Long-Term Outcomes among Patients with Prolonged Disorders of Consciousness
Abstract
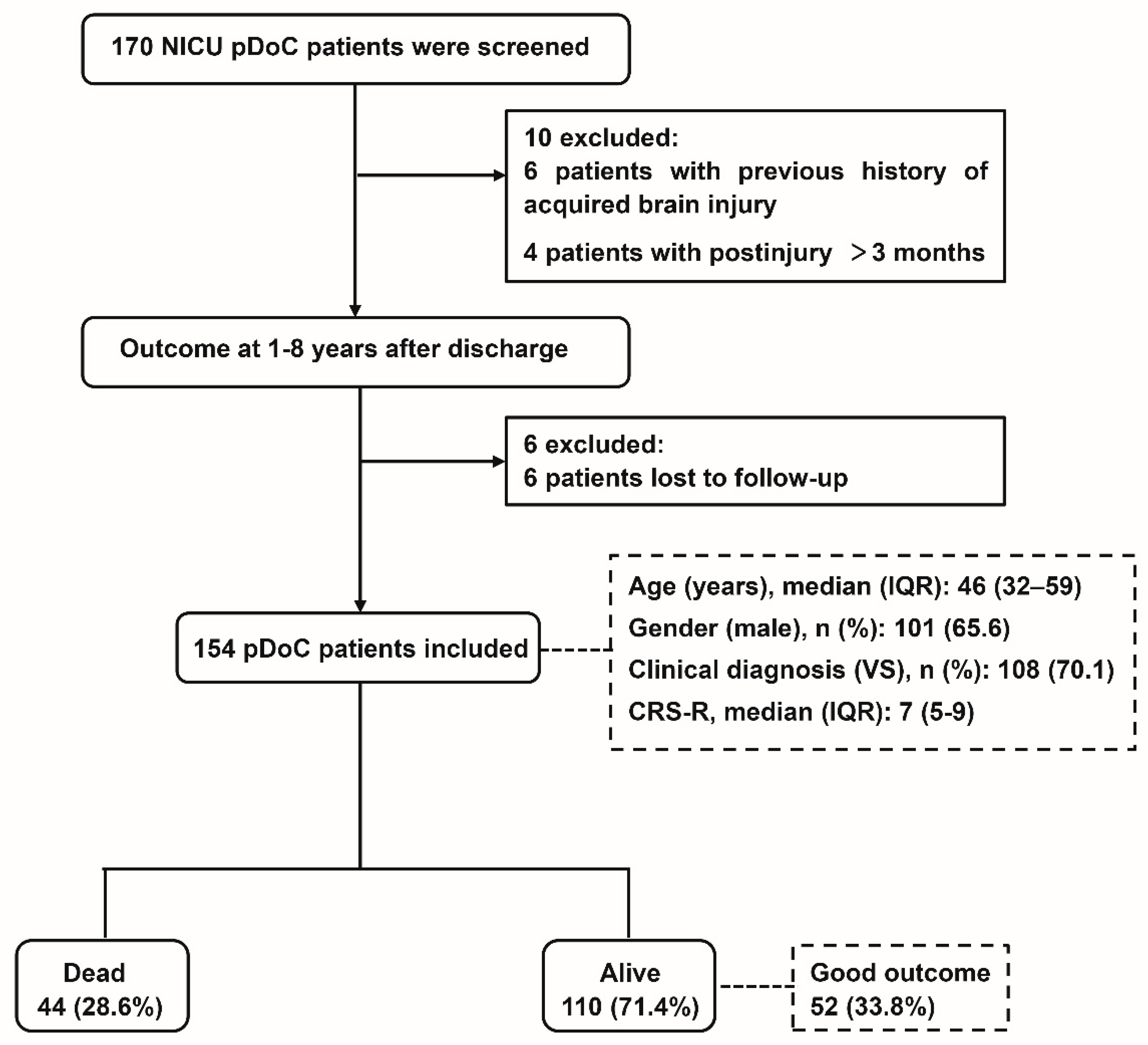
:1. Introduction
2. Materials and Methods
2.1. Study Setting and Participants
2.2. Data Acquisition
2.3. Definition of Outcome
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; Von Wild, K.R.; Zeman, A.; et al. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; Zasler, N.D. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 2018, 91, 450–460. [Google Scholar]
- Lammi, M.H.; Smith, V.H.; Tate, R.L.; Taylor, C.M. The minimally conscious state and recovery potential: A follow-up study 2 to 5 years after traumatic brain injury. Arch. Phys. Med. Rehabil. 2005, 86, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Estraneo, A.; Moretta, P.; Loreto, V.; Lanzillo, B.; Santoro, L.; Trojano, L. Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology 2010, 75, 239–245. [Google Scholar] [CrossRef]
- Luauté, J.; Maucort-Boulch, D.; Tell, L.; Quelard, F.; Sarraf, T.; Iwaz, J.; Boisson, D.; Fischer, C. Long-term outcomes of chronic minimally conscious and vegetative states. Neurology 2010, 75, 246–252. [Google Scholar] [CrossRef]
- Steppacher, I.; Kaps, M.; Kissler, J. Will time heal? A long-term follow-up of severe disorders of consciousness. Ann. Clin. Transl. Neurol. 2014, 1, 401–408. [Google Scholar] [CrossRef]
- Baricich, A.; de Sire, A.; Antoniono, E.; Gozzerino, F.; Lamberti, G.; Cisari, C.; Invernizzi, M. Recovery from vegetative state of patients with a severe brain injury: A 4-year real-practice prospective cohort study. Funct. Neurol. 2017, 32, 131–136. [Google Scholar] [CrossRef]
- Estraneo, A.; De Bellis, F.; Masotta, O.; Loreto, V.; Fiorenza, S.; Sapio, M.L.; Trojano, L. Demographical and clinical indices for long-term evolution of patients in vegetative or in minimally conscious state. Brain Inj. 2019, 33, 1633–1639. [Google Scholar] [CrossRef]
- Yelden, K.; Duport, S.; James, L.M.; Kempny, A.; Farmer, S.F.; Leff, A.P.; Playford, E.D. Late recovery of awareness in prolonged disorders of consciousness –a cross-sectional cohort study. Disabil. Rehabil. 2018, 40, 2433–2438. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-G.; Li, R.; Zhang, Y.; Hao, J.-H.; Du, J.-B.; Guo, A.-S.; Song, W.-Q. Recovery from prolonged disorders of consciousness: A dual-center prospective cohort study in China. World J. Clin. Cases 2020, 8, 2520–2529. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.-G.; Li, L.; Wei, D.; Xu, X.-X.; Zhao, R.; Jing, Y.-Y.; Su, Y.-Y.; Xiong, L.-Z.; Zhao, G.; Jiang, W. Development of a simple score to predict outcome for unresponsive wakefulness syndrome. Crit. Care 2014, 18, R37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estraneo, A.; Magliacano, A.; Fiorenza, S.; Formisano, R.; Grippo, A.; Angelakis, E.; Cassol, H.; Thibaut, A.; Gosseries, O.; Lamberti, G.; et al. Risk factors for 2-year mortality in patients with prolonged disorders of consciousness: An international multicentre study. Eur. J. Neurol. 