Transcutaneous Electrical Cranial-Auricular Acupoint Stimulation Modulating the Brain Functional Connectivity of Mild-to-Moderate Major Depressive Disorder: An fMRI Study Based on Independent Component Analysis
Abstract
:1. Introduction
2. Materials and Methods
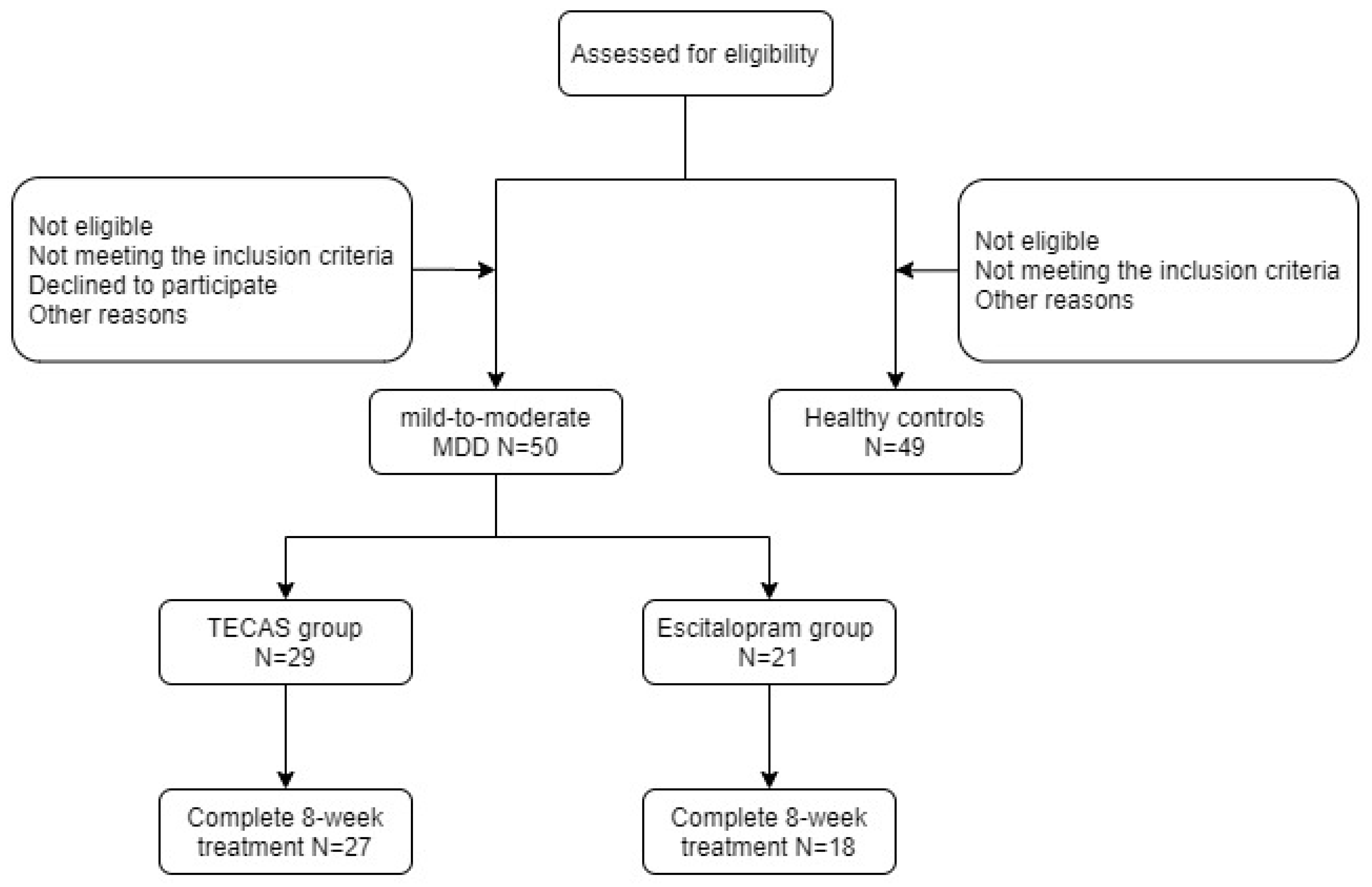
2.1. Participants
2.2. Clinical Scales
2.3. Intervention Procedures
2.4. Data Acquisition and Preprocessing of fMRI
2.5. Independent Component Analysis
2.6. Statistical Analysis
3. Results
3.1. Clinical and Demographic Data
3.2. Clinical Scales for the Patients in the TECAS and Escitalopram Data
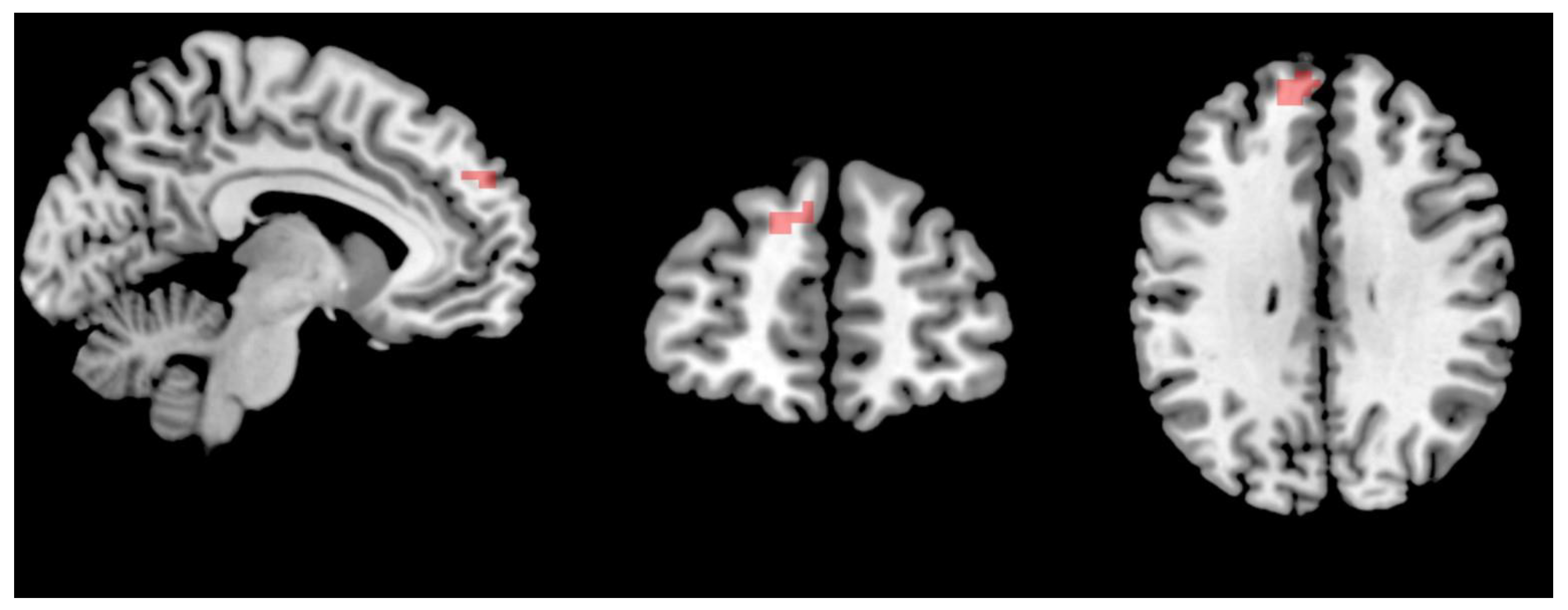
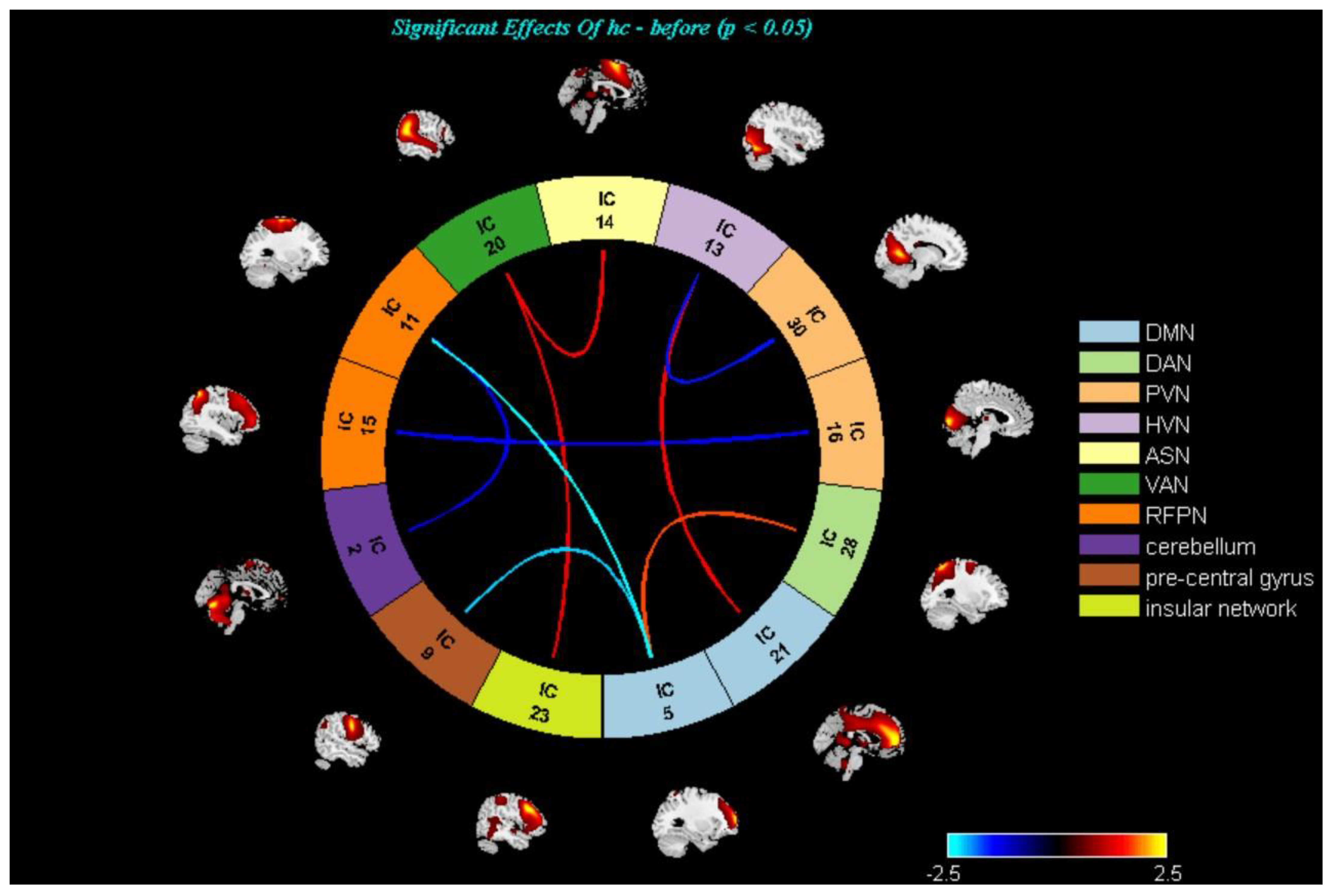
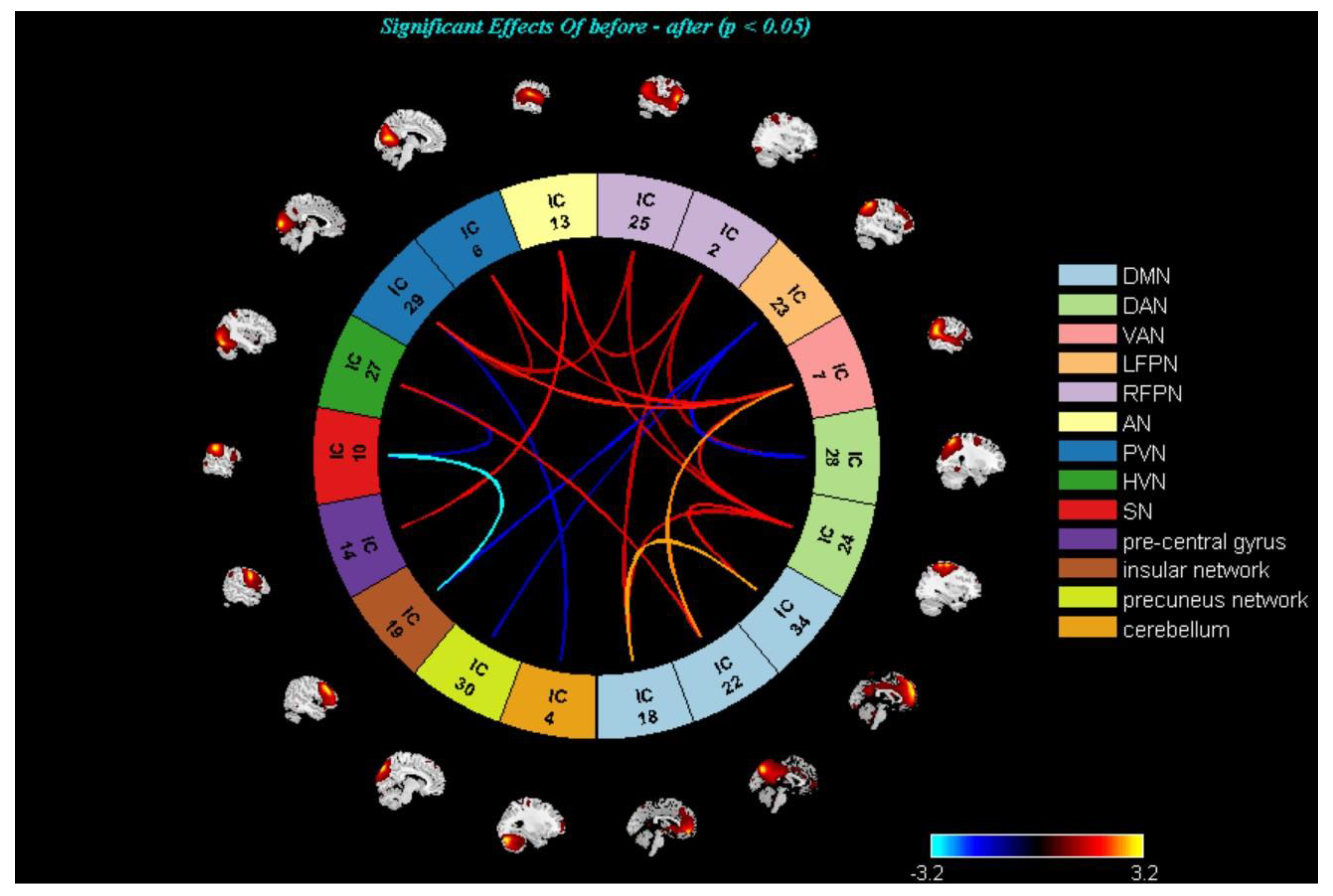
3.3. Changes in the Brain Networks and Correlation Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gore, F.M.; Bloem, P.J.; Patton, G.C.; Ferguson, J.; Joseph, V.; Coffey, C.; Sawyer, S.M.; Mathers, C.D. Global burden of disease in young people aged 10–24 years: A systematic analysis. Lancet 2011, 377, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.E.; Cafiero, E.T.; Jané-Llopis, E.; Abrahams-Gessel, S.; Bloom, L.R.; Fathima, S.; Feigl, A.B.; Gaziano, T.; Mowafi, M.; Pandya, A.; et al. The Global Economic Burden of Noncommunicable Diseases; World Economic Forum: Geneva, Switzerland, 2011. [Google Scholar]
- WHO. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Calhoun, V.D.; Adali, T.; Pearlson, G.D.; Pekar, J.J. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001, 14, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.J.; Sejnowski, T.J. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995, 7, 1129–1159. [Google Scholar] [CrossRef] [PubMed]
- Shahhosseini, Y.; Miranda, M.F. Functional Connectivity Methods and Their Applications in fMRI Data. Entropy 2022, 24, 390. [Google Scholar] [CrossRef]
- Dong, D.; Ming, Q.; Zhong, X.; Pu, W.; Zhang, X.; Jiang, Y.; Gao, Y.; Sun, X.; Wang, X.; Yao, S. State-independent alterations of intrinsic brain network in current and remitted depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 475–480. [Google Scholar] [CrossRef]
- Luo, L.; Wu, H.; Xu, J.; Chen, F.; Wu, F.; Wang, C.; Wang, J. Abnormal large-scale resting-state functional networks in drug-free major depressive disorder. Brain Imaging Behav. 2021, 15, 96–106. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, S.; Mo, Z.; Chattun, M.R.; Wang, Q.; Wang, L.; Zhu, R.; Shao, J.; Wang, X.; Yao, Z.; et al. Antidepressants normalize brain flexibility associated with multi-dimensional symptoms in major depressive patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 100, 109866. [Google Scholar] [CrossRef]
