Anti-MOG Antibody-Associated Unilateral Cortical Encephalitis with Bilateral Meningeal Involvement: A Case Report
Abstract
:1. Introduction
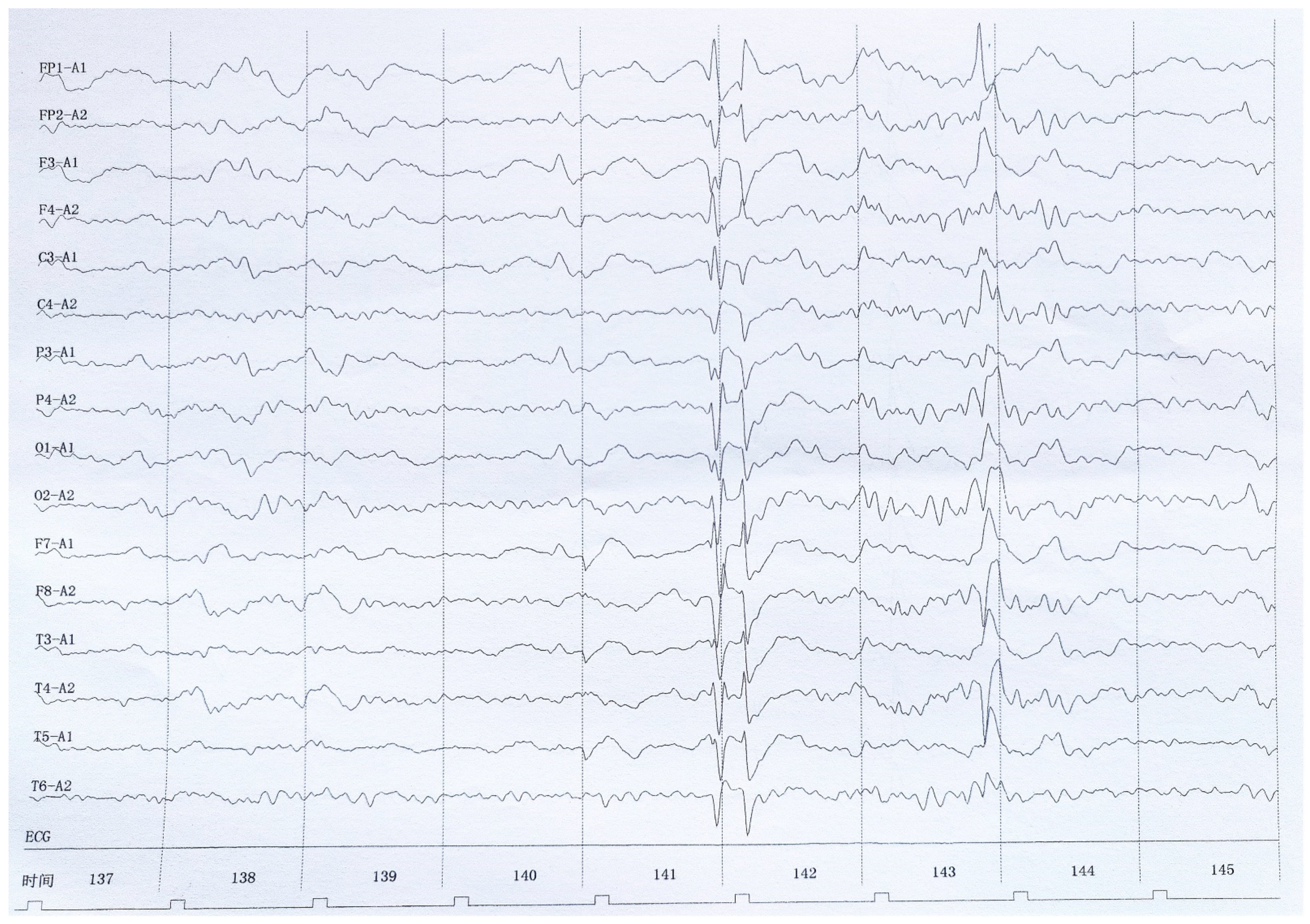
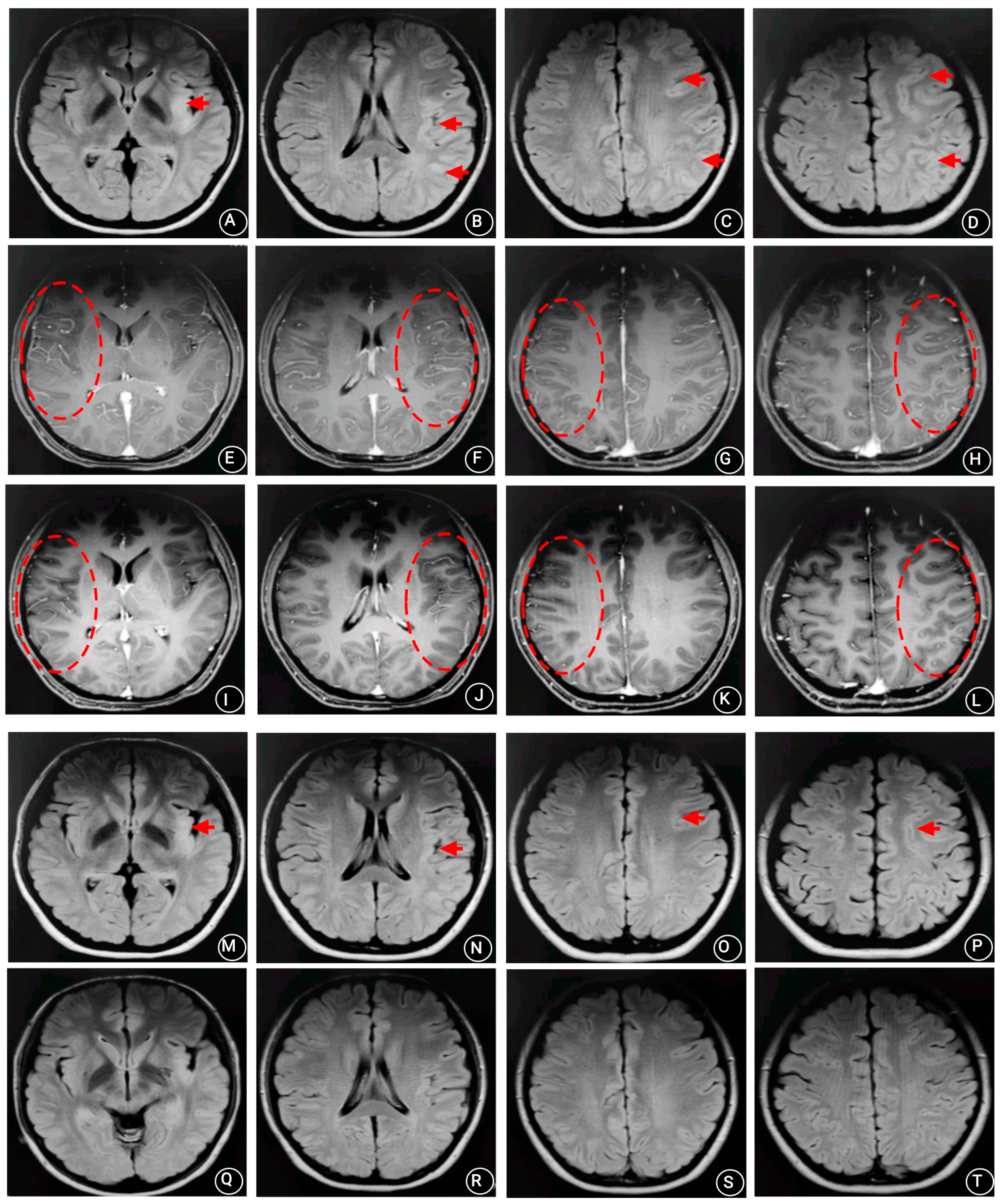
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Höftberger, R.; Guo, Y.; Flanagan, E.P.; Lopez-Chiriboga, A.S.; Endmayr, V.; Hochmeister, S.; Joldic, D.; Pittock, S.J.; Tillema, J.M.; Gorman, M.; et al. The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol. 2020, 139, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.T.; Zheng, Y.; Shen, C.H.; Yang, F.; Fang, W.; Zhang, Y.X.; Ding, M.P. Evaluation of brain and spinal cord lesion distribution criteria at disease onset in distinguishing NMOSD from MS and MOG antibody-associated disorder. Mult. Scler. 2021, 27, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Haddad, N.; Roussel, B.; Pelcovits, A.; Rizvi, S. Optic neuritis, encephalitis and leptomeningeal enhancement in a patient with anti-MOG antibodies: A case study. Mult. Scler. Relat. Disord. 2019, 34, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.J., Jr.; Pröbstel, A.K.; Zamvil, S.S. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat. Rev. Neurosci. 2019, 20, 728–745. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, S.; Gajofatto, A.; Batzu, L.; Delogu, R.; Sechi, G.; Leoni, S.; Pirastru, M.I.; Bonetti, B.; Zanoni, M.; Alberti, D.; et al. Relevance of antibodies to myelin oligodendrocyte glycoprotein in CSF of seronegative cases. Neurology 2019, 93, e1867–e1872. [Google Scholar] [CrossRef]
