A Cross-Sectional Study: Structural and Related Functional Connectivity Changes in the Brain: Stigmata of Adverse Parenting in Patients with Major Depressive Disorder?
Abstract
:1. Introduction
2. Methods
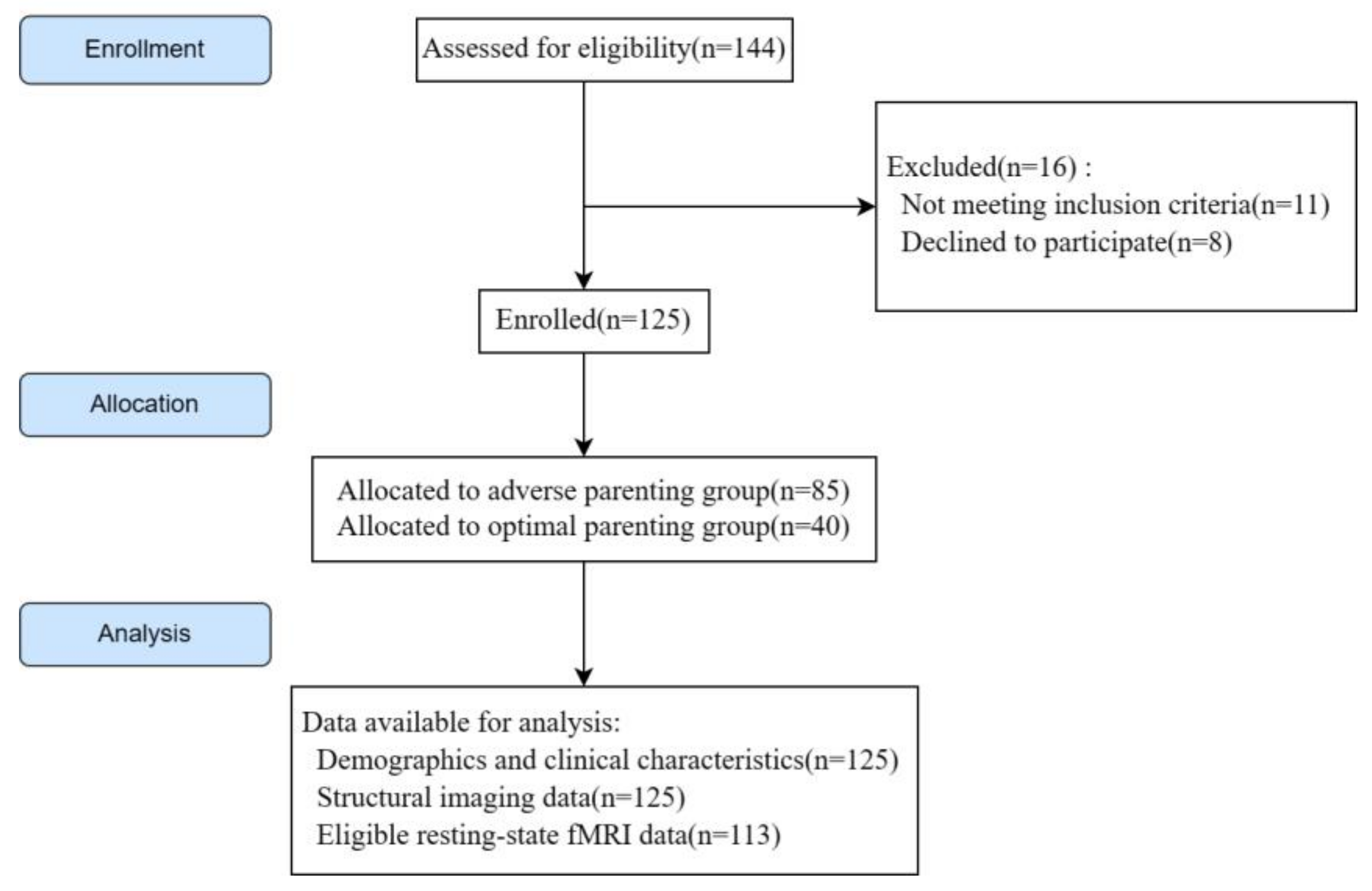
2.1. Study Design, Participants, and Inclusion and Exclusion Criteria
2.2. Assessment Instruments and Grouping Criteria
2.3. MRI Acquisition
2.4. MRI Data Processing
2.5. Voxel-Wise FC Analyses
2.6. Statistical Analyses
3. Results
3.1. Clinical Characteristics
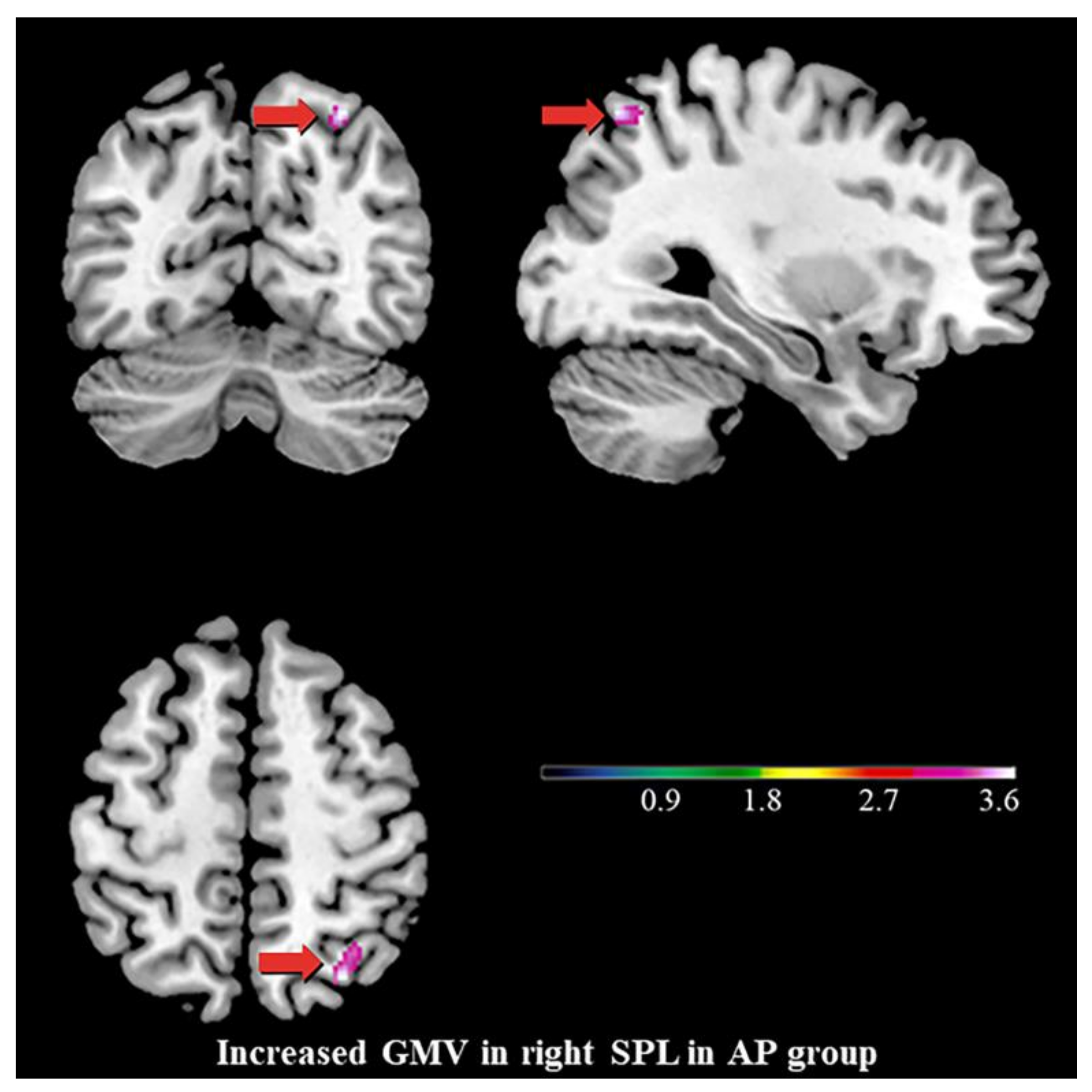
3.2. Differences in GMV
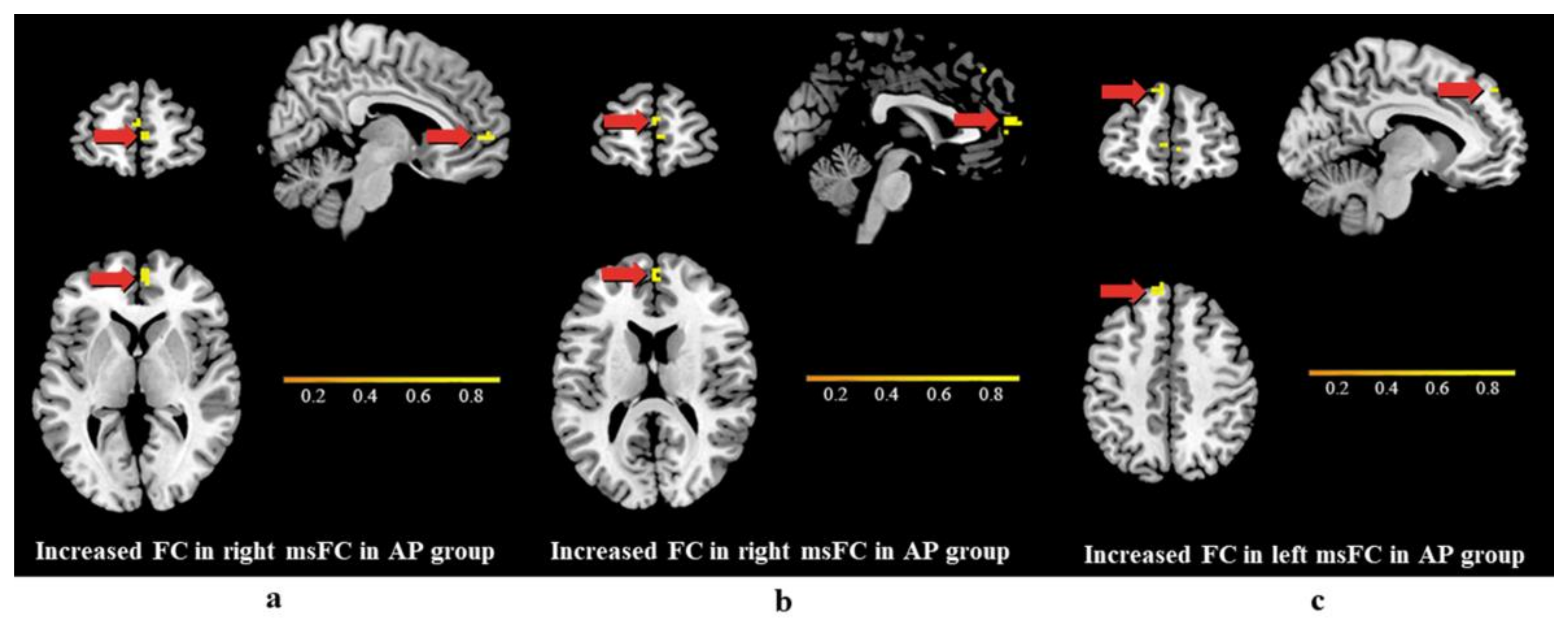
3.3. Differences in Functional Connectivity
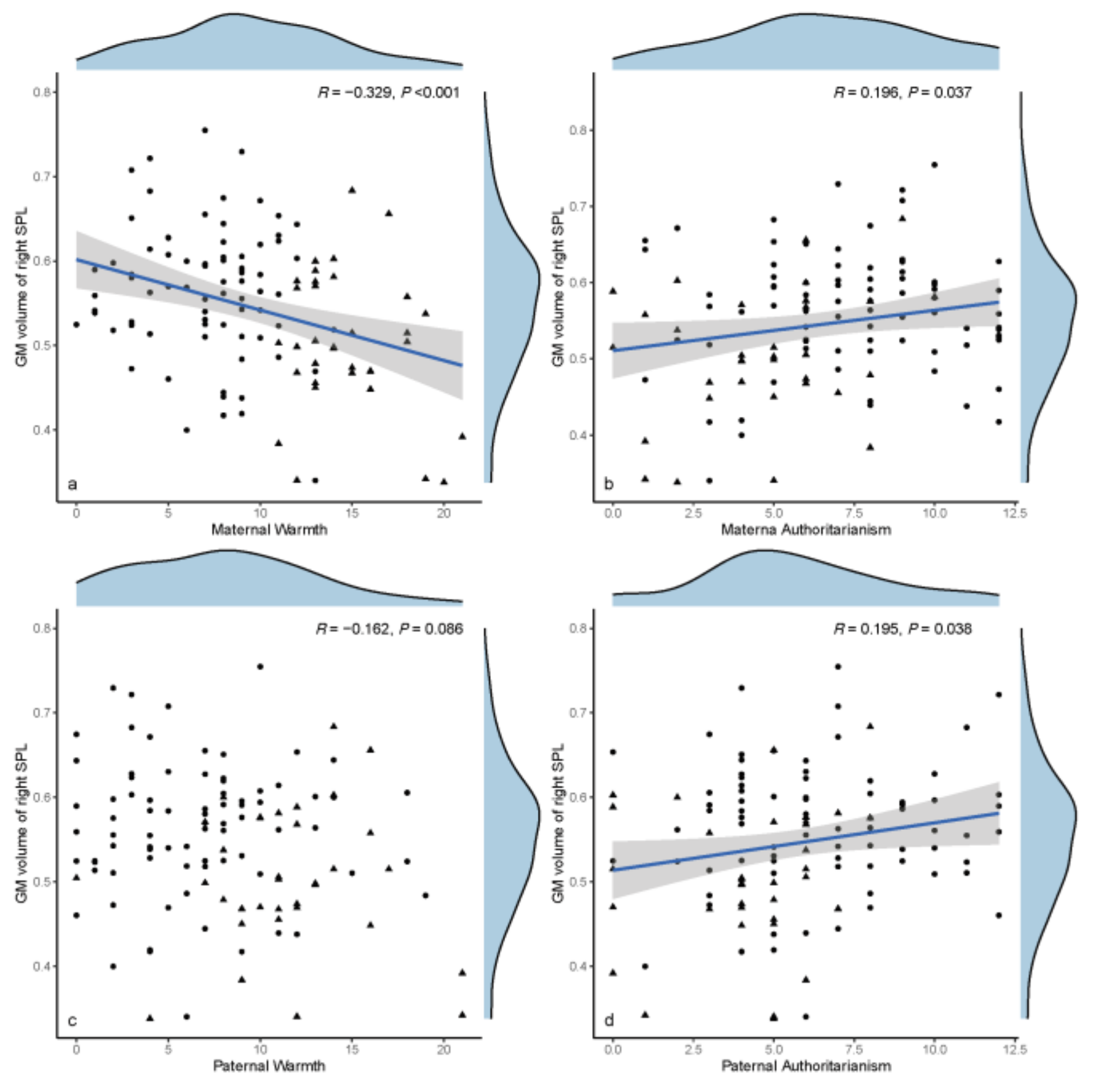
3.4. Exploratory Data Analyses
4. Discussion
4.1. AP in MDD
4.2. Increased GMV of the Right SPL, the Stigmata of Childhood AP?
4.3. Differences in Functional Connectivity
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dagnino, P.; Ugarte, M.J.; Morales, F.; Gonzalez, S.; Saralegui, D.; Ehrenthal, J.C. Risk Factors for Adult Depression: Adverse Childhood Experiences and Personality Functioning. Front. Psychol. 2020, 11, 594698. [Google Scholar] [CrossRef]
- Korotana, L.M.; Dobson, K.S.; Pusch, D.; Josephson, T. A review of primary care interventions to improve health outcomes in adult survivors of adverse childhood experiences. Clin. Psychol. Rev. 2016, 46, 59–90. [Google Scholar] [CrossRef]
- Lovallo, W.R.; Farag, N.H.; Sorocco, K.H.; Cohoon, A.J.; Vincent, A.S. Lifetime adversity leads to blunted stress axis reactivity: Studies from the Oklahoma Family Health Patterns Project. Biol. Psychiatry 2012, 71, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Bellis, M.A.; Hughes, K.; Ford, K.; Ramos Rodriguez, G.; Sethi, D.; Passmore, J. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: A systematic review and meta-analysis. Lancet Public Health 2019, 4, e517–e528. [Google Scholar] [CrossRef]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.; Bellis, M.A.; Hardcastle, K.A.; Sethi, D.; Butchart, A.; Mikton, C.; Jones, L.; Dunne, M.P. The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. Lancet Public Health 2017, 2, e356–e366. [Google Scholar] [CrossRef]
- Parker, G. Parental characteristics in relation to depressive disorders. Br. J. Psychiatry 1979, 134, 138–147. [Google Scholar] [CrossRef]
- Duggan, C.; Sham, P.; Minne, C.; Lee, A.; Murray, R. Quality of parenting and vulnerability to depression: Results from a family study. Psychol. Med. 1998, 28, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Myers, J.; Prescott, C.A. Parenting and adult mood, anxiety and substance use disorders in female twins: An epidemiological, multi-informant, retrospective study. Psychol. Med. 2000, 30, 281–294. [Google Scholar] [CrossRef]
