Temporal Stability of the Dynamic Resting-State Functional Brain Network: Current Measures, Clinical Research Progress, and Future Perspectives
Abstract
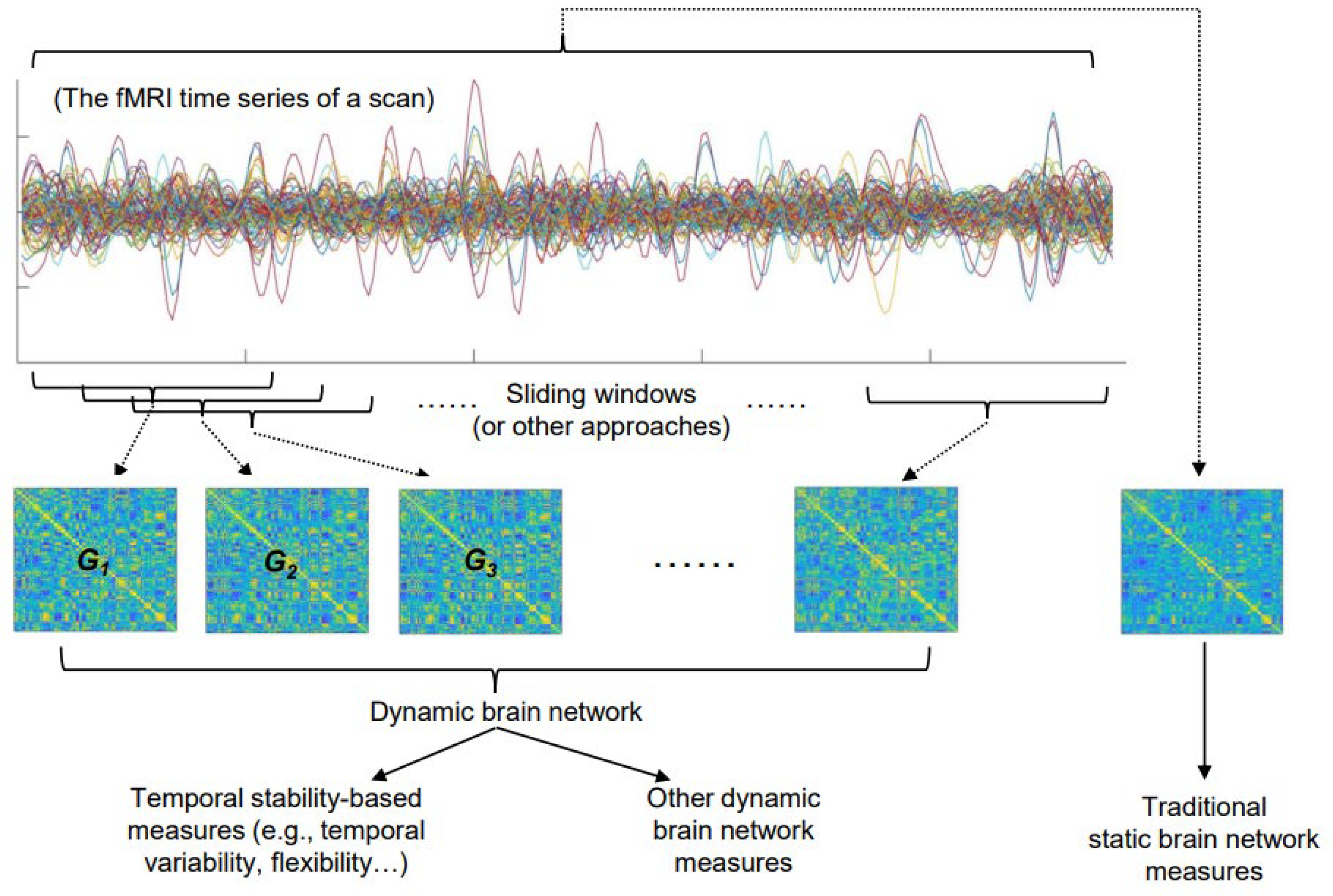
:1. Introduction
2. The Basic Concepts and Commonly Used Measures of the Temporal Stability of a Brain Network
3. Possible Influencing Factors When Analyzing the Temporal Stability of a Brain Network
4. Research Progress on Possible Relationships between the Temporal Stability of Resting-State Brain Networks and Common Psychiatric Disorders
| Reference | Sample | Measure of Temporal Stability | Main Findings on the Temporal Stability in SZ Patients |
|---|---|---|---|
| Zhang et al. [18] | Two datasets: 69 SZ patients/62 HCs and 53 SZ patients/67 HCs | Temporal variability | Decreased stability in subcortical and visual regions; increased stability in default-mode regions |
| Dong et al. [34] | 102 SZ patients/124 HCs | Temporal variability | Decreased stability in visual, sensorimotor, and attention networks, as well as thalamus; increased stability in default-mode and frontal–parietal networks |
| Long et al. [32] | 66 SZ patients/66 HCs | Temporal variability | Decreased temporal stability in sensorimotor, visual, attention, limbic, and subcortical areas; increased stability in default-mode areas |
| Gifford et al. [67] | 55 SZ patients/72 HCs | Flexibility | Decreased stability in cerebellar, subcortical, and fronto-parietal task control networks |
| Guo et al. [70] | 22 SZ patients/60 HCs | Temporal variability | Decreased stability in dFC anchored on the precuneus |
| Wang et al. [68] | 42 SZ patients/35 HCs | Standard deviation | Decreased stability in dFCs among multiple networks |
| Sheng et al. [69] | Two datasets: 51 SZ patients/63 HCs and 36 SZ patients/60 HCs | Temporal variability | Decreased stability in prefrontal cortex, anterior cingulate cortex, temporal cortex, visual cortex, and hippocampus |
| Reference | Sample | Measure of Temporal Stability | Main Findings on the Temporal Stability in MDD Patients |
|---|---|---|---|
| Demirtaş et al. [74] | 27 MDD patients/ 27 HCs | Variance/mean | Increased stability in dFCs between default-mode and fronto-parietal networks |
| Long et al. [17] | 460 MDD patients/ 473 HCs | Temporal variability and temporal clustering coefficient | Decreased stability mainly in default-mode, sensorimotor, and subcortical areas |
| Wise et al. [73] | Two datasets: 20 MDD patients/19 HCs and 19 MDD patients/19 HCs | The standard deviation | Decreased stability within several key default-mode regions |
| Zhao et al. [39] | 55 MDD patients/ 62 HCs | Temporal clustering coefficient | Decreased stability at global level and in sensory perception regions |
| Hou et al. [71] | 77 MDD patients/ 40 HCs | Temporal variability | Decreased stability in inferior occipital gyrus and pallidum |
| Ouyang et al. [40] | 55 MDD patients/ 21 HCs | Temporal clustering coefficient | Decreased stability at global level, and within default-mode and subcortical networks |
| Zhou et al. [30] | 19 MDD patients/ 22 HCs | The variance | Increased stability in dorsolateral prefrontal cortex and precuneus connectivity |
| Tian et al. [75] | 35 MDD patients/ 35 HCs | Flexibility | Increased stability within default-mode and cognitive control networks |
| Han et al. [76] | 61 MDD patients/61 HCs | Flexibility (switching rate) | Increased stability in precuneus, parahippocampal gyrus, dorsal medial prefrontal cortex, anterior insula, amygdala, and striatum |
| Reference | Sample | Measure of Temporal Stability | Main Findings on the Temporal Stability in BD Patients |
|---|---|---|---|
| Nguyen et al. [79] | 15 euthymic BD patients/19 HCs | Standard deviation | Increased stability between medial prefrontal lobe and posterior cingulate gyrus |
| Han et al. [76] | 40 BD patients/61 HCs | Flexibility (switching rate) | Increased stability in precuneus, parahippocampal gyrus, and dorsal medial prefrontal cortex |
