Cortical GABA Levels Are Reduced in Post-Acute COVID-19 Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
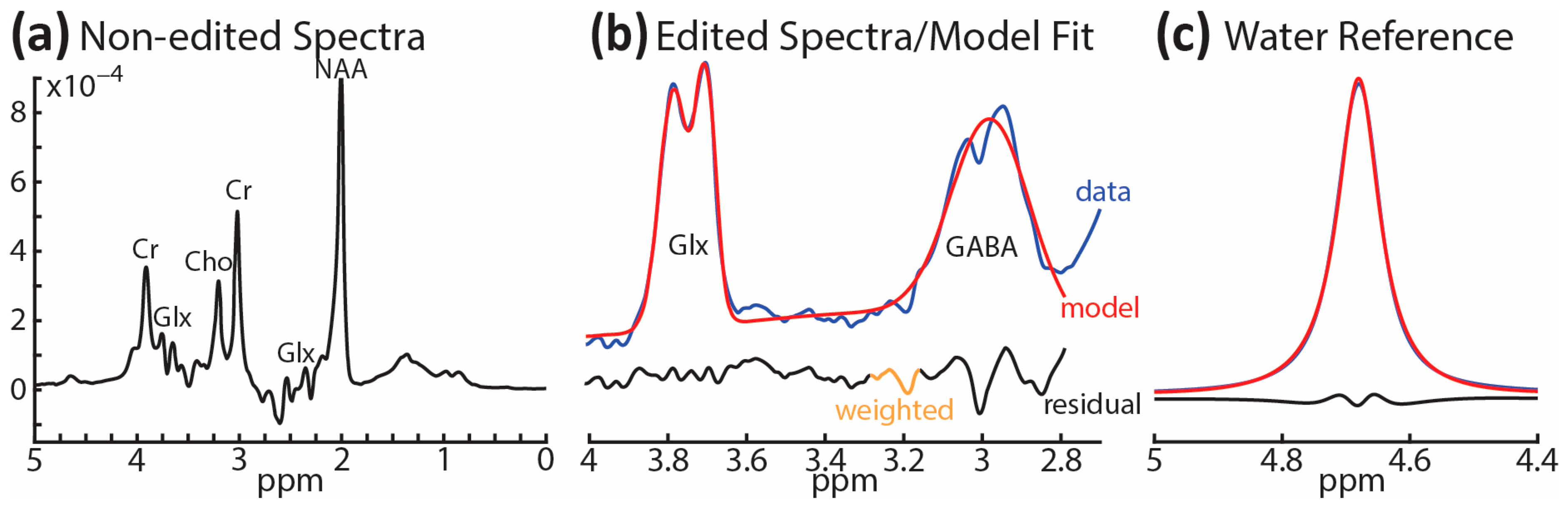
2.3. 1H-MRS and Structural MRI Acquisition
2.4. 1H-MRS Modeling and Analysis, and VOI Tissue Segmentation
2.5. Statistical Analysis
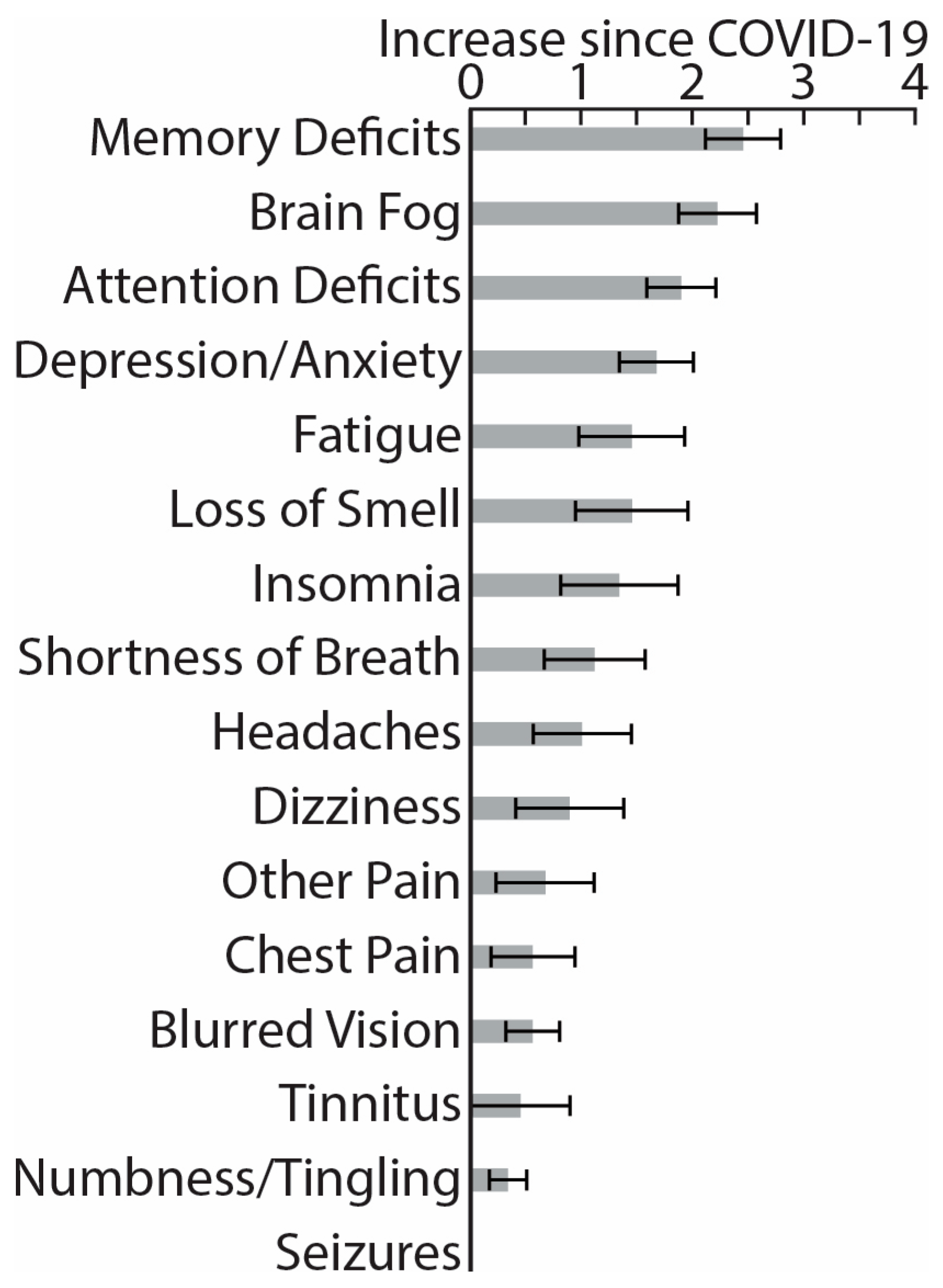
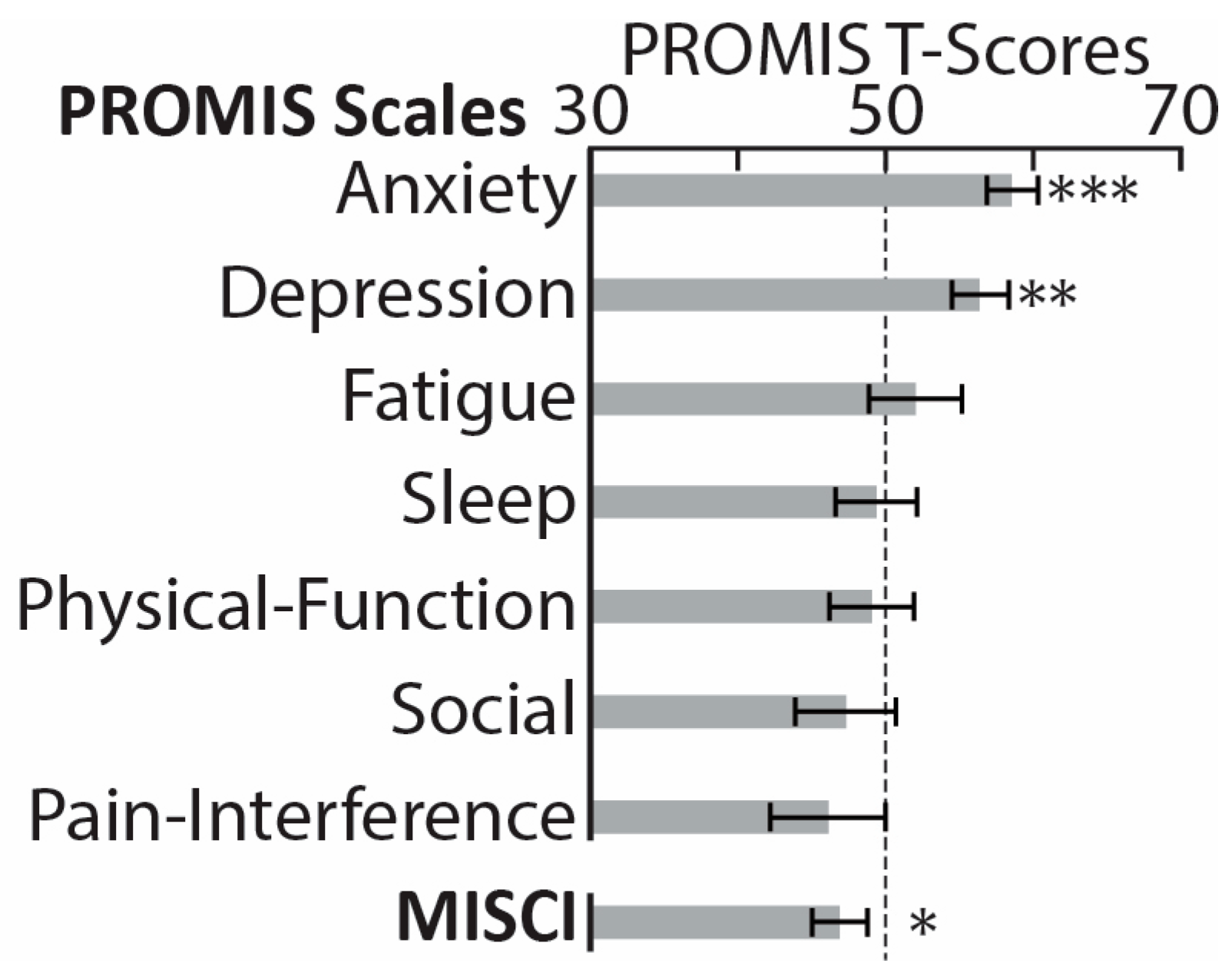
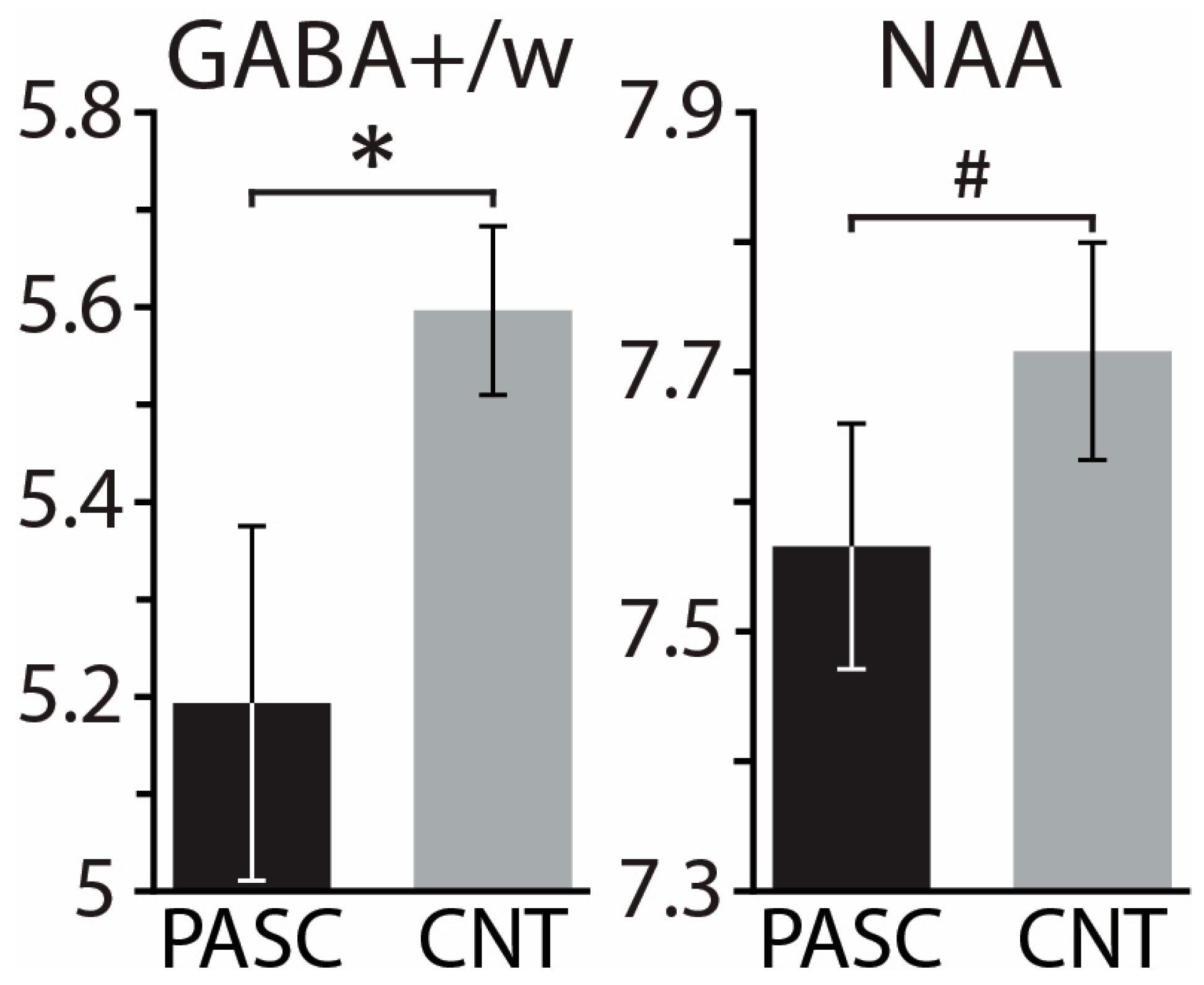
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cutler, D.M.; Summers, L.H. The COVID-19 Pandemic and the $16 Trillion Virus. JAMA 2020, 324, 1495–1496. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.P. Estimating total morbidity burden of COVID-19: Relative importance of death and disability. J. Clin. Epidemiol. 2022, 142, 54–59. [Google Scholar] [CrossRef]
- CDC. Long COVID or Post-COVID Conditions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 3 August 2023).
