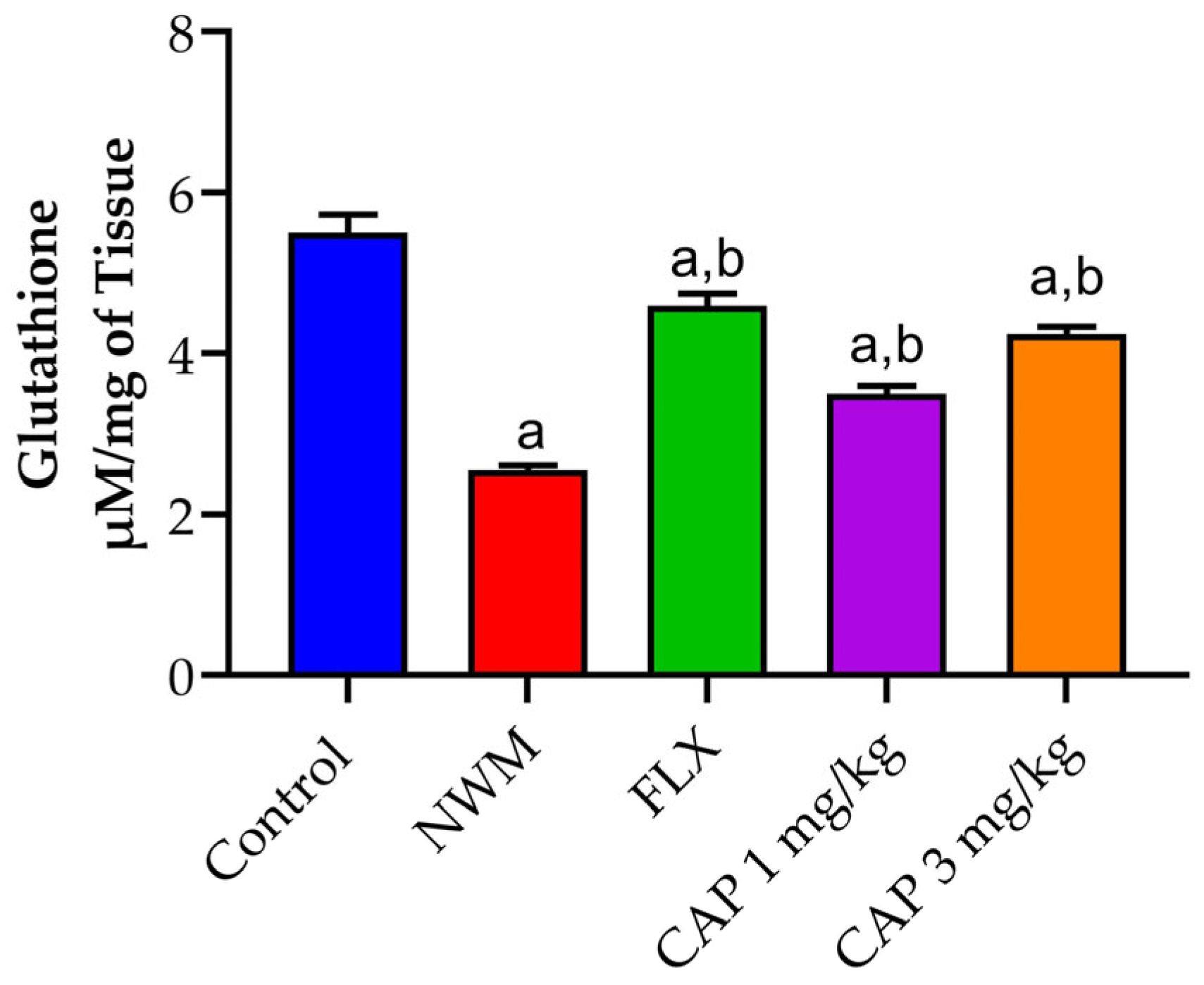
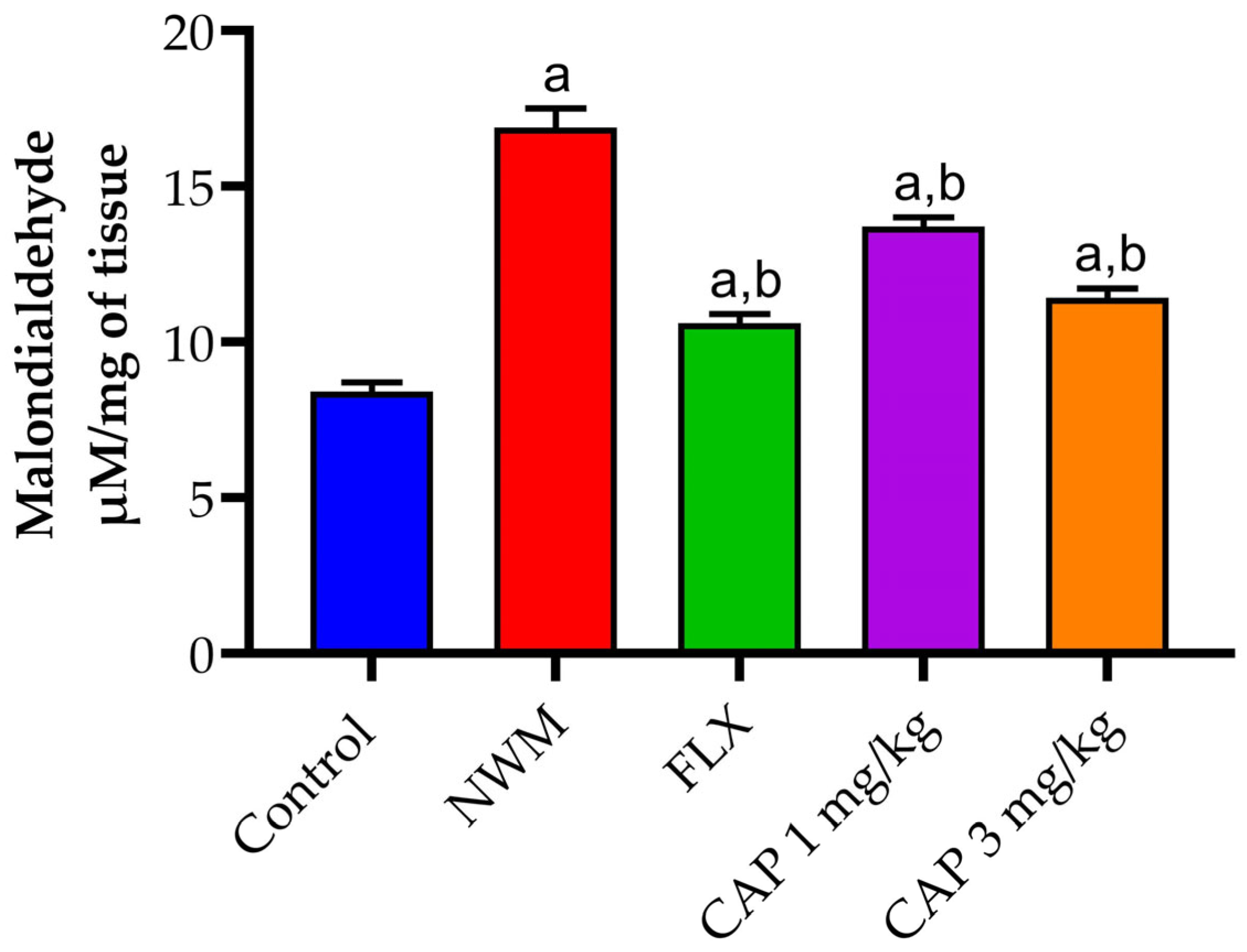
3.3. Antioxidant Markers
3.3.1. SOD
3.3.2. GSH
3.3.3. Lipid Peroxidation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazdin, A.E.; Rabbitt, S.M. Novel models for delivering mental health services and reducing the burdens of mental illness. Clin. Psychol. Sci. 2013, 1, 170–191. [Google Scholar] [CrossRef]
- Saraceno, B.; van Ommeren, M.; Batniji, R.; Cohen, A.; Gureje, O.; Mahoney, J.; Underhill, C. Barriers to improvement of mental health services in low-income and middle-income countries. Lancet 2007, 370, 1164–1174. [Google Scholar] [CrossRef]
- Horwitz, A.V. How an age of anxiety became an age of depression. Milbank Quart. 2010, 88, 112–138. [Google Scholar] [CrossRef] [PubMed]
- Duitama, M.; Vargas-López, V.; Casas, Z.; Albarracin, S.L.; Sutachan, J.J.; Torres, Y.P. TRP channels role in pain associated with neurodegenerative diseases. Front. Neurosci. 2020, 14, 782. [Google Scholar] [CrossRef]
- Chen, J.; Hamers, A.J.; Finsterbusch, M.; Massimo, G.; Zafar, M.; Corder, R.; Colas, R.A.; Dalli, J.; Thiemermann, C.; Ahluwalia, A. Endogenously generated arachidonate-derived ligands for TRPV1 induce cardiac protection in sepsis. FASEB J. 2018, 32, 3816–3831. [Google Scholar] [CrossRef]
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603. [Google Scholar] [PubMed]
- Tizabi, Y.; Hauser, S.R.; Tyler, K.Y.; Getachew, B.; Madani, R.; Sharma, Y.; Manaye, K.F. Effects of nicotine on depressive-like behavior and hippocampal volume of female WKY rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 62–69. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Hetta, H.F. Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef]
- Qadir, A.; Faiyazuddin, M.D.; Hussain, M.D.T.; Alshammari, T.M.; Shakeel, F. Critical steps and energetics involved in a successful development of a stable nanoemulsion. J. Mol. Liq. 2016, 214, 7–18. [Google Scholar] [CrossRef]