- Macêdo, M.A.; Sato, J.R.; Bressan, R.A.; Pan, P.M. Adolescent depression and resting-state fMRI brain networks: A scoping review of longitudinal studies. Braz. J. Psychiatry 2022, 44, 420–433. [Google Scholar] [CrossRef]
- Saris, I.M.J.; Penninx, B.W.J.H.; Dinga, R.; van Tol, M.-J.; Veltman, D.J.; van der Wee, N.J.A.; Aghajani, M. Default Mode Network Connectivity and Social Dysfunction in Major Depressive Disorder. Sci. Rep. 2020, 10, 194. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, M.; Yu, R.; Li, Y.; Yang, Y.; Cui, X.; Zheng, J. Abnormal Default Mode Network Homogeneity in Treatment-Naive Patients with First-Episode Depression. Front. Psychiatry 2018, 9, 697. [Google Scholar] [CrossRef]
- Yokoyama, S.; Okamoto, Y.; Takagaki, K.; Okada, G.; Takamura, M.; Mori, A.; Shiota, S.; Ichikawa, N.; Jinnin, R.; Yamawaki, S. Effects of behavioral activation on default mode network connectivity in subthreshold depression: A preliminary resting-state fMRI study. J. Affect Disord. 2018, 227, 156–163. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Bai, Y.; Liu, W.; Wang, H.; Leung, H.-C.; Tian, P.; Zhang, L.; Guo, F.; Cui, L.-B.; et al. Abnormal resting-state effective connectivity within the default mode network in major depressive disorder: A spectral dynamic causal modeling study. Brain Behav. 2017, 7, 00732. [Google Scholar] [CrossRef]
- Cao, J.; Ai, M.; Chen, X.; Chen, J.; Wang, W.; Kuang, L. Altered resting-state functional network connectivity is associated with suicide attempt in young depressed patients. Psychiatry Res. 2020, 285, 112713. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Yi, J.; Zhu, X.; Zhang, X.; Yang, J.; Yao, S. Default mode network alterations during implicit emotional faces processing in first-episode, treatment-naive major depression patients. Front. Psychol. 2015, 6, 1198. [Google Scholar] [CrossRef]
- Luo, L.; Lei, X.; Zhu, C.; Wu, J.; Ren, H.; Zhan, J.; Qin, Y. Decreased Connectivity in Precuneus of the Ventral Attentional Network in First-Episode, Treatment-Naïve Patients with Major Depressive Disorder: A Network Homogeneity and Independent Component Analysis. Front. Psychiatry 2022, 13, 925253. [Google Scholar] [CrossRef]
- Lu, F.; Cui, Q.; Huang, X.; Li, L.; Duan, X.; Chen, H.; Pang, Y.; He, Z.; Sheng, W.; Han, S.; et al. Anomalous intrinsic connectivity within and between visual and auditory networks in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 100, 109889. [Google Scholar] [CrossRef]