- DHHS. National Research Action Plan on Long COVID; Office of the Assistant Secretary for Health: Washington, DC, USA, 2022.
- Zadeh, F.H.; Wilson, D.R.; Agrawal, D.K. Long COVID: Complications, Underlying Mechanisms, and Treatment Strategies. Arch. Microbiol. Immunol. 2023, 7, 36–61. [Google Scholar] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Kuroda, N.; Sone, D. Neuropsychiatric aspects of long COVID: A comprehensive review. Psychiatry Clin. Neurosci. 2023, 77, 84–93. [Google Scholar] [CrossRef]
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: A systematic review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef] [PubMed]
- Thaweethai, T.; Jolley, S.E.; Karlson, E.W.; Levitan, E.B.; Levy, B.; McComsey, G.A.; McCorkell, L.; Nadkarni, G.N.; Parthasarathy, S.; Singh, U.; et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA 2023, 329, 1934–1946. [Google Scholar] [CrossRef]
- Henneghan, A.M.; Lewis, K.A.; Gill, E.; Kesler, S.R. Cognitive impairment in non-critical, mild-to-moderate COVID-19 survivors. Front. Psychol. 2022, 13, 770459. [Google Scholar] [CrossRef]
- Schild, A.-K.; Goereci, Y.; Scharfenberg, D.; Klein, K.; Lülling, J.; Meiberth, D.; Schweitzer, F.; Stürmer, S.; Zeyen, P.; Sahin, D. Multidomain cognitive impairment in non-hospitalized patients with the post-COVID-19 syndrome: Results from a prospective monocentric cohort. J. Neurol. 2023, 270, 1215–1223. [Google Scholar] [CrossRef]
- Munipalli, B.; Seim, L.; Dawson, N.L.; Knight, D.; Dabrh, A.M.A. Post-acute sequelae of COVID-19 (PASC): A meta-narrative review of pathophysiology, prevalence, and management. SN Compr. Clin. Med. 2022, 4, 90. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Postacute sequelae of COVID-19 at 2 years. Nat. Med. 2023, 29, 2347–2357. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Kondratiuk, A.L.; Pillay, T.D.; Kon, O.M.; Lalvani, A. A conceptual framework to accelerate the clinical impact of evolving research into long COVID. Lancet Infect. Dis. 2021, 21, 756–757. [Google Scholar] [CrossRef]
- Komaroff, A.L.; Bateman, L. Will COVID-19 Lead to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Front. Med. 2020, 7, 606824. [Google Scholar] [CrossRef]
- Bougakov, D.; Podell, K.; Goldberg, E. Multiple Neuroinvasive Pathways in COVID-19. Mol. Neurobiol. 2021, 58, 564–575. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J.; Kamel, H. Effects of COVID-19 on the Nervous System. Cell 2020, 183, 16–27.e11. [Google Scholar] [CrossRef]
- Haroon, E.; Miller, A.H.; Sanacora, G. Inflammation, Glutamate, and Glia: A Trio of Trouble in Mood Disorders. Neuropsychopharmacology 2017, 42, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Maggio, N.; Shavit-Stein, E.; Dori, A.; Blatt, I.; Chapman, J. Prolonged systemic inflammation persistently modifies synaptic plasticity in the hippocampus: Modulation by the stress hormones. Front. Mol. Neurosci. 2013, 6, 46. [Google Scholar] [CrossRef]
- Manganotti, P.; Michelutti, M.; Furlanis, G.; Deodato, M.; Stella, A.B. Deficient GABABergic and glutamatergic excitability in the motor cortex of patients with long-COVID and cognitive impairment. Clin. Neurophysiol. 2023, 151, 83–91. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Shaikh, M.F.; Shah, S.; Kumari, Y.; Othman, I. Role of inflammation in epilepsy and neurobehavioral comorbidities: Implication for therapy. Eur. J. Pharmacol. 2018, 837, 145–155. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Mondelli, V.; Pariante, C.M. What can neuroimmunology teach us about the symptoms of long-COVID? Oxf. Open Immunol. 2021, 2, iqab004. [Google Scholar] [CrossRef]
- Heneka, M.; Golenbock, D.; Latz, E.; Morgan, D.; Brown, R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res. Ther. 2020, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.; Podell, K.; Sodickson, D.K.; Fieremans, E. The brain after COVID-19: Compensatory neurogenesis or persistent neuroinflammation? EClinicalMedicine 2021, 31, 100684. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; Palladini, M.; De Lorenzo, R.; Magnaghi, C.; Poletti, S.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; Benedetti, F.; COVID-19 BioB Outpatient Clinic Study Group. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021, 94, 138–147. [Google Scholar] [CrossRef]
- Loftis, J.M.; Firsick, E.; Shirley, K.; Adkins, J.L.; Le-Cook, A.; Sano, E.; Hudson, R.; Moorman, J. Inflammatory and mental health sequelae of COVID-19. Compr. Psychoneuroendocrinology 2023, 15, 100186. [Google Scholar] [CrossRef]
- Braga, J.; Lepra, M.; Kish, S.J.; Rusjan, P.M.; Nasser, Z.; Verhoeff, N.; Vasdev, N.; Bagby, M.; Boileau, I.; Husain, M.I. Neuroinflammation After COVID-19 With Persistent Depressive and Cognitive Symptoms. JAMA Psychiatry 2023, 80, 787–795. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Zhang, Z.; Wang, Z.; Li, H. Cognitive impairment after long COVID-19: Current evidence and perspectives. Front. Neurol. 2023, 14, 1239182. [Google Scholar] [CrossRef] [PubMed]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Maes, M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: A proof of concept and mechanism study. Mol. Psychiatry 2023, 28, 564–578. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef]
- Amzica, F.; Lopes da Silva, F.H. Cellular substrates of brain rhythms. In Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields; Schomer, D., Lopes da Silva, F.H., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 33–63. [Google Scholar]
- Buzsaki, G. Rhythms of the Brain; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Knight, R.T. Neuroscience. Neural networks debunk phrenology. Science 2007, 316, 1578–1579. [Google Scholar] [CrossRef][Green Version]
- Haider, B.; Duque, A.; Hasenstaub, A.R.; McCormick, D.A. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J. Neurosci. 2006, 26, 4535–4545. [Google Scholar] [CrossRef]