| Wang et al. [81] | 51 depressed BD patients/52 HCs | Standard deviation | Increased stability between default-mode and central executive networks |
| Long et al. [32] | 53 BD patients/66 HCs | Temporal variability | Decreased stability in dFCs within subcortical areas and between thalamus and sensorimotor areas |
| Liang et al. [80] | 18 BD patients/19 HC | Standard deviation | Increased stability in dFC between posterior cingulate cortex and medial prefrontal cortex |
| Luo et al. [82] | 106 depressed BD patients/130 HCs | Standard deviation | Decreased stability between posterior cingulate cortex/precuneus and inferior parietal lobule |
5. Discussion: Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Reference | Diagnostic Criteria for SZ | Was Drug Abuse History Excluded? | Were Other Severe Psychiatric or Somatic Disorders Excluded? |
|---|---|---|---|
| Zhang et al. [18] | DSM-IV | Yes | Yes |
| Dong et al. [34] | DSM-IV | Yes | Yes |
| Long et al. [32] | DSM-IV | Yes | Yes |
| Gifford et al. [67] | DSM-IV | Yes | Yes |
| Guo et al. [70] | DSM-IV | Yes | Yes |
| Wang et al. [68] | DSM-IV | Yes | Yes |
| Sheng et al. [69] | DSM-IV | Yes | Yes |
| Reference | Diagnostic Criteria for MDD | Was Drug Abuse History Excluded? | Were Other Severe Psychiatric or Somatic Disorders Excluded? |
|---|---|---|---|
| Demirtaş et al. [74] | DSM-IV | Yes | Yes |
| Long et al. [17] | DSM-IV | Not mentioned | Not mentioned |
| Wise et al. [73] | DSM-IV | Yes | Yes |
| Zhao et al. [39] | DSM-IV | Yes | Yes |
| Hou et al. [71] | DSM-IV | Yes | Yes |
| Ouyang et al. [40] | DSM-IV | Yes | Yes |
| Zhou et al. [30] | DSM-IV | Yes | Yes |
| Tian et al. [75] | DSM-IV | Yes | Yes |
| Han et al. [76] | DSM-IV | Yes | Yes |
| Reference | Diagnostic Criteria for BD | Was Drug Abuse History Excluded? | Were Other Severe Psychiatric or Somatic Disorders Excluded? |
|---|---|---|---|
| Nguyen et al. [79] | DSM-IV | Yes | Yes |
| Han et al. [76] | DSM-IV | Yes | Yes |
| Wang et al. [81] | DSM-IV | Yes | Yes |
| Long et al. [32] | DSM-IV | Yes | Yes |
| Liang et al. [80] | DSM-IV | Yes | Yes |
| Luo et al. [82] | DSM-V | Yes | Yes |
References
- Canario, E.; Chen, D.; Biswal, B. A Review of Resting-State FMRI and Its Use to Examine Psychiatric Disorders. Psychoradiology 2021, 1, 42–53. [Google Scholar] [CrossRef]
- Lin, Z.; Long, Y.; Wu, Z.; Xiang, Z.; Ju, Y.; Liu, Z. Associations between Brain Abnormalities and Common Genetic Variants for Schizophrenia: A Narrative Review of Structural and Functional Neuroimaging Findings. Ann. Palliat. Med. 2021, 10, 10031–10052. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zhou, H.; Cannon, T.D. Functional Connectome-Wide Associations of Schizophrenia Polygenic Risk. Mol. Psychiatry 2021, 26, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhang, L.; Li, L.; Ji, E.; Han, X.; Huang, G.; Liang, Z.; Shi, L.; Yang, H.; Zhang, Z. Abnormal Transitions of Dynamic Functional Connectivity States in Bipolar Disorder: A Whole-Brain Resting-State FMRI Study. J. Affect. Disord. 2021, 289, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Macoveanu, J.; Stougaard, M.E.; Kjærstad, H.L.; Knudsen, G.M.; Vinberg, M.; Kessing, L.V.; Miskowiak, K.W. Trajectory of Aberrant Reward Processing in Patients with Bipolar Disorder—A Longitudinal FMRI Study. J. Affect. Disord. 2022, 312, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, X.; Chen, Z.B.; Li, L.; Li, X.Y.; Castellanos, F.X.; Bai, T.J.; Bo, Q.J.; Cao, J.; Chang, Z.K.; et al. Disrupted Intrinsic Functional Brain Topology in Patients with Major Depressive Disorder. Mol. Psychiatry 2021, 26, 7363–7371. [Google Scholar] [CrossRef]
- Yan, C.G.; Chen, X.; Li, L.; Castellanos, F.X.; Bai, T.J.; Bo, Q.J.; Cao, J.; Chen, G.M.; Chen, N.X.; Chen, W.; et al. Reduced Default Mode Network Functional Connectivity in Patients with Recurrent Major Depressive Disorder. Proc. Natl. Acad. Sci. USA 2019, 116, 9078–9083. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, R.M.; Womelsdorf, T.; Gati, J.S.; Everling, S.; Menon, R.S. Resting-State Networks Show Dynamic Functional Connectivity in Awake Humans and Anesthetized Macaques. Hum. Brain Mapp. 2013, 34, 2154–2177. [Google Scholar] [CrossRef]