- Martens, M.A.G.; Filippini, N.; Harmer, C.J.; Godlewska, B.R. Resting-state functional connectivity patterns as biomarkers of treatment response to escitalopram in patients with major depressive disorder. Psychopharmacology 2021, 239, 3447–3460. [Google Scholar] [CrossRef]
- Belge, J.-B.; Mulders, P.C.; Van Oort, J.; Van Diermen, L.; Poljac, E.; Sabbe, B.; de Timary, P.; Constant, E.; Sienaert, P.; Schrijvers, D.; et al. Movement, mood and cognition: Preliminary insights into the therapeutic effects of electroconvulsive therapy for depression through a resting-state connectivity analysis. J. Affect Disord. 2021, 290, 117–127. [Google Scholar] [CrossRef]
- Tang, N.; Sun, C.; Wang, Y.; Li, X.; Liu, J.; Chen, Y.; Sun, L.; Rao, Y.; Li, S.; Qi, S.; et al. Clinical Response of Major Depressive Disorder Patients with Suicidal Ideation to Individual Target-Transcranial Magnetic Stimulation. Front. Psychiatry 2021, 12, 768819. [Google Scholar] [CrossRef]
- Teng, C.; Liu, T.; Zhang, N.; Zhong, Y.; Wang, C. Cognitive behavioral therapy may rehabilitate abnormally functional communication pattern among the triple-network in major depressive disorder: A follow-up study. J. Affect Disord. 2022, 304, 28–39. [Google Scholar] [CrossRef]
- American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder, 3rd ed.; American Psychiatric Association: Washington, VA, USA, 2010. [Google Scholar]
- Qaseem, A.; Barry, M.J.; Kansagara, D. Clinical Guidelines Committee of the American College of Physicians. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: A clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2016, 164, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Gartlehner, G.; Gaynes, B.N.; Amick, M.H.R.; Asher, G.N.; Morgan, M.L.C.; Coker-Schwimmer, M.E.; Forneris, C.; Boland, B.E.; Lux, L.J.; Gaylord, S.; et al. Comparative benefits and harms of antidepressant, psychological, complementary, and exercise treatments for major depression: An evidence report for a clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2016, 164, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Moret, C.; Isaac, M.; Briley, M. Review: Problems associated with long-term treatment with selective serotonin reuptake inhibitors. J. Psychopharmacol. 2009, 23, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Dubicka, B.; Hadley, S.; Roberts, C. Suicidal behaviour in youths with depression treated with new-generation antidepressants: Meta-analysis. Br. J. Psychiatry 2006, 189, 393–398. [Google Scholar] [CrossRef]
- Ribeiro, Â.; Ribeiro, J.P.; von Doellinger, O. Depression and psychodynamic psychotherapy. Braz. J. Psychiatry 2018, 40, 105–109. [Google Scholar] [CrossRef]