- Tatti, R.; Haley, M.S.; Swanson, O.K.; Tselha, T.; Maffei, A. Neurophysiology and Regulation of the Balance Between Excitation and Inhibition in Neocortical Circuits. Biol. Psychiatry 2017, 81, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes. Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Sohal, V.S.; Rubenstein, J.L. Excitation-inhibition balance as a framework for investigating mechanisms in neuropsychiatric disorders. Mol. Psychiatry 2019, 24, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Selten, M.; van Bokhoven, H.; Nadif Kasri, N. Inhibitory control of the excitatory/inhibitory balance in psychiatric disorders. F1000Res 2018, 7, 23. [Google Scholar] [CrossRef]
- Gao, R.; Penzes, P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr. Mol. Med. 2015, 15, 146–167. [Google Scholar] [CrossRef]
- Marin, O. Interneuron dysfunction in psychiatric disorders. Nat. Rev. Neurosci. 2012, 13, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Prichard, J.W.; Shulman, R.G. NMR spectroscopy of brain metabolism in vivo. Annu. Rev. Neurosci. 1986, 9, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Radda, G.K.; Rajagopalan, B.; Taylor, D.J. Biochemistry in vivo: An appraisal of clinical magnetic resonance spectroscopy. Magn. Reson. Q. 1989, 5, 122–151. [Google Scholar]
- Bluml, S. Magnetic resonance spectroscopy: Basics. In MR Spectroscopy of Pediatric Brain Disorders; Bluml, S., Panigrahy, A., Eds.; Springer: New York, NY, USA, 2012; pp. 11–23. [Google Scholar]
- Cox, I.J. Development and applications of in vivo clinical magnetic resonance spectroscopy. Prog. Biophys. Mol. Biol. 1996, 65, 45–81. [Google Scholar] [CrossRef]
- Ende, G. Proton Magnetic Resonance Spectroscopy: Relevance of Glutamate and GABA to Neuropsychology. Neuropsychol. Rev. 2015, 25, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.D.; Saleh, M.G.; Edden, R.A. Edited (1) H magnetic resonance spectroscopy in vivo: Methods and metabolites. Magn. Reson. Med. 2017, 77, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.; Kwak, H.; Cheong, E.; Lee, C.J. GABA tone regulation and its cognitive functions in the brain. Nat. Rev. Neurosci. 2023, 24, 523–539. [Google Scholar] [CrossRef]
- Tremblay, R.; Lee, S.; Rudy, B. GABAergic interneurons in the neocortex: From cellular properties to circuits. Neuron 2016, 91, 260–292. [Google Scholar] [CrossRef]
- Markram, H.; Toledo-Rodriguez, M.; Wang, Y.; Gupta, A.; Silberberg, G.; Wu, C. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 2004, 5, 793–807. [Google Scholar] [CrossRef]
- Roux, L.; Buzsaki, G. Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology 2015, 88, 10–23. [Google Scholar] [CrossRef]
- DeFelipe, J.; Alonso-Nanclares, L.; Arellano, J.I. Microstructure of the neocortex: Comparative aspects. J. Neurocytol. 2002, 31, 299–316. [Google Scholar] [CrossRef]
- Kolasinski, J.; Logan, J.P.; Hinson, E.L.; Manners, D.; Zand, A.P.D.; Makin, T.R.; Emir, U.E.; Stagg, C.J. A mechanistic link from GABA to cortical architecture and perception. Curr. Biol. 2017, 27, 1685–1691.e1683. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O’Shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef]
- Schur, R.R.; Draisma, L.W.; Wijnen, J.P.; Boks, M.P.; Koevoets, M.G.; Joels, M.; Klomp, D.W.; Kahn, R.S.; Vinkers, C.H. Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of (1) H-MRS studies. Hum. Brain Mapp. 2016, 37, 3337–3352. [Google Scholar] [CrossRef]
- Mescher, M.; Merkle, H.; Kirsch, J.; Garwood, M.; Gruetter, R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998, 11, 266–272. [Google Scholar] [CrossRef]
- Petroff, O.A.; Hyder, F.; Rothman, D.L.; Mattson, R.H. Homocarnosine and seizure control in juvenile myoclonic epilepsy and complex partial seizures. Neurology 2001, 56, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, K.; Alderson Myers, A.B.; Arienzo, D.; Sereno, M.I.; Mason, G.F. Cortical GABA levels are reduced in young adult binge drinkers: Association with recent alcohol consumption and sex. Neuroimage Clin. 2022, 35, 103091. [Google Scholar] [CrossRef] [PubMed]
- Rae, C.D. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem. Res. 2014, 39, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.G.; Chang, L.; Liang, H.; Ryan, M.C.; Cunningham, E.; Garner, J.; Wilson, E.; Levine, A.R.; Kottilil, S.; Ernst, T. Ongoing oxidative stress in individuals with post-acute sequelae of COVID-19. Neuroimmune Pharmacol. Ther. 2023, 2, 89–94. [Google Scholar] [CrossRef]
- Sanacora, G.; Mason, G.F.; Krystal, J.H. Impairment of GABAergic transmission in depression: New insights from neuroimaging studies. Crit. Rev. Neurobiol. 2000, 14, 23–45. [Google Scholar] [CrossRef]
- Sanacora, G.; Gueorguieva, R.; Epperson, C.N.; Wu, Y.-T.; Appel, M.; Rothman, D.L.; Krystal, J.H.; Mason, G.F. Subtype-specific alterations of γ-aminobutyric acid and glutamatein patients with major depression. Arch. Gen. Psychiat. 2004, 61, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G.; van der Veen, J.W.; Tumonis, T.; Meyers, N.; Shen, J.; Drevets, W.C. Reduced prefrontal glutamate/glutamine and γ-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch. Gen. Psychiat. 2007, 64, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Bhagwagar, Z.; Wylezinska, M.; Jezzard, P.; Evans, J.; Ashworth, F.; Sule, A.; Matthews, P.M.; Cowen, P.J. Reduction in occipital cortex γ-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biol. Psychiat. 2007, 61, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Shungu, D.C.; Mao, X.; Nestadt, P.; Kelly, C.; Collins, K.A.; Murrough, J.W.; Charney, D.S.; Mathew, S.J. Amino acid neurotransmitters assessed by 1H MRS: Relationship to treatment-resistance in major depressive disorder. Biol. Psychiat. 2009, 65, 792. [Google Scholar] [CrossRef] [PubMed]
- Crowley, T.; Cryan, J.F.; Downer, E.J.; O’Leary, O.F. Inhibiting neuroinflammation: The role and therapeutic potential of GABA in neuro-immune interactions. Brain Behav. Immun. 2016, 54, 260–277. [Google Scholar] [CrossRef]
- Tian, J.; Kaufman, D.L. The GABA and GABA-Receptor System in Inflammation, Anti-Tumor Immune Responses, and COVID-19. Biomedicines 2023, 11, 254. [Google Scholar] [CrossRef]
- Sklinda, K.; Górecki, A.; Dorobek, M.; Walecki, J.; Modrzyńska, A.; Mruk, B. Ischaemic background of brain fog in long haul COVID-19–a nuclear magnetic resonance spectroscopy-based metabonomic analysis. Preliminary results. Pol. J. Radiol. 2021, 86, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, S.; Lin, A.; Stanwell, P. Glutamate and glutamine: A review of in vivo MRS in the human brain. NMR Biomed. 2013, 26, 1630–1646. [Google Scholar] [CrossRef]