- Chang, C.; Glover, G.H. Time-Frequency Dynamics of Resting-State Brain Connectivity Measured with FMRI. Neuroimage 2010, 50, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Cavanna, F.; Vilas, M.G.; Palmucci, M.; Tagliazucchi, E. Dynamic Functional Connectivity and Brain Metastability during Altered States of Consciousness. Neuroimage 2018, 180, 383–395. [Google Scholar] [CrossRef] [Green Version]
- Shunkai, L.; Chen, P.; Zhong, S.; Chen, G.; Zhang, Y.; Zhao, H.; He, J.; Su, T.; Yan, S.; Luo, Y.; et al. Alterations of Insular Dynamic Functional Connectivity and Psychological Characteristics in Unmedicated Bipolar Depression Patients with a Recent Suicide Attempt. Psychol. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Preti, M.G.; Bolton, T.A.; van de Ville, D. The Dynamic Functional Connectome: State-of-the-Art and Perspectives. Neuroimage 2017, 160, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liao, X.; Xia, M.; He, Y. Chronnectome Fingerprinting: Identifying Individuals and Predicting Higher Cognitive Functions Using Dynamic Brain Connectivity Patterns. Hum. Brain Mapp. 2018, 39, 902–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, Y.; Ouyang, X.; Yan, C.; Wu, Z.; Huang, X.; Pu, W.; Cao, H.; Liu, Z.; Palaniyappan, L. Evaluating Test–Retest Reliability and Sex-/Age-Related Effects on Temporal Clustering Coefficient of Dynamic Functional Brain Networks. Hum. Brain Mapp. 2023. [Google Scholar] [CrossRef] [PubMed]
- Omidvarnia, A.; Zalesky, A.; Mansour, L.S.; van de Ville, D.; Jackson, G.D.; Pedersen, M. Temporal Complexity of FMRI Is Reproducible and Correlates with Higher Order Cognition. Neuroimage 2021, 230, 117760. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, W.; Zhao, X.; Sha, M.; Liu, Y.; Zhang, X.; Ma, J.; Ni, H.; Ming, D. Age-Related Decline in the Variation of Dynamic Functional Connectivity: A Resting State Analysis. Front. Aging Neurosci. 2017, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Cao, H.; Yan, C.; Chen, X.; Li, L.; Castellanos, F.X.; Bai, T.; Bo, Q.; Chen, G.; Chen, N.; et al. Altered Resting-State Dynamic Functional Brain Networks in Major Depressive Disorder: Findings from the REST-Meta-MDD Consortium. Neuroimage Clin. 2020, 26, 102163. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, W.; Liu, Z.; Zhang, K.; Lei, X.; Yao, Y.; Becker, B.; Liu, Y.; Kendrick, K.M.; Lu, G.; et al. Neural, Electrophysiological and Anatomical Basis of Brain-Network Variability and Its Characteristic Changes in Mental Disorders. Brain 2016, 139, 2307–2321. [Google Scholar] [CrossRef] [Green Version]
- Rubinov, M.; Sporns, O. Complex Network Measures of Brain Connectivity: Uses and Interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Liu, D.; Tang, S.; Wu, Z.; Yang, J.; Liu, Z.; Wu, G.; Sariah, A.; Ouyang, X.; Long, Y. Changes in Brain Network Properties in Major Depressive Disorder Following Electroconvulsive Therapy: A Combined Static and Dynamic Functional Magnetic Resonance Imaging Study. Ann. Palliat. Med. 2022, 11, 1969–1980. [Google Scholar] [CrossRef]
- Sizemore, A.E.; Bassett, D.S. Dynamic Graph Metrics: Tutorial, Toolbox, and Tale. Neuroimage 2018, 180, 417–427. [Google Scholar] [CrossRef]
- Long, Y.; Chen, C.; Deng, M.; Huang, X.; Tan, W.; Zhang, L.; Fan, Z.; Liu, Z. Psychological Resilience Negatively Correlates with Resting-State Brain Network Flexibility in Young Healthy Adults: A Dynamic Functional Magnetic Resonance Imaging Study. Ann. Transl. Med. 2019, 7, 809. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Q.; Xie, A.; Pang, Y.; Sheng, W.; Tang, Q.; Li, D.; Huang, J.; He, Z.; Wang, Y.; et al. Abnormal Dynamic Functional Connectivity Density in Patients with Generalized Anxiety Disorder. J. Affect. Disord. 2020, 261, 49–57. [Google Scholar] [CrossRef]