2022, 29, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Hammond, F.M.; Giacino, J.T.; Richardson, R.N.; Sherer, M.; Zafonte, R.D.; Whyte, J.; Arciniegas, D.B.; Tang, X. Disorders of Consciousness due to Traumatic Brain Injury: Functional Status Ten Years Post-Injury. J. Neurotrauma 2019, 36, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Kalmar, K.; Whyte, J. The JFK Coma Recovery Scale-Revised: Measurement characteristics and diagnostic utility. Arch. Phys. Med. Rehabil. 2004, 85, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Leitinger, M.; Höller, Y.; Kalss, G.; Rohracher, A.; Novak, H.F.; Höfler, J.; Dobesberger, J.; Kuchukhidze, G.; Trinka, E. Epidemiology-Based Mortality Score in Status Epilepticus (EMSE). Neurocritical Care 2014, 22, 273–282. [Google Scholar] [CrossRef]
- Fugate, J.E.; Wijdicks, E.F.; Mandrekar, J.; Claassen, D.O.; Manno, E.M.; White, R.D.; Bell, M.R.; Rabinstein, A.A. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann. Neurol. 2010, 68, 907–914. [Google Scholar] [CrossRef]
- Synek, V.M. Prognostically important EEG coma patterns in diffuse anoxic and traumatic encephalopathies in adults. J. Clin. Neurophysiol 1988, 5, 161–174. [Google Scholar] [CrossRef]
- Azabou, E.; Magalhaes, E.; Braconnier, A.; Yahiaoui, L.; Moneger, G.; Heming, N.; Annane, D.; Mantz, J.; Chrétien, F.; Durand, M.-C.; et al. Early Standard Electroencephalogram Abnormalities Predict Mortality in Septic Intensive Care Unit Patients. PLoS ONE 2015, 10, e0139969. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Marmarou, A.; Lapane, K.; Turf, E.; Wilson, L. A method for reducing misclassification in the extended Glasgow Outcome Score. J. Neurotrauma 2010, 27, 843–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claassen, J.; Doyle, K.; Matory, A.; Couch, C.; Burger, K.M.; Velazquez, A.; Okonkwo, J.U.; King, J.-R.; Park, S.; Agarwal, S.; et al. Detection of Brain Activation in Unresponsive Patients with Acute Brain Injury. N. Engl. J. Med. 2019, 380, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- de Biase, S.; Gigli, G.L.; Lorenzut, S.; Bianconi, C.; Sfreddo, P.; Rossato, G.; Basaldellaa, F.; Fuccaroa, M.; Coricac, A.; Tononc, D.; et al. The importance of polysomnography in the evaluation of prolonged disorders of consciousness: Sleep recordings more adequately correlate than stimulus-related evoked potentials with patients’ clinical status. Sleep Med. 2014, 15, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Avantaggiato, P.; Molteni, E.; Formica, F.; Gigli, G.L.; Valente, M.; Lorenzut, S.; de Biase, S.; Arcieri, S.; Locatelli, F.; Strazzer, S. Polysomnographic Sleep Patterns in Children and Adolescents in Unresponsive Wakefulness Syndrome. J. Head. Trauma Rehabil. 2015, 30, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, P.B.; Conte, M.M.; Fridman, E.A.; Voss, H.U.; Victor, J.D.; Schiff, N.D. Preservation of electroencephalographic organization in patients with impaired consciousness and imaging-based evidence of command-following. Ann. Neurol. 2014, 76, 869–879. [Google Scholar] [CrossRef] [Green Version]