- Alam, P.; Ansari, M.J.; Anwer, M.K.; Raish, M.; Kamal, Y.K.T.; Shakeel, F. Wound healing effects of nanoemulsion containing clove essential oil. Art. Cells Nanomed. Biotechnol. 2017, 45, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Shakeel, F.; Anwer, M.K.; Foudah, A.I.; Alqarni, M.H. Wound healing study of eucalyptus essential oil containing nanoemulsion in rat model. J. Oleo Sci. 2018, 67, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Salem-Bekhit, M.M.; Haq, N.; Alshehri, S. Nanoemulsification improves the pharmaceutical properties and bioactivities of niaouli essential oil (Melaleuca quinquenervia L.). Molecules 2021, 26, 4750. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, W.A.; Alam, P.; Alshetaili, A.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. Product development studies of cranberry seed oil nanoemulsion. Processes 2022, 10, 393. [Google Scholar] [CrossRef]
- Al-Samydai, A.; Alshaer, W.; Al-Dujaili, E.A.S.; Azzam, S.; Alburjai, T. Preparation, characterization, and anticancer effects of capsaicin-loaded nanoliposomes. Nutrients 2021, 13, 3995. [Google Scholar] [CrossRef] [PubMed]
- Shamsheer, R.; Sunoqrot, S.; Kasabri, V.; Shalabi, D.; Alkhateeb, R.; Alhiari, Y.; Ababneh, R.; Ikhmais, B.; Abumansour, H. Preparation and characterization of capsaicin encapsulated polymeric micelles and studies of synergism with nicotinic acids as potential anticancer nanomedicines. J. Pharm. Bioall. Sci. 2023, 15, 107–125. [Google Scholar] [CrossRef]
- Agrawal, U.; Gupta, M.; Vyas, S.P. Capsaicin delivery into the skin with lipidic nanoparticles for the treatment of psoriasis. Art. Cells Nanomed. Biotechnol. 2015, 43, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Anantaworasakul, P.; Chaiyana, W.; Michniak-Kohn, B.B.; Rungseevijitprapa, W.; Ampasavate, C. Enhanced transdermal delivery of concentrated capsaicin from chili extract-loaded lipid nanoparticles with reduced skin irritation. Pharmaceutics 2020, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Kunjiappan, S.; Sankaranarayanan, M.; Kumar, B.K.; Pavadai, P.; Babkiewicz, E.; Maszczyk, P.; Glodkowska-Mrowka, E.; Arunachalam, S.; Pandian, S.R.K.; Ravishankar, V. Capsaicin-loaded solid lipid nanoparticles: Design, biodistribution, in silico modeling and in vitro cytotoxicity evaluation. Nanotechnology 2021, 32, E095101. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Jiang, X.-Y.; Zhu, Y.; Omari-Siaw, E.; Deng, W.-W.; Yu, J.-N.; Xu, X.-M.; Zhang, W.-M. Oral delivery of capsaicin using MPEG-PCL nanoparticles. Acta Pharmacol. Sin. 2015, 36, 139–148. [Google Scholar] [CrossRef]