- Perris, C.; Arrindell, W.A.; Perris, H.; Eisemann, M.; van der Ende, J.; von Knorring, L. Perceived depriving parental rearing and depression. Br. J. Psychiatry 1986, 148, 170–175. [Google Scholar] [CrossRef]
- Lancaster, G.; Rollinson, L.; Hill, J. The measurement of a major childhood risk for depression: Comparison of the Parental Bonding Instrument (PBI) ‘Parental Care’ and the Childhood Experience of Care and Abuse (CECA) ‘Parental Neglect’. J. Affect. Disord. 2007, 101, 263–267. [Google Scholar] [CrossRef]
- Oakley-Browne, M.A.; Joyce, P.R.; Wells, J.E.; Bushnell, J.A.; Hornblow, A.R. Adverse parenting and other childhood experience as risk factors for depression in women aged 18–44 years. J. Affect. Disord. 1995, 34, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, Y.; Cai, Y.; Chen, J.; Shen, Y.; Ni, S.; Wei, Y.; Qiu, Y.; Zhu, X.; Liu, Y.; et al. Perceived parenting and risk for major depression in Chinese women. Psychol. Med. 2012, 42, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Long, E.C.; Aggen, S.H.; Gardner, C.; Kendler, K.S. Differential parenting and risk for psychopathology: A monozygotic twin difference approach. Soc. Psychiatry Psychiatr. Epidemiol. 2015, 50, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L. Parent-child interaction and children’s depression: The relationships between parent-child interaction and children’s depressive symptoms in Taiwan. J. Adolesc. 2003, 26, 447–457. [Google Scholar] [CrossRef]
- Anno, K.; Shibata, M.; Ninomiya, T.; Iwaki, R.; Kawata, H.; Sawamoto, R.; Kubo, C.; Kiyohara, Y.; Sudo, N.; Hosoi, M. Paternal and maternal bonding styles in childhood are associated with the prevalence of chronic pain in a general adult population: The Hisayama Study. BMC Psychiatry 2015, 15, 181. [Google Scholar] [CrossRef]
- McCrory, E.; De Brito, S.A.; Viding, E. Research review: The neurobiology and genetics of maltreatment and adversity. J. Child Psychol. Psychiatry 2010, 51, 1079–1095. [Google Scholar] [CrossRef]
- Mao, Y.; Xiao, H.; Ding, C.; Qiu, J. The role of attention in the relationship between early life stress and depression. Sci. Rep. 2020, 10, 6154. [Google Scholar] [CrossRef]
- Gilbert, R.; Widom, C.S.; Browne, K.; Fergusson, D.; Webb, E.; Janson, S. Burden and consequences of child maltreatment in high-income countries. Lancet 2009, 373, 68–81. [Google Scholar] [CrossRef]
- Miguel, P.M.; Pereira, L.O.; Silveira, P.P.; Meaney, M.J. Early environmental influences on the development of children’s brain structure and function. Dev. Med. Child Neurol. 2019, 61, 1127–1133. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Weissman, D.; Bitran, D. Childhood Adversity and Neural Development: A Systematic Review. Annu. Rev. Dev. Psychol. 2019, 1, 277–312. [Google Scholar] [CrossRef]