- Schönauer, M.; Pöhlchen, D. Sleep spindles. Curr. Biol. 2018, 28, R1129–R1130. [Google Scholar] [CrossRef] [Green Version]
- Urakami, Y. Relationship between, sleep spindles and clinical recovery in patients with traumatic brain injury: A simultaneous EEG and MEG study. Clin. EEG Neurosci. 2012, 43, 39–47. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Hamilton, J.A.; Perrin, P.B.; Campbell, T.A.; Danish, S.J.; Goldstein, A.L. Predicting emergence from a disorder of consciousness using the Coma Recovery Scale–Revised. Neuropsychol. Rehabil. 2020, 30, 266–280. [Google Scholar] [CrossRef]
- Portaccio, E.; Morrocchesi, A.; Romoli, A.M.; Hakiki, B.; Taglioli, M.P.; Lippi, E.; Di Renzone, M.; Grippo, A.; Macchi, C. Score on Coma Recovery Scale-Revised at admission predicts outcome at discharge in intensive rehabilitation after severe brain injury. Brain Inj. 2018, 32, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Portaccio, E.; Morrocchesi, A.; Romoli, A.M.; Hakiki, B.; Taglioli, M.P.; Lippi, E.; Di Renzone, M.; Grippo, A.; Macchi, C.; Atzori, T.; et al. Improvement on the Coma Recovery Scale–Revised During the First Four Weeks of Hospital Stay Predicts Outcome at Discharge in Intensive Rehabilitation after Severe Brain Injury. Arch. Phys. Med. Rehabil. 2018, 99, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Nekrasova, J.; Kanarskii, M.; Borisov, I.; Pradhan, P.; Shunenkov, D.; Vorobiev, A.; Smirnova, M.; Pasko, V.; Petrova, M.; Luginina, E.; et al. One-Year Demographical and Clinical Indices of Patients with Chronic Disorders of Consciousness. Brain Sci. 2021, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Faugeras, F.; Rohaut, B.; Valente, M.; Sitt, J.; Demeret, S.; Bolgert, F.; Weiss, N.; Grinea, A.; Marois, C.; Quirins, M.; et al. Survival and consciousness recovery are better in the minimally conscious state than in the vegetative state. Brain Inj. 2018, 32, 72–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, D.I.; Polyak, M.; Coughlan, D.; Nichols, M.; Roche, A. Natural history of recovery from brain injury after prolonged disorders of consciousness: Outcome of patients admitted to inpatient rehabilitation with 1–4 year follow-up. Prog. Brain Res. 2009, 177, 73–88. [Google Scholar] [PubMed]
- Strauss, D.J.; Ashwal, S.; Day, S.M.; Shavelle, R.M. Life expectancy of children in vegetative and minimally conscious states. Pediatr. Neurol. 2000, 23, 312–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avesani, R.; Gambini, M.G.; Albertini, G. The vegetative state: A report of two cases with a long-term follow-up. Brain Inj. 2006, 20, 333–338. [Google Scholar] [CrossRef]
- Sazbon, L.; Groswasser, Z. Time-related sequelae of TBI in patients with prolonged post-comatose unawareness (PC-U) state. Brain Inj. 1991, 5, 3–8. [Google Scholar] [CrossRef]