- Hazem, N.M.; ElKashef, W.F.; El-Sherbiny, I.M.; Eman, A.A.; Shaalan, D.; Sobh, M. Anticarcinogenic effects of capsaicin-loaded nanoparticles on in vitro hepatocellular carcinoma. Curr. Chem. Biol. 2021, 15, 188–201. [Google Scholar] [CrossRef]
- Carriles, R.; Zavala-Garcia, L.E.; Nova-Coronel, S.; Sanchez-Arreguin, A.; Lopez, M.G.; Sanchez-Segura, L. Post-synthesis nanostructuration of BSA-capsaicin nanoparticles generated by sucrose excipient. Sci. Rep. 2021, 11, E7549. [Google Scholar] [CrossRef]
- Mudhol, S.; Peddha, M.S. Development of capsaicin loaded nanoparticles based microneedle patch for transdermaldrug delivery. J. Drug Deliv. Sci. Technol. 2023, 80, E104120. [Google Scholar] [CrossRef]
- Wang, X.-R.; Gao, S.-Q.; Niu, X.-Q.; Li, L.-J.; Ying, X.-Y.; Hu, Z.-J.; Gao, J.-Q. Capsaicin-loaded nanolipoidal carriers for topical application: Design, characterization, and in vitro/in vivo evaluation. Int. J. Nanomed. 2015, 12, 3881–3898. [Google Scholar] [CrossRef] [PubMed]
- Anantaworasakul, P.; Anuchapreeda, S.; Yotsawimonwat, S.; Naksuriya, O.; Lekawanvijit, S.; Tovanabutra, N.; Anantaworasakul, P.; Wattanasri, W.; Buranapreecha, N.; Ampasavate, C. Nanomaterial lipid-based carrier for non-invasive capsaicin delivery; manufacturing scale-up and human irritation assessment. Molecules 2020, 25, 5575. [Google Scholar] [CrossRef] [PubMed]
- Amruthraj, N.J.; Raj, J.P.P.; Lebel, A. Capsaicin-capped silver nanoparticles: Its kinetics, characterization and biocompatibility assay. Appl. Nanosci. 2015, 5, 403–409. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, J.A.; Kim, J.T.; Cha, D.S.; Cho, J.H.; Park, H.J.; Shin, G.H. Preparation of a capsaicin-loaded nanoemulsion for improving skin penetration. J. Food Agric. Chem. 2014, 62, 725–732. [Google Scholar] [CrossRef]
- Dudi, M.; Kumar, R.; Saini, P.; Sharma, A.; Pandey, S.K.; Kaur, H.; Kumar, R.; Mehta, S.K. Ultrasonication induced osthole loaded nanoemulsion: A medium with superior antioxidant activity, antimicrobial activity and improved cell viability against caco-2 cells. J. Drug Deliv. Sci. Technol. 2023, 84, E104484. [Google Scholar] [CrossRef]