- Aghamohammadi-Sereshki, A.; Coupland, N.J.; Silverstone, P.H.; Huang, Y.; Hegadoren, K.M.; Carter, R.; Seres, P.; Malykhin, N.V. Effects of childhood adversity on the volumes of the amygdala subnuclei and hippocampal subfields in individuals with major depressive disorder. J. Psychiatry Neurosci. 2021, 46, E186–E195. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zollei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
- Finn, E.S.; Shen, X.; Scheinost, D.; Rosenberg, M.D.; Huang, J.; Chun, M.M.; Papademetris, X.; Constable, R.T. Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015, 18, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.D.; Finn, E.S.; Scheinost, D.; Papademetris, X.; Shen, X.; Constable, R.T.; Chun, M.M. A neuromarker of sustained attention from whole-brain functional connectivity. Nat. Neurosci. 2016, 19, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.T.; Rosenberg, M.D.; Scheinost, D.; Constable, R.T.; Chun, M.M. Resting-state functional connectivity predicts neuroticism and extraversion in novel individuals. Soc. Cogn. Affect. Neurosci. 2018, 13, 224–232. [Google Scholar] [CrossRef]
- Cheng, T.W.; Mills, K.L.; Miranda Dominguez, O.; Zeithamova, D.; Perrone, A.; Sturgeon, D.; Feldstein Ewing, S.W.; Fisher, P.A.; Pfeifer, J.H.; Fair, D.A.; et al. Characterizing the impact of adversity, abuse, and neglect on adolescent amygdala resting-state functional connectivity. Dev. Cogn. Neurosci. 2021, 47, 100894. [Google Scholar] [CrossRef]
- Fadel, E.; Boeker, H.; Gaertner, M.; Richter, A.; Kleim, B.; Seifritz, E.; Grimm, S.; Wade-Bohleber, L.M. Differential Alterations in Resting State Functional Connectivity Associated with Depressive Symptoms and Early Life Adversity. Brain Sci. 2021, 11, 591. [Google Scholar] [CrossRef]
- Albertina, E.A.; Barch, D.M.; Karcher, N.R. Internalizing Symptoms and Adverse Childhood Experiences Associated with Functional Connectivity in a Middle Childhood Sample. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022. [CrossRef]
- Andrews-Hanna, J.R. The brain’s default network and its adaptive role in internal mentation. Neuroscientist 2012, 18, 251–270. [Google Scholar] [CrossRef]
- Davis, K.; Hirsch, E.; Gee, D.; Andover, M.; Roy, A.K. Mediating role of the default mode network on parental acceptance/warmth and psychopathology in youth. Brain Imaging Behav. 2022, 16, 2229–2238. [Google Scholar] [CrossRef]
- Vossel, S.; Geng, J.J.; Fink, G.R. Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist 2014, 20, 150–159. [Google Scholar] [CrossRef]
- Houtepen, L.C.; Heron, J.; Suderman, M.J.; Tilling, K.; Howe, L.D. Adverse childhood experiences in the children of the Avon Longitudinal Study of Parents and Children (ALSPAC). Wellcome Open Res. 2018, 3, 106. [Google Scholar] [CrossRef]