- Inglese, M.; Rusinek, H.; George, I.C.; Babb, J.S.; Grossman, R.I.; Gonen, O. Global average gray and white matter N-acetylaspartate concentration in the human brain. Neuroimage 2008, 41, 270–276. [Google Scholar] [CrossRef]
- Urenjak, J.; Williams, S.R.; Gadian, D.G.; Noble, M. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J. Neurosci. 1993, 13, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Moffett, J.R.; Ross, B.; Arun, P.; Madhavarao, C.N.; Namboodiri, A.M. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog. Neurobiol. 2007, 81, 89–131. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.V.; Adalsteinsson, E.; Spielman, D.M.; Hurd, R.E.; Pfefferbaum, A. N-acetylaspartate—A marker of neuronal integrity. Ann. Neurol. 2001, 50, 823. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.M.; La, P.L.; Walker, R.; Harris, A.D. Magnetic resonance spectroscopy of traumatic brain injury and subconcussive hits: A systematic review and meta–analysis. J. Neurotrauma 2022, 39, 1455–1476. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Li, H.; Lin, F.; Zheng, W.; Zhang, H.; Wu, R. Neurochemical and microstructural alterations in bipolar and depressive disorders: A multimodal magnetic resonance imaging study. Front. Neurol. 2023, 14, 1089067. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Zhang, Y.; Guan, J.; Zhang, J.; Xu, C.; Shen, Z.; Xiao, B.; Liang, C.; Chen, K. Differential neurometabolite alterations in brains of medication-free individuals with bipolar disorder and those with unipolar depression: A two-dimensional proton magnetic resonance spectroscopy study. Bipolar Disord. 2016, 18, 583–590. [Google Scholar] [CrossRef]
- Chang, L.; Munsaka, S.M.; Kraft-Terry, S.; Ernst, T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J. Neuroimmune Pharmacol. 2013, 8, 576–593. [Google Scholar] [CrossRef]
- Paslakis, G.; Träber, F.; Roberz, J.; Block, W.; Jessen, F. N-acetyl-aspartate (NAA) as a correlate of pharmacological treatment in psychiatric disorders: A systematic review. Eur. Neuropsychopharmacol. 2014, 24, 1659–1675. [Google Scholar] [CrossRef]
- Robinson, R.T.; Drafts, B.C.; Fisher, J.L. Fluoxetine increases GABAA receptor activity through a novel modulatory site. J. Pharmacol. Exp. Ther. 2003, 304, 978–984. [Google Scholar] [CrossRef]
- Pereira, F.C.; Rolo, M.R.; Marques, E.; Mendes, V.M.; Ribeiro, C.F.; Ali, S.F.; Morgadinho, T.; Macedo, T.R. Acute increase of the glutamate–glutamine cycling in discrete brain areas after administration of a single dose of amphetamine. Ann. N. Y. Acad. Sci. 2008, 1139, 212–221. [Google Scholar] [CrossRef]
- Klok, F.A.; Boon, G.; Barco, S.; Endres, M.; Geelhoed, J.J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef] [PubMed]
- Premraj, L.; Kannapadi, N.V.; Briggs, J.; Seal, S.M.; Battaglini, D.; Fanning, J.; Suen, J.; Robba, C.; Fraser, J.; Cho, S.-M. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: A meta-analysis. J. Neurol. Sci. 2022, 434, 120162. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-term sequelae of COVID-19: A systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Jennings, G.; Monaghan, A.; Xue, F.; Mockler, D.; Romero-Ortuño, R. A systematic review of persistent symptoms and residual abnormal functioning following acute COVID-19: Ongoing symptomatic phase vs. post-COVID-19 syndrome. J. Clin. Med. 2021, 10, 5913. [Google Scholar] [CrossRef] [PubMed]
- Carle, A.C.; Riley, W.; Hays, R.D.; Cella, D. Confirmatory Factor Analysis of the Patient Reported Outcomes Measurement Information System (PROMIS) Adult Domain Framework Using Item Response Theory Scores. Med. Care 2015, 53, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Kratz, A.L.; Schilling, S.G.; Goesling, J.; Williams, D.A. Development and initial validation of a brief self-report measure of cognitive dysfunction in fibromyalgia. J. Pain 2015, 16, 527–536. [Google Scholar] [CrossRef]
- Rubega, M.; Ciringione, L.; Bertuccelli, M.; Paramento, M.; Sparacino, G.; Vianello, A.; Masiero, S.; Vallesi, A.; Formaggio, E.; Del Felice, A. High-density EEG sleep correlates of cognitive and affective impairment at 12-month follow-up after COVID-19. Clin. Neurophysiol. 2022, 140, 126–135. [Google Scholar] [CrossRef]
- Saucier, J.; Jose, C.; Beroual, Z.; Al-Qadi, M.; Chartrand, S.; Libert, E.; Losier, M.-C.; Cooling, K.; Girouard, G.; Jbilou, J. Cognitive inhibition deficit in long COVID-19: An exploratory study. Front. Neurol. 2023, 14, 1125574. [Google Scholar] [CrossRef]
- Rothrock, N.; Amtmann, D.; Cook, K. Development and validation of an interpretive guide for PROMIS scores. J. Patient Rep. Outcomes 2020, 4, 16. [Google Scholar] [CrossRef]
- Percze, A.R.; Nagy, A.; Polivka, L.; Barczi, E.; Czaller, I.; Kovats, Z.; Varga, J.T.; Ballai, J.H.; Muller, V.; Horvath, G. Fatigue, sleepiness and sleep quality are SARS-CoV-2 variant independent in patients with long COVID symptoms. Inflammopharmacology 2023, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, S.; Kokonda, M.; Sultana, R.; Duong, B.B.; Nagy, S.E.; Zaidan, M.F.; Baig, M.M.; Grigg, B.V.; Seashore, J.; Deer, R.R. Association between sleep quality and mental health among patients at a post-COVID-19 recovery clinic. Brain Sci. 2022, 12, 586. [Google Scholar] [CrossRef] [PubMed]
- Lauria, A.; Carfì, A.; Benvenuto, F.; Bramato, G.; Ciciarello, F.; Rocchi, S.; Rota, E.; Salerno, A.; Stella, L.; Tritto, M. Neuropsychological measures of post-COVID-19 cognitive status. Front. Psychol. 2023, 14, 1136667. [Google Scholar] [CrossRef] [PubMed]
- Kerr, W.C.; Ye, Y.; Martinez, P.; Karriker-Jaffe, K.J.; Patterson, D.; Greenfield, T.K.; Mulia, N. Longitudinal assessment of drinking changes during the pandemic: The 2021 COVID-19 follow-up study to the 2019 to 2020 National Alcohol Survey. Alcohol. Clin. Exp. Res. 2022, 46, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Castaldelli-Maia, J.M.; Segura, L.E.; Martins, S.S. The concerning increasing trend of alcohol beverage sales in the US during the COVID-19 pandemic. Alcohol 2021, 96, 37–42. [Google Scholar] [CrossRef] [PubMed]
- QualtricsXM. Available online: https://www.qualtrics.com/ (accessed on 30 June 2023).