- Sun, Y.; Collinson, S.L.; Suckling, J.; Sim, K. Dynamic Reorganization of Functional Connectivity Reveals Abnormal Temporal Efficiency in Schizophrenia. Schizophr. Bull. 2019, 45, 659–669. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Zheng, C.Y.; Handwerker, D.A.; Bandettini, P.A.; Calhoun, V.D.; Mitra, S.; Gonzalez-Castillo, J. Efficacy of Different Dynamic Functional Connectivity Methods to Capture Cognitively Relevant Information. Neuroimage 2019, 188, 502–514. [Google Scholar] [CrossRef]
- Allen, E.A.; Damaraju, E.; Plis, S.M.; Erhardt, E.B.; Eichele, T.; Calhoun, V.D. Tracking Whole-Brain Connectivity Dynamics in the Resting State. Cerebr. Cortex 2014, 24, 663–676. [Google Scholar] [CrossRef]
- Leonardi, N.; Richiardi, J.; Gschwind, M.; Simioni, S.; Annoni, J.M.; Schluep, M.; Vuilleumier, P.; van de Ville, D. Principal Components of Functional Connectivity: A New Approach to Study Dynamic Brain Connectivity during Rest. Neuroimage 2013, 83, 937–950. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, J.; Yang, Y.; Zhao, S.; Guo, L.; Han, J.; Hu, X. Test–Retest Reliability of Dynamic Functional Connectivity in Naturalistic Paradigm Functional Magnetic Resonance Imaging. Hum. Brain Mapp. 2022, 43, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Choe, A.S.; Nebel, M.B.; Barber, A.D.; Cohen, J.R.; Xu, Y.; Pekar, J.J.; Caffo, B.; Lindquist, M.A. Comparing Test-Retest Reliability of Dynamic Functional Connectivity Methods. Neuroimage 2017, 158, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yuan, Z.; Yingliang, D.; Chaoyong, X.; Ning, Z.; Chun, W. Differential Patterns of Dynamic Functional Connectivity Variability in Major Depressive Disorder Treated with Cognitive Behavioral Therapy. J. Affect. Disord. 2021, 291, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Baum, S.A.; Adduru, V.R.; Biswal, B.B.; Michael, A.M. Test-Retest Reliability of Dynamic Functional Connectivity in Resting State FMRI. Neuroimage 2018, 183, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Liu, Z.; Chan, C.K.Y.; Wu, G.; Xue, Z.; Pan, Y.; Chen, X.; Huang, X.; Li, D.; Pu, W. Altered Temporal Variability of Local and Large-Scale Resting-State Brain Functional Connectivity Patterns in Schizophrenia and Bipolar Disorder. Front. Psychiatry 2020, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Z.; Rolls, E.T.; Chen, Q.; Yao, Y.; Yang, W.; Wei, D.; Zhang, Q.; Zhang, J.; Feng, J.; et al. Verbal Creativity Correlates with the Temporal Variability of Brain Networks during the Resting State. Cerebr. Cortex 2019, 29, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Duan, M.; Wang, Y.; Zhang, X.; Jia, X.; Li, Y.; Xin, F.; Yao, D.; Luo, C. Reconfiguration of Dynamic Functional Connectivity in Sensory and Perceptual System in Schizophrenia. Cerebr. Cortex 2019, 29, 3577–3589. [Google Scholar] [CrossRef]
- Bassett, D.S.; Wymbs, N.F.; Porter, M.A.; Mucha, P.J.; Carlson, J.M.; Grafton, S.T. Dynamic Reconfiguration of Human Brain Networks during Learning. Proc. Natl. Acad. Sci. USA 2011, 108, 7641–7646. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, M.; Zalesky, A.; Omidvarnia, A.; Jackson, G.D. Multilayer Network Switching Rate Predicts Brain Performance. Proc. Natl. Acad. Sci. USA 2018, 115, 13376–13381. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Liu, Z.; Cao, H.; Yang, J.; Wu, Z.; Long, Y. Childhood Trauma Is Linked to Decreased Temporal Stability of Functional Brain Networks in Young Adults. J. Affect. Disord. 2021, 290, 23–30. [Google Scholar] [CrossRef]
- Betzel, R.F.; Satterthwaite, T.D.; Gold, J.I.; Bassett, D.S. Positive Affect, Surprise, and Fatigue Are Correlates of Network Flexibility. Sci. Rep. 2017, 7, 520. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Zhang, Y.; Chen, N.; Li, Y.; Guo, H.; Guo, M.; Yao, Z.; Hu, B. Altered Temporal Reachability Highlights the Role of Sensory Perception Systems in Major Depressive Disorder. Prog Neuropsychopharmacol. Biol. Psychiatry 2021, 112, 110426. [Google Scholar] [CrossRef]