- Narita, K.; Fujihara, K.; Takei, Y.; Suda, M.; Aoyama, Y.; Uehara, T.; Majima, T.; Kosaka, H.; Amanuma, M.; Fukuda, M.; et al. Associations among parenting experiences during childhood and adolescence, hypothalamus-pituitary-adrenal axis hypoactivity, and hippocampal gray matter volume reduction in young adults. Hum. Brain Mapp. 2012, 33, 2211–2223. [Google Scholar] [CrossRef]
- Narita, K.; Takei, Y.; Suda, M.; Aoyama, Y.; Uehara, T.; Kosaka, H.; Amanuma, M.; Fukuda, M.; Mikuni, M. Relationship of parental bonding styles with gray matter volume of dorsolateral prefrontal cortex in young adults. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Kang, L.; Guo, X.; Liu, H.; Yao, L.; Bai, H.; Chen, C.; Hu, M.; Du, L.; Du, H.; et al. Discrepancies between self-rated depression and observed depression severity: The effects of personality and dysfunctional attitudes. Gen. Hosp. Psychiatry 2021, 70, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Li, R.; Liu, H.; Ma, S.; Sun, S.; Zhang, N.; Yao, L.; Wang, Y.; Zong, X.; Ai, C.; et al. Nonsuicidal self-injury in undergraduate students with major depressive disorder: The role of psychosocial factors. J. Affect. Disord. 2021, 290, 102–108. [Google Scholar] [CrossRef] [PubMed]
- World Medical, A. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- Kendler, K.S. Parenting: A genetic-epidemiologic perspective. Am. J. Psychiatry 1996, 153, 11–20. [Google Scholar] [CrossRef]
- Trajkovic, G.; Starcevic, V.; Latas, M.; Lestarevic, M.; Ille, T.; Bukumiric, Z.; Marinkovic, J. Reliability of the Hamilton Rating Scale for Depression: A meta-analysis over a period of 49 years. Psychiatry Res. 2011, 189, 1–9. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999, 282, 1737–1744. [Google Scholar] [CrossRef]
- Maier, W.; Buller, R.; Philipp, M.; Heuser, I. The Hamilton Anxiety Scale: Reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 1988, 14, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Snaith, R.P.; Hamilton, M.; Morley, S.; Humayan, A.; Hargreaves, D.; Trigwell, P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br. J. Psychiatry 1995, 167, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Proust-Lima, C.; Amieva, H.; Dartigues, J.F.; Jacqmin-Gadda, H. Sensitivity of four psychometric tests to measure cognitive changes in brain aging-population-based studies. Am. J. Epidemiol. 2007, 165, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Parker, G. Parental representations of patients with anxiety neurosis. Acta Psychiatr. Scand. 1981, 63, 33–36. [Google Scholar] [CrossRef]