- Marignier, R.; Hacohen, Y.; Cobo-Calvo, A.; Pröbstel, A.K.; Aktas, O.; Alexopoulos, H.; Amato, M.P.; Asgari, N.; Banwell, B.; Bennett, J. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021, 20, 762–772. [Google Scholar] [CrossRef]
- Hennes, E.M.; Baumann, M.; Schanda, K.; Anlar, B.; Bajer-Kornek, B.; Blaschek, A.; Brantner-Inthaler, S.; Diepold, K.; Eisenkölbl, A.; Gotwald, T.; et al. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology 2017, 89, 900–908. [Google Scholar] [CrossRef]
- Gilani, A.; Kleinschmidt-DeMasters, B.K. Childhood Small-Vessel Primary Angiitis of the Central Nervous System: Overlap with MOG-Associated Disease. Pediatr. Dev. Pathol. 2022, 15, 18–29. [Google Scholar] [CrossRef]
- Spadaro, M.; Winklmeier, S.; Beltrán, E.; Macrini, C.; Höftberger, R.; Schuh, E.; Thaler, F.S.; Gerdes, L.A.; Laurent, S.; Gerhards, R. Pathogenicity of human antibodies against myelin oligodendrocyte glycoprotein. Ann. Neurol. 2018, 84, 315–328. [Google Scholar] [CrossRef]
- Chen, J.J.; Flanagan, E.P.; Bhatti, M.T.; Jitprapaikulsan, J.; Dubey, D.; Lopez Chiriboga, A.S.S.; Fryer, J.P.; Weinshenker, B.G.; McKeon, A.; Tillema, J.M.; et al. Steroid-sparing maintenance immunotherapy for MOG-IgG associated disorder. Neurology 2020, 95, e111–e120. [Google Scholar] [CrossRef]
- Salama, S.; Khan, M.; Pardo, S.; Izbudak, I.; Levy, M. MOG antibody-associated encephalomyelitis/encephalitis. Mult. Scler. J. 2019, 25, 1427–1433. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, Z.; Li, D.; Yang, X.; Ding, Y.; Gao, L.; Liu, A.; Song, Y.; Li, C.; Gao, R.; et al. Clinical and Imaging Features of Patients with Encephalitic Symptoms and Myelin Oligodendrocyte Glycoprotein Antibodies. Front. Immunol. 2021, 12, 722404. [Google Scholar] [CrossRef]