- Peters, K.; Unger, R.E.; Kirkpatrick, C.J.; Gatti, A.M.; Monari, E. Effects of nano-scaled particles on endothelial cell function in vitro: Studies on viability, proliferation and inflammation. J. Mater. Sci. Mater. Med. 2004, 15, 321–325. [Google Scholar] [CrossRef]
- Abushal, A.S.; Aleanizy, F.S.; Alqahtani, F.Y.; Shakeel, F.; Iqbal, M.; Haq, N.; Alsarra, I.A. Self-nanoemulsifying drug delivery system (SNEDDS) of apremilast: In vitro evaluation and pharmacokinetics studies. Molecules 2022, 27, 3085. [Google Scholar] [CrossRef]
- Guo, C.-L.; Chen, H.-Y.; Cui, B.-L.; Chen, Y.-H.; Zhou, Y.-F.; Peng, X.-S.; Wang, Q. Development of a HPLC method for the quantitative determination of capsaicin in collagen sponge. Int. J. Anal. Chem. 2015, 2015, E912631. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; Alkahtani, H.M.; El Tahir, E.; Ansari, S.A.; Alharbi, A.; Al-Bdrawy, A.; Shakeel, F.; Haq, N.; Al-Rasheed, L.S.; et al. Chitosan loaded RNA polymerase inhibitor nanoparticles increased attenuation in toxin release from Streptococcus pneumonia. Saudi Pharm. J. 2023, 31, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Ruengdech, A.; Siripatrawan, U. Improving encapsulating efficiency, stability, and antioxidant activity of catechin nanoemulsion using foam mat freeze-drying: The effect of wall material types and concentrations. LWT Food Sci. Technol. 2022, 162, E113478. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, E3769. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The mouse forced swim test. J. Vis. Exp. 2012, 59, E3638. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, W.C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, E52434. [Google Scholar]
- Khan, R.A.; Khan, M.R.; Sahreen, H. Brain antioxidant markers, cognitive performance and acetylcholinesterase activity of rats: Efficiency of Sonchus asper. Behav. Brain Funct. 2012, 8, E21. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Ind. J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Mehdi, S.; Manohar, K.; Shariff, A.; Kinattingal, N.; Wani, S.U.D.; Alshehri, S.; Imam, M.T.; Shakeel, F.; Krishna, K.L. Omega-3 fatty acids supplementation in the treatment of depression: An observational study. J. Pers. Med. 2023, 13, 224. [Google Scholar] [CrossRef]
- Muntteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Alzoghaibi, M.A.; Al-Mofleh, I.A.; Al-Jebreen, A.M. Antioxidant activities for superoxide dismutase in patients with Crohn’s disease. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 59–62. [Google Scholar] [CrossRef]
- Morris, G.; Anderson, G.; Dean, O.; Berk, M.; Galecki, P.; Martin-Subero, M.; Maes, M. The glutathione system: A new drug target in neuroimmune disorders. Mol. Neurobiol. 2014, 50, 1059–1084. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Furman, R.; Axelsen, P.H.; Shchepinov, M.S. Free radical chain reactions and polyunsaturated fatty acids in brain lipids. ACS Omega 2022, 7, 25337–25345. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Yin, T.; Shinozaki, K.; Lampe, J.W.; Stevens, J.F.; Becker, L.B.; Kim, J. Comprehensive analysis of phospholipids in the brain, heart, kidney, and liver: Brain phospholipids are least enriched with polyunsaturated fatty acids. Mol. Cell. Biochem. 2018, 442, 187–201. [Google Scholar] [CrossRef]
- Jove, M.; Mota-Martorell, N.; Obis, E.; Sol, J.; Martin-Gari, M.; Ferrer, I.; Portero-Otin, M.; Pamplona, R. Lipid adaptations against oxidative challenge in the healthy adult human brain. Antioxidants 2023, 12, 177. [Google Scholar] [CrossRef]
- Wani, A.L.; Bhat, S.A.; Ara, A. Omega-3 fatty acid and the treatment of depression: A review of scientific evidence. Integer. Med. Res. 2015, 4, 132–141. [Google Scholar] [CrossRef]
- Mehdi, S.; Manohar, K.; Shariff, A.; Wani, S.U.D.; Almuqbil, M.; Alshehri, S.; Shakeel, F.; Imam, M.T.; Krishna, K.L. Analysis of antidepressants utilization for patients visiting psychiatric out-patient clinic in a tertiary care hospital. Healthcare 2022, 10, 2081. [Google Scholar] [CrossRef]
- Basavaraju, S.M.; Mudhol, S.; Peddha, M.S.; Wani, S.U.D.; Krishna, K.L.; Mehdi, S.; Kinattingal, N. Nanoemulsion-based piperine to enhance bioavailability for the treatment of LPS-induced depression-like behaviour in mice. Neurosci. Lett. 2023, 814, E137441. [Google Scholar] [CrossRef]