- Plantes, M.M.; Prusoff, B.A.; Brennan, J.; Parker, G. Parental representations of depressed outpatients from a U.S.A. sample. J. Affect. Disord. 1988, 15, 149–155. [Google Scholar] [CrossRef]
- Parker, G. Parental reports of depressives. An investigation of several explanations. J. Affect. Disord. 1981, 3, 131–140. [Google Scholar] [CrossRef]
- Maldjian, J.A.; Laurienti, P.J.; Kraft, R.A.; Burdette, J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003, 19, 1233–1239. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, C.; Liang, M.; Li, J.; Tian, L.; Zhou, Y.; Qin, W.; Li, K.; Jiang, T. Whole brain functional connectivity in the early blind. Brain 2007, 130, 2085–2096. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, Z.; Zhang, B.; Zhang, W.; Wang, J.; Ni, W.; Miao, Y.; Liu, J.; Bi, Y. Enhancement of Impaired Olfactory Neural Activation and Cognitive Capacity by Liraglutide, but Not Dapagliflozin or Acarbose, in Patients with Type 2 Diabetes: A 16-Week Randomized Parallel Comparative Study. Diabetes Care 2022, 45, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Nichols, T.E. Multiple testing corrections, nonparametric methods, and random field theory. Neuroimage 2012, 62, 811–815. [Google Scholar] [CrossRef]
- Kiefner-Burmeister, A.; Hinman, N. The Role of General Parenting Style in Child Diet and Obesity Risk. Curr. Nutr. Rep. 2020, 9, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, L.; Fang, F. Psychometric properties of the Chinese version of the Parental Bonding Instrument. Int. J. Nurs. Stud. 2011, 48, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Amianto, F.; Martini, M.; Olandese, F.; Davico, C.; Abbate-Daga, G.; Fassino, S.; Vitiello, B. Affectionless control: A parenting style associated with obesity and binge eating disorder in adulthood. Eur. Eat. Disord. Rev. 2021, 29, 178–192. [Google Scholar] [CrossRef]
- Goschin, S.; Briggs, J.; Blanco-Lutzen, S.; Cohen, L.J.; Galynker, I. Parental affectionless control and suicidality. J. Affect. Disord. 2013, 151, 1–6. [Google Scholar] [CrossRef]
- Otani, K.; Suzuki, A.; Matsumoto, Y.; Sadahiro, R.; Enokido, M. Affectionless control by the same-sex parents increases dysfunctional attitudes about achievement. Compr. Psychiatry 2014, 55, 1411–1414. [Google Scholar] [CrossRef]
- Chao, R.K. Extending research on the consequences of parenting style for Chinese Americans and European Americans. Child Dev. 2001, 72, 1832–1843. [Google Scholar] [CrossRef]
- Rohner, R.P.; Pettengill, S.M. Perceived parental acceptance-rejection and parental control among Korean adolescents. Child Dev. 1985, 56, 524–528. [Google Scholar] [CrossRef]
- Handa, H.; Ito, A.; Tsuda, H.; Ohsawa, I.; Ogawa, T. Low level of parental bonding might be a risk factor among women with prolonged depression: A preliminary investigation. Psychiatry Clin. Neurosci. 2009, 63, 721–729. [Google Scholar] [CrossRef]