- Yao, T.; Zeng, Q.; Xie, Y.; Bi, F.; Zhang, L.; Xiao, B.; Zhou, J. Clinical analysis of adult MOG antibody-associated cortical encephalitis. Mult. Scler. Relat. Disord. 2022, 60, 103727. [Google Scholar] [CrossRef]
- Budhram, A.; Mirian, A.; Le, C.; Hosseini-Moghaddam, S.M.; Sharma, M.; Nicolle, M.W. Unilateral cortical FLAIR-hyperintense Lesions in Anti-MOG-associated Encephalitis with Seizures (FLAMES): Characterization of a distinct clinico-radiographic syndrome. J. Neurol. 2019, 266, 2481–2487. [Google Scholar] [CrossRef]
- Nie, H.; Gao, H.; Li, Y.; Shen, Y. Unilateral cerebral cortical encephalitis (CCE) with positive anti-MOG antibodies: A case report. Medicine 2021, 100, e26087. [Google Scholar] [CrossRef]
- Lin, S.; Long, W.; Wen, J.; Su, Q.; Liao, J.; Hu, Z. Myelin oligodendrocyte glycoprotein antibody-associated aseptic meningitis without neurological parenchymal lesions:A novel phenotype. Mult. Scler. Relat. Disord. 2022, 23, 104126. [Google Scholar] [CrossRef]
- Gombolay, G.Y.; Gadde, J.A. Aseptic meningitis and leptomeningeal enhancement associated with anti-MOG antibodies: A review. J. Neuroimmunol. 2021, 15, 577653. [Google Scholar] [CrossRef]
- Satukijchai, C.; Mariano, R.; Messina, S.; Sa, M.; Woodhall, M.R.; Robertson, N.P.; Ming, L.; Wassmer, E.; Kneen, R.; Huda, S.; et al. Factors Associated with Relapse and Treatment of Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease in the United Kingdom. JAMA Netw. Open. 2022, 5, e2142780. [Google Scholar] [CrossRef]
- Epstein, S.E.; Levin, S.; Onomichi, K.; Langston, C.; Yeshokumar, A.; Fabian, M.; Sand, I.K.; Klineova, S.; Lublin, F.; Dykstra, K.; et al. Myelin oligodendrocyte glycoprotein (MOG) antibody-mediated disease: The difficulty of predicting relapses. Mult. Scler. Relat. Disord. 2021, 56, 103229. [Google Scholar] [CrossRef]