- Cuijpers, P.; Karyotaki, E.; Eckshtain, D.; Ng, M.Y.; Corteselli, K.A.; Noma, H.; Quero, S.; Weisz, J.R. Psychotherapy for Depression Across Different Age Groups: A Systematic Review and Meta-analysis. JAMA Psychiatry 2020, 77, 694–702. [Google Scholar] [CrossRef]
- Bottomley, J.M.; LeReun, C.; Diamantopoulos, A.; Mitchell, S.; Gaynes, B.N. Vagus nerve stimulation (VNS) therapy in patients with treatment resistant depression: A systematic review and meta-analysis. Compr. Psychiatry 2020, 98, 152156. [Google Scholar] [CrossRef]
- Nahas, Z.; Marangell, L.B.; Husain, M.M.; Rush, A.J.; Sackeim, H.A.; Lisanby, S.H.; Martinez, J.M.; George, M.S. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J. Clin. Psychiatry 2005, 66, 1097–1104. [Google Scholar] [CrossRef]
- Rush, A.J.; Sackeim, H.A.; Marangell, L.B.; George, M.S.; Brannan, S.K.; Davis, S.M.; Lavori, P.; Howland, R.; Kling, M.A.; Rittberg, B.; et al. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: A naturalistic study. Biol. Psychiatry 2005, 58, 355–363. [Google Scholar] [CrossRef]
- Hein, E.; Nowak, M.; Kiess, O.; Biermann, T.; Bayerlein, K.; Kornhuber, J.; Kraus, T. Auricular transcutaneous electrical nerve stimulation in depressed patients: A randomized controlled pilot study. J. Neural Transm. 2013, 120, 821–827. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.Y.; Hu, X.Y.; Hao, F. literature research on rule of points selection for depression treated by acupuncture. J. Clin. Acupunct. Moxibust. 2019, 35, 53–56. [Google Scholar]
- Jin, H.; Zheng, X.Y.; Chen, Y.; Sun, Z.; Zhang, Q.; Zhang, M. A Survey of Research on the Treatment of Depression with Acupuncture Baihui and Yintang. Chin. J. Ethnomed. Ethnopharm. 2021, 30, 62–66. [Google Scholar]
- Duan, G.; He, Q.; Pang, Y.; Chen, W.; Liao, H.; Liu, H.; Tan, L.; Liu, Y.; Tao, J.; Zhang, J.; et al. Altered amygdala resting-state functional connectivity following acupuncture stimulation at BaiHui (GV20) in first-episode drug-Naïve major depressive disorder. Brain Imaging Behav. 2020, 14, 2269–2280. [Google Scholar] [CrossRef]
- Fang, J.; Rong, P.; Hong, Y.; Fan, Y.; Liu, J.; Wang, H.; Zhang, G.; Chen, X.; Shi, S.; Wang, L.; et al. Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biol. Psychiatry 2016, 79, 266–273. [Google Scholar] [CrossRef]
- Rong, P.; Liu, J.; Wang, L.; Liu, R.; Fang, J.; Zhao, J.; Zhao, Y.; Wang, H.; Vangel, M.; Sun, S.; et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: A nonrandomized controlled pilot study. J. Affect Disord. 2016, 195, 172–179. [Google Scholar] [CrossRef]