- Brown, J.M.; Lansdall, C.J.; Wiggins, J.; Dawson, K.E.; Hunter, K.; Rowe, J.B.; Parker, R.A. The Test Your Memory for Mild Cognitive Impairment (TYM-MCI). J. Neurol. Neurosurg. Psychiatry 2017, 88, 1045–1051. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence (WASI—II); The Psychological Corporation: San Antonio, TX, USA, 1999. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Coutlee, C.G.; Politzer, C.S.; Hoyle, R.H.; Huettel, S.A. An abbreviated impulsiveness scale constructed through confirmatory factor analysis of the Barratt Impulsiveness Scale version 11. Arch. Sci. Psychol. 2014, 2, 1–12. [Google Scholar] [CrossRef]
- Hoyle, R.H.; Stephenson, M.T.; Palmgreen, P.; Lorch, E.P.; Donohew, R.L. Reliability and validity of a brief measure of sensation seeking. Pers. Indiv. Differ. 2002, 32, 401–414. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Lowe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr. Ann. 2002, 32, 509–515. [Google Scholar] [CrossRef]
- Mullins, P.G.; Chen, H.; Xu, J.; Caprihan, A.; Gasparovic, C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magn. Reson. Med. 2008, 60, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.; Loo, R.S.; Puts, N.A.J.; Edden, R.A.E.; Harris, A.D. Designing GABA-edited magnetic resonance spectroscopy studies: Considerations of scan duration, signal-to-noise ratio and sample size. J. Neurosci. Methods 2018, 303, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Edden, R.A.; Puts, N.A.; Harris, A.D.; Barker, P.B.; Evans, C.J. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging 2014, 40, 1445–1452. [Google Scholar] [CrossRef]
- Cuypers, K.; Hehl, M.; van Aalst, J.; Chalavi, S.; Mikkelsen, M.; Van Laere, K.; Dupont, P.; Mantini, D.; Swinnen, S.P. Age-related GABAergic differences in the primary sensorimotor cortex: A multimodal approach combining PET, MRS and TMS. Neuroimage 2021, 226, 117536. [Google Scholar] [CrossRef]
- Puts, N.A.J.; Heba, S.; Harris, A.D.; Evans, C.J.; McGonigle, D.J.; Tegenthoff, M.; Schmidt-Wilcke, T.; Edden, R.A.E. GABA Levels in Left and Right Sensorimotor Cortex Correlate across Individuals. Biomedicines 2018, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Bhagwagar, Z.; Wylezinska, M.; Taylor, M.; Jezzard, P.; Matthews, P.M.; Cowen, P.J. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am. J. Psychiatry 2004, 161, 368–370. [Google Scholar] [CrossRef]
- Peek, A.; Rebbeck, T.; Leaver, A.; Foster, S.L.; Refshauge, K.; Puts, N.; Oeltzschner, G.; Andronesi, O.C.; Barker, P.B.; Bogner, W. A comprehensive guide to MEGA-PRESS for GABA measurement. Anal. Biochem. 2023, 669, 115113. [Google Scholar] [CrossRef]
- Gasparovic, C.; Song, T.; Devier, D.; Bockholt, H.J.; Caprihan, A.; Mullins, P.G.; Posse, S.; Jung, R.E.; Morrison, L.A. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn. Reson. Med. 2006, 55, 1219–1226. [Google Scholar] [CrossRef]
- Mullins, P.G.; McGonigle, D.J.; O’Gorman, R.L.; Puts, N.A.; Vidyasagar, R.; Evans, C.J.; Cardiff Symposium on MRS of GABA; Edden, R.A. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 2014, 86, 43–52. [Google Scholar] [CrossRef]
- SPSS. IBM SPSS Statistics for Windows; SPSS Inc.: Chicago, IL, USA, 2017. [Google Scholar]
- MacKinnon, D.P.; Fairchild, A.J.; Fritz, M.S. Mediation analysis. Annu. Rev. Psychol. 2007, 58, 593–614. [Google Scholar] [CrossRef]
- Hayes, A.F. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Commun. Monogr. 2009, 76, 408–420. [Google Scholar] [CrossRef]
- Cheetham, N.J.; Penfold, R.; Giunchiglia, V.; Bowyer, V.; Sudre, C.H.; Canas, L.S.; Deng, J.; Murray, B.; Kerfoot, E.; Antonelli, M. The effects of COVID-19 on cognitive performance in a community-based cohort: A COVID symptom study biobank prospective cohort study. EClinicalMedicine 2023, 62, 102086. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, W.; Huang, S.; Huang, Y.; Chen, Y.; Zhang, H.; Guo, H.; Liu, J. Two-year follow-up of brain structural changes in patients who recovered from COVID-19: A prospective study. Psychiatry Res. 2023, 319, 114969. [Google Scholar] [CrossRef] [PubMed]
- Nouraeinejad, A. Brain fog as a Long-term Sequela of COVID-19. SN Compr. Clin. Med. 2022, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Stefanou, M.-I.; Palaiodimou, L.; Bakola, E.; Smyrnis, N.; Papadopoulou, M.; Paraskevas, G.P.; Rizos, E.; Boutati, E.; Grigoriadis, N.; Krogias, C. Neurological manifestations of long-COVID syndrome: A narrative review. Ther. Adv. Chronic Dis. 2022, 13, 20406223221076890. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Mäki, H.; Rosanova, M.; Casarotto, S.; Canali, P.; Casali, A.G.; Tononi, G.; Massimini, M. Human cortical excitability increases with time awake. Cereb. Cortex 2013, 23, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Cantone, M.; Lanuzza, B.; Pennisi, M.; Bella, R.; Pennisi, G.; Ferri, R. Distinctive patterns of cortical excitability to transcranial magnetic stimulation in obstructive sleep apnea syndrome, restless legs syndrome, insomnia, and sleep deprivation. Sleep Med. Rev. 2015, 19, 39–50. [Google Scholar] [CrossRef]