- Bremner, J.D.; Vythilingam, M.; Vermetten, E.; Southwick, S.M.; McGlashan, T.; Nazeer, A.; Khan, S.; Vaccarino, L.V.; Soufer, R.; Garg, P.K.; et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am. J. Psychiatry 2003, 160, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.B.; Koverola, C.; Hanna, C.; Torchia, M.G.; McClarty, B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol. Med. 1997, 27, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimaki, M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Iob, E.; Lacey, R.; Steptoe, A. The long-term association of adverse childhood experiences with C-reactive protein and hair cortisol: Cumulative risk versus dimensions of adversity. Brain Behav. Immun. 2020, 87, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Carvalho, L.A.; Pariante, C.M. Glucocorticoids, cytokines and brain abnormalities in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 722–729. [Google Scholar] [CrossRef]
- Milbocker, K.A.; Campbell, T.S.; Collins, N.; Kim, S.; Smith, I.F.; Roth, T.L.; Klintsova, A.Y. Glia-Driven Brain Circuit Refinement Is Altered by Early-Life Adversity: Behavioral Outcomes. Front. Behav. Neurosci. 2021, 15, 786234. [Google Scholar] [CrossRef]
- Emoto, K.; Hensch, T.K.; Yuzaki, M. “Scrap & build” functional circuits: Molecular and cellular basis of neural remodeling. Neurosci. Res. 2021, 167, 1–2. [Google Scholar] [CrossRef]
- Espinosa, J.S.; Stryker, M.P. Development and plasticity of the primary visual cortex. Neuron 2012, 75, 230–249. [Google Scholar] [CrossRef]
- Huh, C.Y.L.; Abdelaal, K.; Salinas, K.J.; Gu, D.; Zeitoun, J.; Figueroa Velez, D.X.; Peach, J.P.; Fowlkes, C.C.; Gandhi, S.P. Long-term Monocular Deprivation during Juvenile Critical Period Disrupts Binocular Integration in Mouse Visual Thalamus. J. Neurosci. 2020, 40, 585–604. [Google Scholar] [CrossRef]
- Vainchtein, I.D.; Chin, G.; Cho, F.S.; Kelley, K.W.; Miller, J.G.; Chien, E.C.; Liddelow, S.A.; Nguyen, P.T.; Nakao-Inoue, H.; Dorman, L.C.; et al. Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science 2018, 359, 1269–1273. [Google Scholar] [CrossRef]
- Blakemore, S.J.; Choudhury, S. Brain development during puberty: State of the science. Dev. Sci. 2006, 9, 11–14. [Google Scholar] [CrossRef]
- Luby, J.L.; Baram, T.Z.; Rogers, C.E.; Barch, D.M. Neurodevelopmental Optimization after Early-Life Adversity: Cross-Species Studies to Elucidate Sensitive Periods and Brain Mechanisms to Inform Early Intervention. Trends Neurosci. 2020, 43, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Modrono, C.; Navarrete, G.; Nicolle, A.; Gonzalez-Mora, J.L.; Smith, K.W.; Marling, M.; Goel, V. Developmental grey matter changes in superior parietal cortex accompany improved transitive reasoning. Think. Reason. 