- Zeng, Y.; Xia, J.; Chen, Z.; Tian, X.; Ren, Y. Transcutaneous electrical acupoint stimulation (TEAS) for cancer-related fatigue: Study protocol for a systematic review and meta-analysis. BMJ Open 2021, 11, e049318. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, Y.; Luo, L.Y.; Wu, J.Y.; Yang, S.J.; Zhang, N.; Zhang, Z.J. Transcutaneous electrical acupoint stimulation for post-traumatic stress disorder: Assessor-blinded, randomized controlled study. Psychiatry Clin. Neurosci. 2019, 73, 179–186. [Google Scholar] [CrossRef]
- Paketci, S. Interpretation of the Montgomery-Åsberg Depression Rating Scale (MADRS). Br. J. Psychiatry 2021, 219, 620–621. [Google Scholar] [CrossRef]
- Hamilton, M. The Hamilton Rating Scale for Depression; Springer: Cham, Switzerland, 1986; pp. 143–152. [Google Scholar]
- Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef]
- Shiozawa, P.; da Silva, M.E.; Netto, G.T.M.; Taiar, I.; Cordeiro, Q. Effect of a 10- day trigeminal nerve stimulation (TNS) protocol for treating major depressive disorder: A phase II, sham-controlled, randomized clinical trial. Epilepsy Behav. 2015, 44, 23–26. [Google Scholar] [CrossRef]
- Cimpianu, C.L.; Strube, W.; Falkai, P.; Palm, U.; Hasan, A. Vagus nerve stimulation in psychiatry: A systematic review of the available evidence. J. Neural Transm. 2017, 124, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S. Acupuncture: Neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003, 26, 17–22. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Ng, R.; Man, S.C.; Li, T.Y.; Wong, W.; Tan, Q.R.; Wong, H.K.; Chung, K.F.; Wong, M.T.; Tsang, W.K. Dense cranial electroacupuncture stimulation for major depressive disorder—A single-blind, randomized, controlled study. PLoS ONE 2012, 7, e29651. [Google Scholar]
- Jia, X.-Z.; Wang, J.; Sun, H.-Y.; Zhang, H.; Liao, W.; Wang, Z.; Yan, C.-G.; Song, X.-W.; Zang, Y.-F. RESTplus: An improved toolkit for resting-state functional magnetic resonance imaging data processing. Sci. Bull. 2019, 64, 953–954. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.A.; Damaraju, E.; Plis, S.M.; Erhardt, E.B.; Eichele, T.; Calhoun, V.D. Tracking whole-brain connectivity dynamics in the resting-state. Cereb. Cortex. 2014, 24, 663–676. [Google Scholar] [CrossRef]
- Jafri, M.J.; Pearlson, G.D.; Stevens, M.; Calhoun, V.D. A method for functional network connectivity among spatially in dependent resting-state components in schizophrenia. Neuroimage 2008, 39, 1666–1681. [Google Scholar] [CrossRef]