| S. No. | Group (n = 6) | Treatment |
|---|---|---|
| 1 | Control | NC (2 mg/kg [s.c.] thrice a day up to 7 days + CMC 1% [10 mL/kg p.o] once in a day up to 14 days) was administered |
| 2 | NWM | NC (2 mg/kg [s.c.] thrice a day up to 7 days + CMC 1% [10 mL/kg p.o] once in a day up to 14 days) was administered |
| 3 | FLX | NC (2 mg/kg [s.c.] thrice a day up to 7 days + FLX [20 mg/kg p.o] once in a day up to 14 days) was administered |
| 4 | CAP 1 mg/kg | NC (2 mg/kg [s.c.] thrice a day up to 7 days + CAP low dose [1 mg/kg p.o] once in a day up to 14 days) was administered |
| 5 | CAP 3 mg/kg | NC (2 mg/kg [s.c.] thrice a day up to 7 days + CAP [3 mg/kg p.o] once in a day up to 14 days) was administered |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnamoorthy, N.K.; Roohi, T.F.; Peddha, M.S.; Kinattingal, N.; Wani, S.U.D.; Krishna, K.L.; Shakeel, F.; Mehdi, S. Evaluation of Antidepressant Activity of Capsaicin Nanoemulsion in Nicotine Withdrawal-Induced Depression in Mice. Brain Sci. 2023, 13, 1668. https://doi.org/10.3390/brainsci13121668
Krishnamoorthy NK, Roohi TF, Peddha MS, Kinattingal N, Wani SUD, Krishna KL, Shakeel F, Mehdi S. Evaluation of Antidepressant Activity of Capsaicin Nanoemulsion in Nicotine Withdrawal-Induced Depression in Mice. Brain Sciences. 2023; 13(12):1668. https://doi.org/10.3390/brainsci13121668
Chicago/Turabian StyleKrishnamoorthy, Naveen Kumar, Tamsheel Fatima Roohi, Muthukumar Serva Peddha, Nabeel Kinattingal, Shahid Ud Din Wani, Kamsagara Linganna Krishna, Faiyaz Shakeel, and Seema Mehdi. 2023. "Evaluation of Antidepressant Activity of Capsaicin Nanoemulsion in Nicotine Withdrawal-Induced Depression in Mice" Brain Sciences 13, no. 12: 1668. https://doi.org/10.3390/brainsci13121668
APA StyleKrishnamoorthy, N. K., Roohi, T. F., Peddha, M. S., Kinattingal, N., Wani, S. U. D., Krishna, K. L., Shakeel, F., & Mehdi, S. (2023). Evaluation of Antidepressant Activity of Capsaicin Nanoemulsion in Nicotine Withdrawal-Induced Depression in Mice. Brain Sciences, 13(12), 1668. https://doi.org/10.3390/brainsci13121668