2019, 25, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Linkersdorfer, J.; Jurcoane, A.; Lindberg, S.; Kaiser, J.; Hasselhorn, M.; Fiebach, C.J.; Lonnemann, J. The association between gray matter volume and reading proficiency: A longitudinal study of beginning readers. J. Cogn. Neurosci. 2015, 27, 308–318. [Google Scholar] [CrossRef]
- Devous, M.D., Sr.; Altuna, D.; Furl, N.; Cooper, W.; Gabbert, G.; Ngai, W.T.; Chiu, S.; Scott, J.M., 3rd; Harris, T.S.; Payne, J.K.; et al. Maturation of speech and language functional neuroanatomy in pediatric normal controls. J. Speech Lang. Hear. Res. 2006, 49, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Whitford, T.J.; Rennie, C.J.; Grieve, S.M.; Clark, C.R.; Gordon, E.; Williams, L.M. Brain maturation in adolescence: Concurrent changes in neuroanatomy and neurophysiology. Hum. Brain Mapp. 2007, 28, 228–237. [Google Scholar] [CrossRef]
- Dosenbach, N.U.; Nardos, B.; Cohen, A.L.; Fair, D.A.; Power, J.D.; Church, J.A.; Nelson, S.M.; Wig, G.S.; Vogel, A.C.; Lessov-Schlaggar, C.N.; et al. Prediction of individual brain maturity using fMRI. Science 2010, 329, 1358–1361. [Google Scholar] [CrossRef]
- Kharitonova, M.; Martin, R.E.; Gabrieli, J.D.; Sheridan, M.A. Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Dev. Cogn. Neurosci. 2013, 6, 61–71. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Jacobus, J.; Sorg, S.F.; Jernigan, T.L.; Tapert, S.F. Early adolescent cortical thinning is related to better neuropsychological performance. J. Int. Neuropsychol. Soc. 2013, 19, 962–970. [Google Scholar] [CrossRef]
- Sowell, E.R.; Thompson, P.M.; Leonard, C.M.; Welcome, S.E.; Kan, E.; Toga, A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 2004, 24, 8223–8231. [Google Scholar] [CrossRef] [PubMed]
- Tooley, U.A.; Bassett, D.S.; Mackey, A.P. Environmental influences on the pace of brain development. Nat. Rev. Neurosci. 2021, 22, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; Short, A.K.; Othy, S.; Kooiker, C.L.; Shao, M.; Gunn, B.G.; Beck, J.; Bai, X.; Law, S.M.; Savage, J.C.; et al. Early stress-induced impaired microglial pruning of excitatory synapses on immature CRH-expressing neurons provokes aberrant adult stress responses. Cell Rep. 2022, 38, 110600. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.L. Neuroinflammation, Early-Life Adversity, and Brain Development. Harv. Rev. Psychiatry 2022, 30, 24–39. [Google Scholar] [CrossRef]
- Ivy, A.S.; Rex, C.S.; Chen, Y.; Dube, C.; Maras, P.M.; Grigoriadis, D.E.; Gall, C.M.; Lynch, G.; Baram, T.Z. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci. 2010, 30, 13005–13015. [Google Scholar] [CrossRef]