- Inoue, T.; Sasai, K.; Kitagawa, T.; Nishimura, A.; Inada, I. Randomized, double-blind, placebo-controlled study to assess the efficacy and safety of vortioxetine in Japanese patients with major depressive disorder. Psychiatry Clin. Neurosci. 2020, 74, 140–148. [Google Scholar] [CrossRef]
- Carstens, L.; Hartling, C.; Stippl, A.; Domke, A.-K.; Herrera-Mendelez, A.L.; Aust, S.; Gärtner, M.; Bajbouj, M.; Grimm, S. A symptom-based approach in predicting ECT outcome in depressed patients employing MADRS single items. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 1275–1284. [Google Scholar] [CrossRef]
- O’Reardon, J.P.; Solvason, H.B.; Janicak, P.G.; Sampson, S.; Isenberg, K.E.; Nahas, Z.; McDonald, W.M.; Avery, D.; Fitzgerald, P.B.; Loo, C.; et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biol. Psychiatry 2007, 62, 1208–1216. [Google Scholar] [CrossRef]
- Carneiro, A.M.; Fernandes, F.; Moreno, R.A. Hamilton depression rating scale and montgomery-asberg depression rat ing scale in depressed and bipolar I patients: Psychometric properties in a Brazilian sample. Health Qual. Life Outcomes 2015, 13, 42. [Google Scholar] [CrossRef]
- Yang, S.; Qin, Z.; Yang, X.; Chan, M.Y.; Zhang, S.; Rong, P.; Hou, X.; Jin, G.; Xu, F.; Liu, Y.; et al. Transcutaneous Electrical Cranial-Auricular Acupoint Stimulation vs. Escitalopram for Patients with MMD (TECAS): Study Design for a Randomized Controlled, Non-inferiority Trial. Front. Psychiatry 2022, 13, 829932. [Google Scholar] [CrossRef]
- Gusnard, D.A.; Raichle, M.E.; Raichle, M.E. Searching for a baseline: Functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001, 2, 685–694. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Ford, J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76. [Google Scholar] [CrossRef]
- Kaiser, R.H.; Andrews-Hanna, J.R.; Wager, T.D.; Pizzagalli, D.A. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry 2015, 72, 603–611. [Google Scholar] [CrossRef]
- Mulders, P.C.; van Eijndhoven, P.F.; Schene, A.H.; Beckmann, C.F.; Tendolkar, I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci. Biobehav. Rev. 2015, 56, 330–344. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Mantini, D.; Perrucci, M.G.; Del Gratta, C.; Romani, G.L.; Corbetta, M. Electrophysiological signatures of resting-state networks in the human brain. Proc. Natl. Acad. Sci. USA 2007, 104, 13170–13175. [Google Scholar] [CrossRef]
- Cole, M.W.; Repovš, G.; Anticevic, A. The frontoparietal control system: A central role in mental health. Neuroscientist 2014, 20, 652–664. [Google Scholar] [CrossRef]