- Salama, S.; Khan, M.; Shanechi, A.; Levy, M.; Izbudak, I. MRI differences between MOG antibody disease and AQP4 NMOSD. Mult. Scler. J. 2020, 26, 1854–1865. [Google Scholar] [CrossRef]
- Sugimoto, T.; Ishibashi, H.; Hayashi, M.; Tachiyama, K.; Fujii, H.; Kaneko, K.; Takahashi, T.; Kurokawa, K.; Yamawaki, T. A case of anti-MOG antibody-positive unilaterally dominant meningoencephalitis followed by longitudinally extensive transverse myelitis. Mult. Scler. Relat. Disord. 2018, 25, 128–130. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, B.; Li, S.; Liu, B.; Zhang, J.; Feng, Y. Anti-MOG Antibody-Associated Unilateral Cortical Encephalitis with Bilateral Meningeal Involvement: A Case Report. Brain Sci. 2023, 13, 283. https://doi.org/10.3390/brainsci13020283
Ren B, Li S, Liu B, Zhang J, Feng Y. Anti-MOG Antibody-Associated Unilateral Cortical Encephalitis with Bilateral Meningeal Involvement: A Case Report. Brain Sciences. 2023; 13(2):283. https://doi.org/10.3390/brainsci13020283
Chicago/Turabian StyleRen, Bo, Shiying Li, Bin Liu, Jinxia Zhang, and Yaqing Feng. 2023. "Anti-MOG Antibody-Associated Unilateral Cortical Encephalitis with Bilateral Meningeal Involvement: A Case Report" Brain Sciences 13, no. 2: 283. https://doi.org/10.3390/brainsci13020283
APA StyleRen, B., Li, S., Liu, B., Zhang, J., & Feng, Y. (2023). Anti-MOG Antibody-Associated Unilateral Cortical Encephalitis with Bilateral Meningeal Involvement: A Case Report. Brain Sciences, 13(2), 283. https://doi.org/10.3390/brainsci13020283