- Plante, D.T.; Jensen, J.E.; Schoerning, L.; Winkelman, J.W. Reduced γ-aminobutyric acid in occipital and anterior cingulate cortices in primary insomnia: A link to major depressive disorder? Neuropsychopharmacology 2012, 37, 1548–1557. [Google Scholar] [CrossRef]
- Winkelman, J.W.; Buxton, O.M.; Jensen, J.E.; Benson, K.L.; O’Connor, S.P.; Wang, W.; Renshaw, P.F. Reduced brain GABA in primary insomnia: Preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). Sleep 2008, 31, 1499–1506. [Google Scholar] [CrossRef]
- Roth, T. A physiologic basis for the evolution of pharmacotherapy for insomnia. J. Clin. Psychiatry 2007, 68, 13. [Google Scholar]
- Godfrey, K.E.; Gardner, A.C.; Kwon, S.; Chea, W.; Muthukumaraswamy, S.D. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: A systematic review and meta-analysis. J. Psychiatr. Res. 2018, 105, 33–44. [Google Scholar] [CrossRef]
- Luscher, B.; Fuchs, T. GABAergic control of depression-related brain states. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 73, pp. 97–144. [Google Scholar]
- Fogaça, M.V.; Duman, R.S. Cortical GABAergic dysfunction in stress and depression: New insights for therapeutic interventions. Front. Cell. Neurosci. 2019, 13, 87. [Google Scholar] [CrossRef]
- Cutler, A.J.; Mattingly, G.W.; Maletic, V. Understanding the mechanism of action and clinical effects of neuroactive steroids and GABAergic compounds in major depressive disorder. Transl. Psychiatry 2023, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Mason, G.F.; Rothman, D.L.; Krystal, J.H. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am. J. Psychiatry 2002, 159, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Mason, G.F.; Rothman, D.L.; Hyder, F.; Ciarcia, J.J.; Ostroff, R.B.; Berman, R.M.; Krystal, J.H. Increased cortical GABA concentrations in depressed patients receiving ECT. Am. J. Psychiatry 2003, 160, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, C.G.; Niciu, M.J.; Fenton, L.R.; Fasula, M.K.; Jiang, L.; Black, A.; Rothman, D.L.; Mason, G.F.; Sanacora, G. Decreased occipital cortical glutamate levels in response to successful cognitive-behavioral therapy and pharmacotherapy for major depressive disorder. Psychother. Psychosom. 2014, 83, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Caverzasi, E.; Pichiecchio, A.; Poloni, G.U.; Calligaro, A.; Pasin, M.; Palesi, F.; Castellazzi, G.; Pasquini, M.; Biondi, M.; Barale, F. Magnetic resonance spectroscopy in the evaluation of treatment efficacy in unipolar major depressive disorder: A review of the literature. Funct. Neurol. 2012, 27, 13. [Google Scholar]
- Nutt, D.; Wilson, S.; Paterson, L. Sleep disorders as core symptoms of depression. Dialogues Clin. Neurosci. 2022, 10, 329–336. [Google Scholar] [CrossRef]
- Mollayeva, T.; Thurairajah, P.; Burton, K.; Mollayeva, S.; Shapiro, C.M.; Colantonio, A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med. Rev. 2016, 25, 52–73. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Roohi, E.; Jaafari, N.; Hashemian, F. On inflammatory hypothesis of depression: What is the role of IL-6 in the middle of the chaos? J. Neuroinflammation 2021, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Nakhaee, H.; Zangiabadian, M.; Bayati, R.; Rahmanian, M.; Ghaffari Jolfayi, A.; Rakhshanderou, S. The effect of antidepressants on the severity of COVID-19 in hospitalized patients: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0267423. [Google Scholar] [CrossRef]
- Lu, Y.; Ho, C.S.; Liu, X.; Chua, A.N.; Wang, W.; McIntyre, R.S.; Ho, R.C. Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS ONE 2017, 12, e0186700. [Google Scholar] [CrossRef]
- Cui, C.; Shurtleff, D.; Harris, R.A. Neuroimmune mechanisms of alcohol and drug addiction. Int. Rev. Neurobiol. 2014, 118, 1–12. [Google Scholar] [CrossRef]
- Hodes, G.E.; Kana, V.; Menard, C.; Merad, M.; Russo, S.J. Neuroimmune mechanisms of depression. Nat. Neurosci. 2015, 18, 1386–1393. [Google Scholar] [CrossRef]
- Tzingounis, A.V.; Wadiche, J.I. Glutamate transporters: Confining runaway excitation by shaping synaptic transmission. Nat. Rev. Neurosci. 2007, 8, 935–947. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Franklin, T.; Iwata, M.; Duman, R.S. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016, 17, 497–511. [Google Scholar] [CrossRef]
- Versace, V.; Sebastianelli, L.; Ferrazzoli, D.; Romanello, R.; Ortelli, P.; Saltuari, L.; D’Acunto, A.; Porrazzini, F.; Ajello, V.; Oliviero, A. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clin. Neurophysiol. 2021, 132, 1138–1143. [Google Scholar] [CrossRef]
- Furlanis, G.; Buoite Stella, A.; Biaduzzini, F.; Bellavita, G.; Frezza, N.A.; Olivo, S.; Menichelli, A.; Lunardelli, A.; Ajčević, M.; Manganotti, P. Cognitive deficit in post-acute COVID-19: An opportunity for EEG evaluation? Neurol. Sci. 2023, 44, 1491–1498. [Google Scholar] [CrossRef] [PubMed]