- Ouyang, X.; Long, Y.; Wu, Z.; Liu, D.; Liu, Z.; Huang, X. Temporal Stability of Dynamic Default Mode Network Connectivity Negatively Correlates with Suicidality in Major Depressive Disorder. Brain Sci. 2022, 12, 1263. [Google Scholar] [CrossRef]
- Liao, W.; Li, J.; Duan, X.; Cui, Q.; Chen, H.; Chen, H. Static and Dynamic Connectomics Differentiate between Depressed Patients with and without Suicidal Ideation. Hum. Brain Mapp. 2018, 39, 4105–4118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhao, L.; Ji, J.; Ma, B.; Zhao, Z.; Wu, M.; Zheng, W.; Zhang, Z. Temporal Grading Index of Functional Network Topology Predicts Pain Perception of Patients with Chronic Back Pain. Front. Neurol. 2022, 13, 899254. [Google Scholar] [CrossRef] [PubMed]
- de Lacy, N.; McCauley, E.; Kutz, J.N.; Calhoun, V.D. Sex-Related Differences in Intrinsic Brain Dynamism and Their Neurocognitive Correlates. Neuroimage 2019, 202, 116116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cahill, N.D.; Arbabshirani, M.R.; White, T.; Baum, S.A.; Michael, A.M. Sex and Age Effects of Functional Connectivity in Early Adulthood. Brain Connect. 2016, 6, 700–713. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Wang, J.; Yan, C.; He, Y. Hemisphere- and Gender-Related Differences in Small-World Brain Networks: A Resting-State Functional MRI Study. Neuroimage 2011, 54, 191–202. [Google Scholar] [CrossRef]
- Mao, N.; Zheng, H.; Long, Z.; Yao, L.; Wu, X. Gender Differences in Dynamic Functional Connectivity Based on Resting-State FMRI. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, (EMBS), Jeju, Korea, 11–15 July 2017; pp. 2940–2943. [Google Scholar]
- Cai, B.; Zhang, G.; Zhang, A.; Hu, W.; Stephen, J.M.; Wilson, T.W.; Calhoun, V.D.; Wang, Y.P. A GICA-TVGL Framework to Study Sex Differences in Resting State FMRI Dynamic Connectivity. J. Neurosci. Methods 2020, 332, 108531. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, D.; Zhang, J.; Zhang, W.; Tao, H.; Ouyang, X.; Wu, G.; Chen, M.; Yu, M.; Zhou, L.; et al. Sex Difference in the Prevalence of Psychotic-like Experiences in Adolescents: Results from a Pooled Study of 21,248 Chinese Participants. Psychiatry Res. 2022, 317, 114894. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, D.; Tian, Y.; Du, Y.; Chen, J.; Zhou, H.; Chen, D.; Wang, L.; Alonzo, B.A.; Emily Wu, H.; et al. Sex Difference in Association between Insomnia and Cognitive Impairment in Patients with Chronic Schizophrenia. Schizophr. Res. 2022, 240, 143–149. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, B.; Xiang, Z.; Zou, Z.; Liu, Z.; Long, Y.; Chen, X. Increasing Trends in Mental Health Problems Among Urban Chinese Adolescents: Results from Repeated Cross-Sectional Data in Changsha 2016–2020. Front. Public Health 2022, 10, 1–7. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, Q.; Shi, L.; Li, M.Z.; Gong, W.; Chen, H.; Qiu, J. Tracking the Dynamic Functional Connectivity Structure of the Human Brain across the Adult Lifespan. Hum. Brain Mapp. 2019, 40, 717–728. [Google Scholar] [CrossRef] [Green Version]
- Marusak, H.A.; Calhoun, V.D.; Brown, S.; Crespo, L.M.; Sala-Hamrick, K.; Gotlib, I.H.; Thomason, M.E. Dynamic Functional Connectivity of Neurocognitive Networks in Children. Hum. Brain Mapp. 2017, 38, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chen, S.G.; Hu, D.; Zeng, L.L.; Fan, Y.M.; Chen, X.P.; Shen, H. Predicting Individual Brain Maturity Using Dynamic Functional Connectivity. Front. Hum. Neurosci. 2015, 9, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.; Wu, Z.; Cao, H.; Chen, X.; Wu, G.; Tan, W.; Liu, D.; Yang, J.; Long, Y.; Liu, Z. Age-Related Decrease in Default-Mode Network Functional Connectivity Is Accelerated in Patients with Major Depressive Disorder. Front. Aging Neurosci. 2022, 13, 809853. [Google Scholar] [CrossRef] [PubMed]