- Moreno, R.; Morais, P. Outcome prediction in intensive care: Results of a prospective, multicentre, Portuguese study. Intensive Care Med. 1997, 23, 177–186. [Google Scholar] [CrossRef]
- Pascarella, A. Multicentre registry of brain-injured patients with disorder of consciousness: Rationale and preliminary data. Funct. Neurol. 2018, 33, 19–30. [Google Scholar] [CrossRef]
- Lopez-Rolon, A.; Vogler, J.; Howell, K.; Shock, J.; Czermak, S.; Heck, S.; Straube, A.; Bender, A. Severe disorders of consciousness after acquired brain injury: A single-centre long-term follow-up study. Neurorehabilitation 2017, 40, 509–517. [Google Scholar] [CrossRef]
- Kang, J.; Huang, L.; Tang, Y.; Chen, G.; Ye, W.; Wang, J.; Feng, Z. A dynamic model to predict long-term outcomes in patients with prolonged disorders of consciousness. Aging 2022, 14, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.; Leonardi, M.; Covelli, V.; Giovannetti, A.M.; Sattin, D. Risk factors for mortality in 600 patients in vegetative and minimally conscious states. J. Neurol. 2014, 261, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.-A.; Ledoux, D.; Vanhaudenhuyse, A.; Gosseries, O.; Thibaut, A.; Laureys, S. Prognosis of Patients with Altered State of Consciousness, in Coma and Disorders of Consciousness; Schnakers, C., Laureys, S., Eds.; Springer: London, UK, 2012; pp. 11–23. [Google Scholar]
- Eilander, H.J.; Wijnen, V.J.M.; Scheirs, J.G.M.; De Kort, P.L.M.; Prevo, A.J.H. Children and young adults in a prolonged unconscious state due to severe brain injury: Outcome after an early intensive neurorehabilitation programme. Brain Inj. 2005, 19, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Vos, P.E.; Lamers, K.J.; Hendriks, J.C.; van Haaren, M.; Beems, T.; Zimmerman, C.; van Geel, W.; de Reus, H.; Biert, J.; Verbeek, M.M. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology 2004, 62, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Yuan, Q.; Yang, J.; Wang, W.; Liu, H. The Prognostic Value of Serum Neuron-Specific Enolase in Traumatic Brain Injury: Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e106680. [Google Scholar] [CrossRef] [PubMed]


| Variable | Total (n = 154) | Alive (n = 110) | Dead (n = 44) |
|---|---|---|---|
| Age, y | 46 [32,59] | 45 [31,55] | 54 [33,63] |
| F/M | 53/101 | 35/75 | 18/26 |
| Residence, rural/urban | 107/47 | 79/31 | 28/16 |
| Education, y < 12 / ≥12 | 83/71 | 67/43 | 16/28 |
| Etiology, T/NT | 22/132 | 16/94 | 6/38 |
| Length of stay in the ICU, d | 18 [12,29] | 17 [12,29] | 21 [12,30] |
| TPI, d | 46 [36,64] | 45 [34,60] | 48 [37,78] |
| CRS-R score | 7 [5,9] | 7 [5,9] | 6 [5,8] |
| Clinical diagnosis, VS/MCS | 108/46 | 74/36 | 34/10 |
| APACHE II score | 13 [10,17] | 12 [10,15] | 15 [11,18] |
| CCI, 0/1–2/ ≥3 | 55/66/33 | 38/50/22 | 17/16/11 |
| Motor response, P/A | 103/51 | 76/34 | 27/17 |
| NSE (μg/L), <33/ ≥33 | 69/85 | 53/57 | 16/28 |
| Sleep spindles, P/A | 84/70 | 70/40 | 14/30 |