- Yesilkaya, U.H.; Sen, M.; Balcioglu, Y.H. COVID-19-related cognitive dysfunction may be associated with transient disruption in the DLPFC glutamatergic pathway. J. Clin. Neurosci. 2021, 87, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Rapalino, O.; Weerasekera, A.; Moum, S.J.; Eikermann-Haerter, K.; Edlow, B.L.; Fischer, D.; Torrado-Carvajal, A.; Loggia, M.L.; Mukerji, S.S.; Schaefer, P.W.; et al. Brain MR Spectroscopic Findings in 3 Consecutive Patients with COVID-19: Preliminary Observations. AJNR Am. J. Neuroradiol. 2021, 42, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, A.; Kritzer, M.D.; Eryilmaz, H.; Praschan, N.; Camprodon, J.A.; Fricchione, G.; Chemali, Z. Neurocircuitry hypothesis and clinical experience in treating neuropsychiatric symptoms of postacute sequelae of severe acute respiratory syndrome coronavirus 2. J. Acad. Consult. Liaison Psychiatry 2022, 63, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M. Differential diagnosis and pathogenesis of the neurological signs and symptoms in COVID-19 and long-COVID syndrome. CNS Neurosci. Ther. 2022, 28, 1905–1907. [Google Scholar] [CrossRef] [PubMed]
- Bookstaver, P.B.; Mohorn, P.L.; Shah, A.; Tesh, L.D.; Quidley, A.M.; Kothari, R.; Bland, C.M.; Weissman, S. Management of viral central nervous system infections: A primer for clinicians. J. Cent. Nerv. Syst. Dis. 2017, 9, 1179573517703342. [Google Scholar] [CrossRef]
- Schifitto, G.; Navia, B.A.; Yiannoutsos, C.T.; Marra, C.M.; Chang, L.; Ernst, T.; Jarvik, J.G.; Miller, E.N.; Singer, E.J.; Ellis, R.J. Memantine and HIV-associated cognitive impairment: A neuropsychological and proton magnetic resonance spectroscopy study. Aids 2007, 21, 1877–1886. [Google Scholar] [CrossRef]
- Gonul, A.S.; Kitis, O.; Ozan, E.; Akdeniz, F.; Eker, C.; Eker, O.D.; Vahip, S. The effect of antidepressant treatment on N-acetyl aspartate levels of medial frontal cortex in drug-free depressed patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 120–125. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, W.; Li, Y.; Wu, X.; Shi, X.; Geng, D. Effects of antidepressant treatment on N-acetyl aspartate and choline levels in the hippocampus and thalami of post-stroke depression patients: A study using 1H magnetic resonance spectroscopy. Psychiatry Res. Neuroimaging 2010, 182, 48–52. [Google Scholar] [CrossRef]
- Patel, T.; Blyth, J.C.; Griffiths, G.; Kelly, D.; Talcott, J.B. Moderate relationships between NAA and cognitive ability in healthy adults: Implications for cognitive spectroscopy. Front. Hum. Neurosci. 2014, 8, 39. [Google Scholar] [CrossRef] [PubMed]

| PASC (n = 18) | CNT (n = 20) | F(1,36)/χ2 | p | |
|---|---|---|---|---|
| % Women | 61% | 60% | 0.01 a | 0.944 |
| % White/Non-Hispanic | 83% | 50% | 5.38 a | 0.146 |
| Age | 24.44 ± 5.25 | 23.35 ± 3.66 | 0.57 | 0.457 |
| Intelligence (WASI) | 109.00 ± 12.50 | 111.30 ± 9.03 | 0.87 | 0.359 |
| Stress (PSS) | 19.56 ± 4.57 | 18.80 ± 4.77 | 0.25 | 0.622 |
| Sleep Quality Total (PSQI) | 9.22 ± 3.52 | 5.70 ± 2.39 | 13.25 | <0.001 |
| Impulsivity (ABIS) | ||||
| Attention | 1.73 ± 0.30 | 1.76 ± 0.41 | 0.06 | 0.813 |
| Motor | 1.77 ± 0.37 | 1.60 ± 0.41 | 1.36 | 0.253 |
| Non-Planning | 1.97 ± 0.48 | 1.80 ± 0.57 | 0.72 | 0.404 |
| Sensation Seeking (BSSS) | ||||
| Experience | 7.38 ± 1.28 | 7.65 ± 1.84 | 0.19 | 0.667 |
| Boredom | 6.09 ± 1.30 | 6.35 ± 1.84 | 0.17 | 0.683 |
| Thrill | 6.74 ± 1.87 | 6.30 ± 2.11 | 0.34 | 0.567 |
| Disinhibition | 5.60 ± 1.85 | 5.55 ± 1.79 | 0.01 | 0.942 |
| Anxiety (GAD-7) | 4.50 ± 3.13 | 3.28 ± 3.01 | 1.43 | 0.240 |
| Depression (PHQ-9) | 4.33 ± 3.45 | 2.80 ± 2.89 | 2.22 | 0.145 |
| Avg Drinks/Week | 4.45 ± 4.87 | 1.69 ± 1.46 | 5.88 | 0.020 |
| Memory (TYM-MCI) | 9.60 ± 3.02 |
| VOI | PASC (n = 18) | CNT (n = 20) | F(1,36) | p |
|---|---|---|---|---|
| GM | 0.63 ± 0.05 | 0.62 ± 0.03 | 0.69 | 0.413 |
| WM | 0.29 ± 0.03 | 0.29 ± 0.03 | 0.84 | 0.366 |
| CSF | 0.09 ± 0.03 | 0.09 ± 0.02 | 0.10 | 0.760 |
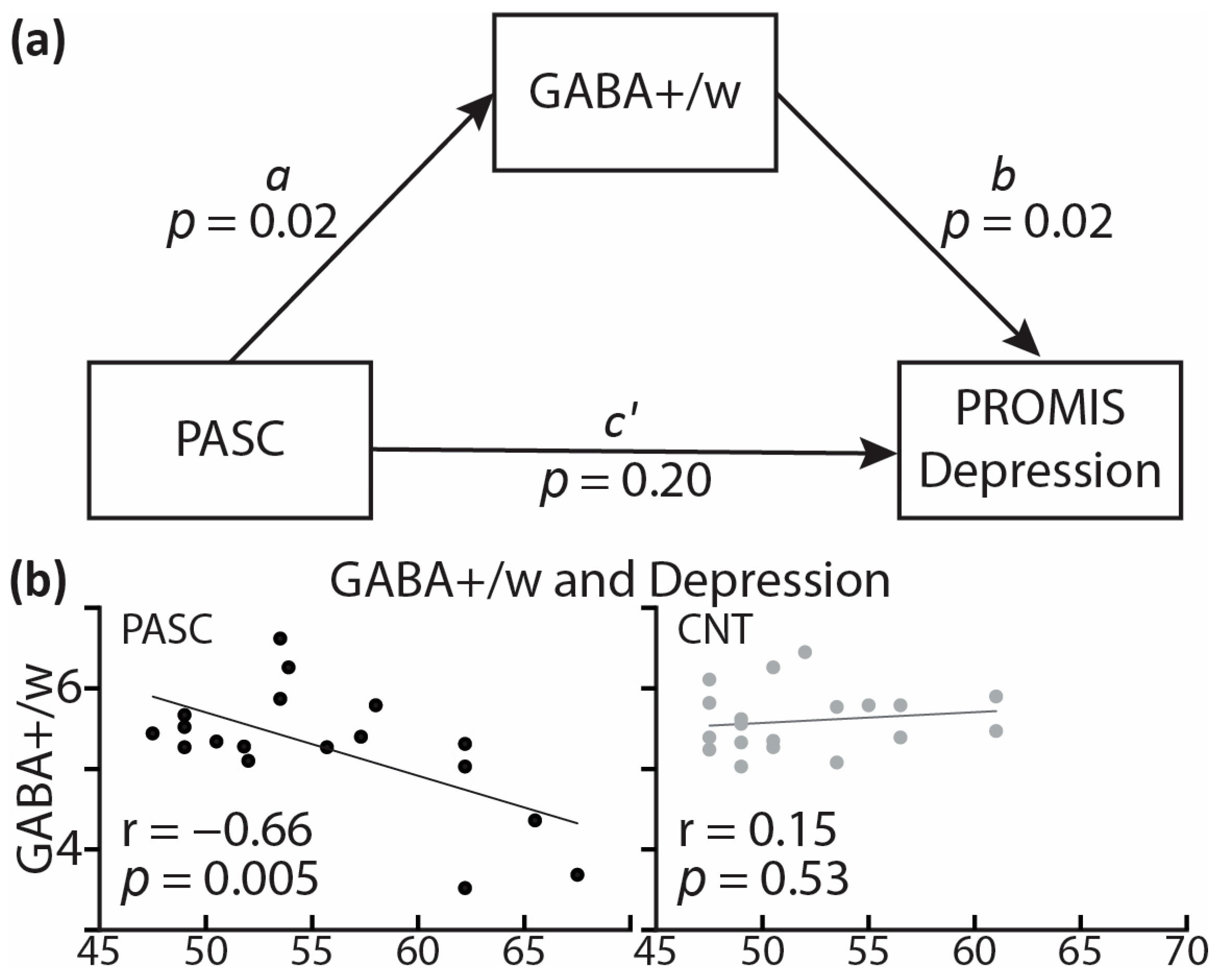
| Parameter | Est. | p | 95% C.I. |
|---|---|---|---|
| a | −0.51 | 0.017 | [−0.92 −0.10] |
| b | −3.94 | 0.023 | [−7.31 −0.58] |
| c’ | 2.67 | 0.202 | [−1.51 6.85] |
| ab | 2.01 | 0.089 | [−0.31 4.33] |
| ab + c’ | 4.68 | 0.026 | [0.60 8.76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinkovic, K.; White, D.R.; Alderson Myers, A.; Parker, K.S.; Arienzo, D.; Mason, G.F. Cortical GABA Levels Are Reduced in Post-Acute COVID-19 Syndrome. Brain Sci. 2023, 13, 1666. https://doi.org/10.3390/brainsci13121666
Marinkovic K, White DR, Alderson Myers A, Parker KS, Arienzo D, Mason GF. Cortical GABA Levels Are Reduced in Post-Acute COVID-19 Syndrome. Brain Sciences. 2023; 13(12):1666. https://doi.org/10.3390/brainsci13121666
Chicago/Turabian StyleMarinkovic, Ksenija, David R. White, Austin Alderson Myers, Katie S. Parker, Donatello Arienzo, and Graeme F. Mason. 2023. "Cortical GABA Levels Are Reduced in Post-Acute COVID-19 Syndrome" Brain Sciences 13, no. 12: 1666. https://doi.org/10.3390/brainsci13121666
APA StyleMarinkovic, K., White, D. R., Alderson Myers, A., Parker, K. S., Arienzo, D., & Mason, G. F. (2023). Cortical GABA Levels Are Reduced in Post-Acute COVID-19 Syndrome. Brain Sciences, 13(12), 1666. https://doi.org/10.3390/brainsci13121666