- Hilger, K.; Fukushima, M.; Sporns, O.; Fiebach, C.J. Temporal Stability of Functional Brain Modules Associated with Human Intelligence. Hum. Brain Mapp. 2020, 41, 362–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, U.; Schäfer, A.; Walter, H.; Erk, S.; Romanczuk-Seiferth, N.; Haddad, L.; Schweiger, J.I.; Grimm, O.; Heinz, A.; Tost, H.; et al. Dynamic Reconfiguration of Frontal Brain Networks during Executive Cognition in Humans. Proc. Natl. Acad. Sci. USA 2015, 112, 11678–11683. [Google Scholar] [CrossRef] [Green Version]
- Douw, L.; Wakeman, D.G.; Tanaka, N.; Liu, H.; Stufflebeam, S.M. State-Dependent Variability of Dynamic Functional Connectivity between Frontoparietal and Default Networks Relates to Cognitive Flexibility. Neuroscience 2016, 339, 12–21. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Zhuang, K.; Li, Y.; Sun, J.; Meng, J.; Zhu, W.; Mao, Y.; Chen, Q.; Chen, X.; Qiu, J. Brain Flexibility Associated with Need for Cognition Contributes to Creative Achievement. Psychophysiology 2019, 56, e13464. [Google Scholar] [CrossRef]
- Liu, D.; Liu, X.; Long, Y.; Xiang, Z.; Wu, Z.; Liu, Z.; Bian, D.; Tang, S. Problematic Smartphone Use Is Associated with Differences in Static and Dynamic Brain Functional Connectivity in Young Adults. Front. Neurosci. 2022, 16, 1010488. [Google Scholar] [CrossRef]
- Li, H.; Li, L.; Li, K.; Li, P.; Xie, W.; Zeng, Y.; Kong, L.; Long, T.; Huang, L.; Liu, X.; et al. Abnormal Dynamic Functional Network Connectivity in Male Obstructive Sleep Apnea with Mild Cognitive Impairment: A Data-Driven Functional Magnetic Resonance Imaging Study. Front. Aging Neurosci. 2022, 14, 977917. [Google Scholar] [CrossRef]
- Savva, A.D.; Kassinopoulos, M.; Smyrnis, N.; Matsopoulos, G.K.; Mitsis, G.D. Effects of Motion Related Outliers in Dynamic Functional Connectivity Using the Sliding Window Method. J. Neurosci. Methods 2020, 330, 108519. [Google Scholar] [CrossRef]
- Hutchison, R.M.; Womelsdorf, T.; Allen, E.A.; Bandettini, P.A.; Calhoun, V.D.; Corbetta, M.; della Penna, S.; Duyn, J.H.; Glover, G.H.; Gonzalez-Castillo, J.; et al. Dynamic Functional Connectivity: Promise, Issues, and Interpretations. Neuroimage 2013, 80, 360–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Specht, K. Current Challenges in Translational and Clinical FMRI and Future Directions. Front. Psychiatry 2020, 10, 924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodkinson, D.J.; O’Daly, O.; Zunszain, P.A.; Pariante, C.M.; Lazurenko, V.; Zelaya, F.O.; Howard, M.A.; Williams, S.C.R. Circadian and Homeostatic Modulation of Functional Connectivity and Regional Cerebral Blood Flow in Humans under Normal Entrained Conditions. J. Cereb. Blood Flow Metab. 2014, 34, 1493–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, K.; Fox, M.D. Towards a Consensus Regarding Global Signal Regression for Resting State Functional Connectivity MRI. Neuroimage 2017, 154, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, N.; van de Ville, D. On Spurious and Real Fluctuations of Dynamic Functional Connectivity during Rest. Neuroimage 2015, 104, 430–436. [Google Scholar] [CrossRef] [Green Version]
- Gifford, G.; Crossley, N.; Kempton, M.J.; Morgan, S.; Dazzan, P.; Young, J.; McGuire, P. Resting State FMRI Based Multilayer Network Configuration in Patients with Schizophrenia. Neuroimage Clin. 2020, 25, 102169. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Sun, Y.; Hu, M.; Chen, A. Aberrant Intra-Salience Network Dynamic Functional Connectivity Impairs Large-Scale Network Interactions in Schizophrenia. Neuropsychologia 2016, 93, 262–270. [Google Scholar] [CrossRef]
- Sheng, D.; Pu, W.; Linli, Z.; Tian, G.L.; Guo, S.; Fei, Y. Aberrant Global and Local Dynamic Properties in Schizophrenia with Instantaneous Phase Method Based on Hilbert Transform. Psychol. Med. 2021. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, W.; Tao, H.; Liu, Z.; Palaniyappan, L. The Instability of Functional Connectivity in Patients with Schizophrenia and Their Siblings: A Dynamic Connectivity Study. Schizophr. Res. 2018, 195, 183–189. [Google Scholar] [CrossRef]