| Synek’s scale, <3/ ≥3 | 90/64 | 67/43 | 23/21 |
| EEG R, P/A | 117/37 | 90/20 | 27/17 |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95%CI) | p | HR (95%CI) | p | |
| Age | 1.014 (0.995–1.033) | 0.160 | ||
| Sex | 0.755 (0.414–1.376) | 0.358 | ||
| Residence | 1.320 (0.713–2.441) | 0.377 | ||
| Education | 2.252 (1.218–4.165) | 0.010 | ||
| Etiology | 0.910 (0.385–2.154) | 0.831 | ||
| Clinical diagnosis | 0.659 (0.325–1.334) | 0.246 | ||
| Length of stay in the ICU | 1.008 (0.997–1.020) | 0.168 | ||
| TPI, d | 0.762(0.411–1.432) | 0.193 | ||
| CRS-R score | 0.958 (0.862–1.066) | 0.435 | ||
| APACHE score | 1.114 (1.045–1.188) | 0.001 | 1.101 (1.031–1.177) | 0.004 |
| APACHE score ≥ 18 | 0.290 (0.153–0.550) | <0.001 | ||
| CCI, 0 | 0.559 | |||
| 1–2 | 0.749 (0.378–1.484) | 0.408 | ||
| ≥3 | 1.106 (0.518–2.362) | 0.794 | ||
| Motor response | 0.797 (0.435–1.463) | 0.465 | ||
| NSE (μg/L) | 0.627 (0.339–1.160) | 0.137 | ||
| Sleep spindles | 0.314 (0.166–0.594) | <0.001 | 0.347 (0.183–0.659) | 0.001 |
| Synek’s scale | 0.789 (0.436–1.425) | 0.432 | ||
| EEG R | 0.416 (0.227–0.764) | 0.005 | ||
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | |
| Age, y | 0.980 (0.960–1.001) | 0.061 | 0.978 (0.955–1.000) | 0.051 |
| Sex, M | 1.281 (0.627–2.615) | 0.497 | ||
| Residence, urban | 0.770 (0.368–1.615) | 0.489 | ||
| Education, y ≥ 12 | 1.127 (0.577–2.201) | 0.726 | ||
| Etiology, T | 2.220 (0.890–5.533) | 0.087 | ||
| Clinical diagnosis, MCS | 2.724 (1.329–5.584) | 0.006 | ||
| Length of stay in the ICU, d | 0.993 (0.976–1.009) | 0.386 | ||
| TPI, d | 1.114 (0.867–1.415) | 0.114 | ||
| CRS-R score | 1.245 (1.097–1.412) | 0.001 | 1.258 (1.095–1.445) | 0.001 |
| CRS-R score ≥ 7 | 2.810 (1.390–5.680) | 0.004 | ||
| CCI, 0 | 0.443 | |||
| 1–2 | 1.000 (0.475–2.104) | 1.000 | ||
| ≥3 | 0.569 (0.213–1.473) | 0.240 | ||
| APACHE score | 0.938 (0.867–1.015) | 0.112 | ||
| Motor response, p | 1.345 (0.652–2.776) | 0.422 | ||
| NSE (μg/L), <33 | 1.221 (0.624–2.386) | 0.560 | ||
| Sleep spindles, p | 2.531 (1.250–5.126) | 0.010 | 2.368 (1.131–4.958) | 0.022 |
| Synek’s scale, <3 | 1.371 (0.690–2.724) | 0.367 | ||
| EEG R, p | 2.679 (1.086–6.609) | 0.032 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Kang, X.-G.; Gao, Q.; Liu, Y.; Song, C.-G.; Shi, X.-J.; Wu, J.-N.; Jiang, W. Long-Term Outcomes among Patients with Prolonged Disorders of Consciousness. Brain Sci. 2023, 13, 194. https://doi.org/10.3390/brainsci13020194
Liu Y, Kang X-G, Gao Q, Liu Y, Song C-G, Shi X-J, Wu J-N, Jiang W. Long-Term Outcomes among Patients with Prolonged Disorders of Consciousness. Brain Sciences. 2023; 13(2):194. https://doi.org/10.3390/brainsci13020194
Chicago/Turabian StyleLiu, Yan, Xiao-Gang Kang, Qiong Gao, Yu Liu, Chang-Geng Song, Xiao-Jing Shi, Jia-Ning Wu, and Wen Jiang. 2023. "Long-Term Outcomes among Patients with Prolonged Disorders of Consciousness" Brain Sciences 13, no. 2: 194. https://doi.org/10.3390/brainsci13020194