- Satz, S.; Halchenko, Y.O.; Ragozzino, R.; Lucero, M.M.; Phillips, M.L.; Swartz, H.A.; Manelis, A. The Relationship Between Default Mode and Dorsal Attention Networks Is Associated with Depressive Disorder Diagnosis and the Strength of Memory Representations Acquired Prior to the Resting State Scan. Front. Hum. Neurosci. 2022, 16, 749767. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Fair, D.A.; Miezin, F.M.; Cohen, A.L.; Wenger, K.K.; Dosenbach, R.A.T.; Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E.; et al. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. USA 2007, 104, 11073–11078. [Google Scholar] [CrossRef]
- Chang, X.; Shen, H.; Wang, L.; Liu, Z.; Xin, W.; Hu, D.; Miao, D. Altered default mode and fronto-parietal network subsystems in patients with schizophrenia and their unaffected siblings. Brain Res. 2014, 1562, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; An, J.; Zeng, L.; Shen, H.; Qiu, S.; Hu, D. Altered functional connectivity among default, attention, and control networks in idiopathic generalized epilepsy. Epilepsy Behav. 2015, 46, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Griffiths, K.R.; Breukelaar, I.A.; Barreiros, A.R.; Chen, W.; Boyce, P.; Hazell, P.; Foster, S.L.; Malhi, G.S.; Harris, A.W.F.; et al. Default-mode and fronto-parietal network connectivity during rest distinguishes asymptomatic patients with bipolar disorder and major depressive disorder. Transl. Psychiatry 2021, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, T.; Yu, C.; Tian, L.; Li, J.; Liu, Y.; Zhou, Y.; Xu, L.; Song, M.; Li, K. Spontaneous activity associated with primary visual cortex: A resting-state FMRI study. Cereb. Cortex. 2008, 18, 697–704. [Google Scholar] [CrossRef]
- Viviani, R. Emotion regulation, attention to emotion, and the ventral attentional network. Front. Hum. Neurosci. 2013, 7, 746. [Google Scholar] [CrossRef]
- Liu, J.; Xu, P.; Zhang, J.; Jiang, N.; Li, X.; Luo, Y. Ventral attention-network effective connectivity predicts individual differences in adolescent depression. J. Affect Disord. 2019, 252, 55–59. [Google Scholar] [CrossRef]
- Sylvester, C.M.; Barch, D.M.; Corbetta, M.; Power, J.D.; Schlaggar, B.L.; Luby, J.L. Resting-state functional connectivity of the ventral attention network in children with a history of depression or anxiety. J. Am. Acad. Child. Adolesc. Psychiatry 2013, 52, 1326–1336.e5. [Google Scholar] [CrossRef]


| Variables | HCs | MDD | Analysis | |
|---|---|---|---|---|
| p Value | χ2 | |||
| Sample size | 49 | 50 | - | - |
| Age (years) a | 46.57 ± 11.87 | 51.08 ± 11.77 | 0.061 | - |
| Sex (M/F) b | 15/34 | 8/42 | - | 0.085 |
| Education level (none/PS/JSH/CD) b | 0/17/9/23 | 10/13/13/14 | - | 0.01 * |
| MADRS a | 1.37 ± 1.67 | 16.48 ± 4.08 | <0.001 | - |