- Hou, Z.; Kong, Y.; He, X.; Yin, Y.; Zhang, Y.; Yuan, Y. Increased Temporal Variability of Striatum Region Facilitating the Early Antidepressant Response in Patients with Major Depressive Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 85, 39–45. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Ford, J.M. Default Mode Network Activity and Connectivity in Psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76. [Google Scholar] [CrossRef] [PubMed]
- Wise, T.; Marwood, L.; Perkins, A.M.; Herane-Vives, A.; Joules, R.; Lythgoe, D.J.; Luh, W.M.; Williams, S.C.R.; Young, A.H.; Cleare, A.J.; et al. Instability of Default Mode Network Connectivity in Major Depression: A Two-Sample Confirmation Study. Transl. Psychiatry 2017, 7, e1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirtaş, M.; Tornador, C.; Falcón, C.; López-Solà, M.; Hernández-Ribas, R.; Pujol, J.; Menchón, J.M.; Ritter, P.; Cardoner, N.; Soriano-Mas, C.; et al. Dynamic Functional Connectivity Reveals Altered Variability in Functional Connectivity among Patients with Major Depressive Disorder. Hum. Brain Mapp. 2016, 37, 2918–2930. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Zhang, S.; Mo, Z.; Chattun, M.R.; Wang, Q.; Wang, L.; Zhu, R.; Shao, J.; Wang, X.; Yao, Z.; et al. Antidepressants Normalize Brain Flexibility Associated with Multi-Dimensional Symptoms in Major Depressive Patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 100, 109866. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Cui, Q.; Wang, X.; Li, L.; Li, D.; He, Z.; Guo, X.; Fan, Y.S.; Guo, J.; Sheng, W.; et al. Resting State Functional Network Switching Rate Is Differently Altered in Bipolar Disorder and Major Depressive Disorder. Hum. Brain Mapp. 2020, 41, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, B.; Yan, C.G. Reproducibility of R-FMRI Metrics on the Impact of Different Strategies for Multiple Comparison Correction and Sample Sizes. Hum. Brain Mapp. 2018, 39, 300–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.; Ouyang, X.; Huang, D.; Wu, Z.; Liu, Z.; He, Z.; Long, Y. Disrupted Intrinsic Functional Brain Network in Patients with Late-Life Depression: Evidence from a Multi-Site Dataset. J. Affect. Disord. 2023, 323, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Kovacevic, S.; Dev, S.I.; Lu, K.; Liu, T.T.; Eyler, L.T. Dynamic Functional Connectivity in Bipolar Disorder Is Associated with Executive Function and Processing Speed: A Preliminary Study. Neuropsychology 2017, 31, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Jiang, X.; Zhu, W.; Shen, Y.; Xue, F.; Li, Y.; Chen, Z. Disturbances of Dynamic Function in Patients With Bipolar Disorder I and Its Relationship With Executive-Function Deficit. Front. Psychiatry 2020, 11, 537981. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Huang, H.; Jia, Y.; Zheng, S.; Zhong, S.; Chen, G.; Huang, L.; Huang, R. Abnormal Dynamic Functional Network Connectivity in Unmedicated Bipolar and Major Depressive Disorders Based on the Triple-Network Model. Psychol. Med. 2020, 50, 465–474. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, G.; Jia, Y.; Zhong, S.; Gong, J.; Chen, F.; Wang, J.; Qi, Z.; Liu, X.; Huang, L.; et al. Shared and Specific Dynamics of Brain Segregation and Integration in Bipolar Disorder and Major Depressive Disorder: A Resting-State Functional Magnetic Resonance Imaging Study. J. Affect. Disord. 2021, 280, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ouyang, X.; Tao, H.; Pu, W.; Fan, Z.; Zeng, C.; Huang, X.; Chen, X.; Liu, J.; Liu, Z.; et al. Connectomic Signatures of Working Memory Deficits in Depression, Mania, and Euthymic States of Bipolar Disorder. J. Affect. Disord. 2020, 274, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Harlalka, V.; Bapi, R.S.; Vinod, P.K.; Roy, D. Atypical Flexibility in Dynamic Functional Connectivity Quantifies the Severity in Autism Spectrum Disorder. Front. Hum. Neurosci. 