| HAMD a | 1.61 ± 2.35 | 13.70 ± 5.45 | <0.001 | - |
| HAMA a | 1.06 ± 1.53 | 13.44 ± 5.40 | <0.001 | - |
| TECAS | Escitalopram | ||||||
|---|---|---|---|---|---|---|---|
| Before | After | p1 Value | Before | After | p2 Value | p3 Value | |
| Sample size | 27 | 18 | |||||
| MADRS ac | 16.33 ± 3.69 | 9.00 ± 4.867 | <0.001 | 16.67 ± 4.88 | 7.72 ± 3.89 | <0.001 | 0.795 △ |
| HAMD ac | 16.41 ± 3.91 | 10.04 ± 4.10 | <0.001 | 14.83 ± 3.33 | 9.11 ± 5.12 | <0.001 | 0.168 △ |
| HAMA ac | 15.26 ± 4.71 | 9.44 ± 4.80 | <0.001 | 15.22 ± 3.69 | 9.61 ± 4.85 | <0.001 | 0.978 △ |
| ER in MADRS | 44.40% | 55.50% | |||||
| ER in HAMD | 33.30% | 38.80% | |||||
| ER in HAMA | 44.40% | 38.80% | |||||
| RR in MADRS a | 0.457 ± 0.246 | 0.526 ± 0.233 | 0.351 | ||||
| RR in HAMD a | 0.363 ± 0.261 | 0.360 ± 0.379 | 0.977 | ||||
| RR in HAMA a | 0.258 ± 0.652 | 0.335 ± 0.356 | 0.648 | ||||
| SERS a | 6.55 ± 3.17 | 5.44 ± 3.601 | 0.321 | ||||
| Age (years) a | 50.63 ± 12.61 | 51.5 ± 11.96 | 0.818 | ||||
| Gender (M/F) b | 6/21 | 2/16 | 0.445 * | ||||
| Education (none/PS/JSH/CD) b | 5/7/4/11 | 5/7/4/11 | 0.119 * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, L.; Zhang, L.; Lv, J.; Liu, Y.; Fang, J.; Rong, P.; Liu, Y. Transcutaneous Electrical Cranial-Auricular Acupoint Stimulation Modulating the Brain Functional Connectivity of Mild-to-Moderate Major Depressive Disorder: An fMRI Study Based on Independent Component Analysis. Brain Sci. 2023, 13, 274. https://doi.org/10.3390/brainsci13020274
Liao L, Zhang L, Lv J, Liu Y, Fang J, Rong P, Liu Y. Transcutaneous Electrical Cranial-Auricular Acupoint Stimulation Modulating the Brain Functional Connectivity of Mild-to-Moderate Major Depressive Disorder: An fMRI Study Based on Independent Component Analysis. Brain Sciences. 2023; 13(2):274. https://doi.org/10.3390/brainsci13020274
Chicago/Turabian StyleLiao, Lifang, Liulu Zhang, Jun Lv, Yingchun Liu, Jiliang Fang, Peijing Rong, and Yong Liu. 2023. "Transcutaneous Electrical Cranial-Auricular Acupoint Stimulation Modulating the Brain Functional Connectivity of Mild-to-Moderate Major Depressive Disorder: An fMRI Study Based on Independent Component Analysis" Brain Sciences 13, no. 2: 274. https://doi.org/10.3390/brainsci13020274
APA StyleLiao, L., Zhang, L., Lv, J., Liu, Y., Fang, J., Rong, P., & Liu, Y. (2023). Transcutaneous Electrical Cranial-Auricular Acupoint Stimulation Modulating the Brain Functional Connectivity of Mild-to-Moderate Major Depressive Disorder: An fMRI Study Based on Independent Component Analysis. Brain Sciences, 13(2), 274. https://doi.org/10.3390/brainsci13020274