2019, 13, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Wu, Z.; Liu, Z.; Liu, D.; Huang, D.; Long, Y. Acute Effect of Betel Quid Chewing on Brain Network Dynamics: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Psychiatry 2021, 12, 701420. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Schäfer, A.; Bassett, D.S.; Rausch, F.; Schweiger, J.I.; Bilek, E.; Erk, S.; Romanczuk-Seiferth, N.; Grimm, O.; Geiger, L.S.; et al. Dynamic Brain Network Reconfiguration as a Potential Schizophrenia Genetic Risk Mechanism Modulated by NMDA Receptor Function. Proc. Natl. Acad. Sci. USA 2016, 113, 12568–12573. [Google Scholar] [CrossRef] [Green Version]
- Gama Marques, J.; Ouakinin, S. Schizophrenia-Schizoaffective-Bipolar Spectra: An Epistemological Perspective. CNS Spectr. 2021, 26, 197–201. [Google Scholar] [CrossRef]
- Marques, J.G.; Arantes-Gonçalves, F. A Perspective on a Possible Relation between the Psychopathology of the Schizophrenia/Schizoaffective Spectrum and Unconjugated Bilirubin: A Longitudinal Protocol Study. Front. Psychiatry 2018, 9, 146. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Pearlson, G.D.; Lin, D.; Sui, J.; Chen, J.; Salman, M.; Tamminga, C.A.; Ivleva, E.I.; Sweeney, J.A.; Keshavan, M.S.; et al. Identifying Dynamic Functional Connectivity Biomarkers Using GIG-ICA: Application to Schizophrenia, Schizoaffective Disorder, and Psychotic Bipolar Disorder. Hum. Brain Mapp. 2017, 38, 2683–2708. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Hao, H.; Wang, S.; Pearlson, G.D.; Calhoun, V.D. Identifying Commonality and Specificity across Psychosis Sub-Groups via Classification Based on Features from Dynamic Connectivity Analysis. Neuroimage Clin. 2020, 27, 102284. [Google Scholar] [CrossRef]
- Marques, J.G. Organic Psychosis Causing Secondary Schizophrenia in One-Fourth of a Cohort of 200 Patients Previously Diagnosed With Primary Schizophrenia. Prim. Care Companion CNS Disord. 2020, 22, 19m02549. [Google Scholar] [CrossRef]
- Long, Y.; Ouyang, X.; Liu, Z.; Chen, X.; Hu, X.; Lee, E.; Chen, E.Y.H.; Pu, W.; Shan, B.; Rohrbaugh, R.M. Associations among Suicidal Ideation, White Matter Integrity and Cognitive Deficit in First-Episode Schizophrenia. Front. Psychiatry 2018, 9, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madjirova, N.P.; Petrova, N.S.; Delchev, N.K. Daily Rhythmicity of Temperature, Pulse and Blood Pressure in Schizophrenic Patients. Schizophr Res. 1995, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Madjirova, N.P.; Tashev, T.G.; Delchev, N.N.; Bakalova, R.G. Interrelationship between Cortisol Levels in Plasma and the Circadian Rhythm of Temperature, Pulse and Blood Pressure in Depressed Patients with Good and Disturbed Sleep. Int. J. Psychophysiol. 1995, 20, 145–154. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Y.; Liu, X.; Liu, Z. Temporal Stability of the Dynamic Resting-State Functional Brain Network: Current Measures, Clinical Research Progress, and Future Perspectives. Brain Sci. 2023, 13, 429. https://doi.org/10.3390/brainsci13030429
Long Y, Liu X, Liu Z. Temporal Stability of the Dynamic Resting-State Functional Brain Network: Current Measures, Clinical Research Progress, and Future Perspectives. Brain Sciences. 2023; 13(3):429. https://doi.org/10.3390/brainsci13030429
Chicago/Turabian StyleLong, Yicheng, Xiawei Liu, and Zhening Liu. 2023. "Temporal Stability of the Dynamic Resting-State Functional Brain Network: Current Measures, Clinical Research Progress, and Future Perspectives" Brain Sciences 13, no. 3: 429. https://doi.org/10.3390/brainsci13030429
APA StyleLong, Y., Liu, X., & Liu, Z. (2023). Temporal Stability of the Dynamic Resting-State Functional Brain Network: Current Measures, Clinical Research Progress, and Future Perspectives. Brain Sciences, 13(3), 429. https://doi.org/10.3390/brainsci13030429