- Malave, L.; van Dijk, M.T.; Anacker, C. Early life adversity shapes neural circuit function during sensitive postnatal developmental periods. Transl. Psychiatry 2022, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, A.; Sheu, Y.S.; Rabi, K.; Suzuki, H.; Navalta, C.P.; Polcari, A.; Teicher, M.H. Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage 2011, 54 (Suppl. S1), S280–S286. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Behrmann, M.; Geng, J.J.; Shomstein, S. Parietal cortex and attention. Curr. Opin. Neurobiol. 2004, 14, 212–217. [Google Scholar] [CrossRef]
- Caspari, N.; Arsenault, J.T.; Vandenberghe, R.; Vanduffel, W. Functional Similarity of Medial Superior Parietal Areas for Shift-Selective Attention Signals in Humans and Monkeys. Cereb. Cortex 2018, 28, 2085–2099. [Google Scholar] [CrossRef]
- Vandenberghe, R.; Gitelman, D.R.; Parrish, T.B.; Mesulam, M.M. Functional specificity of superior parietal mediation of spatial shifting. Neuroimage 2001, 14, 661–673. [Google Scholar] [CrossRef]
- Zhang, S.; Ide, J.S.; Li, C.S. Resting-state functional connectivity of the medial superior frontal cortex. Cereb. Cortex 2012, 22, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y.; Okada, T.; Katsumi, Y.; Hayashi, T.; Yamauchi, H.; Sawamoto, N.; Toma, K.; Nakamura, K.; Hanakawa, T.; Konishi, J.; et al. Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. Neuroimage 1999, 10, 193–199. [Google Scholar] [CrossRef]
- Pozzi, E.; Simmons, J.G.; Bousman, C.A.; Vijayakumar, N.; Bray, K.O.; Dandash, O.; Richmond, S.; Schwartz, O.; Seal, M.; Sheeber, L.; et al. The Influence of Maternal Parenting Style on the Neural Correlates of Emotion Processing in Children. J. Am. Acad Child Adolesc. Psychiatry 2020, 59, 274–282. [Google Scholar] [CrossRef]
- Machner, B.; Braun, L.; Imholz, J.; Koch, P.J.; Munte, T.F.; Helmchen, C.; Sprenger, A. Resting-State Functional Connectivity in the Dorsal Attention Network Relates to Behavioral Performance in Spatial Attention Tasks and May Show Task-Related Adaptation. Front. Hum. Neurosci. 2021, 15, 757128. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, F.X.; Aoki, Y. Intrinsic Functional Connectivity in Attention-Deficit/Hyperactivity Disorder: A Science in Development. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 253–261. [Google Scholar] [CrossRef]
- Zhang, Z.; Luh, W.M.; Duan, W.; Zhou, G.D.; Weinschenk, G.; Anderson, A.K.; Dai, W. Longitudinal effects of meditation on brain resting-state functional connectivity. Sci. Rep. 2021, 11, 11361. [Google Scholar] [CrossRef]
- Lin, H.; Lin, Q.; Li, H.; Wang, M.; Chen, H.; Liang, Y.; Bu, X.; Wang, W.; Yi, Y.; Zhao, Y.; et al. Functional Connectivity of Attention-Related Networks in Drug-Naive Children with ADHD. J. Atten. Disord. 2021, 25, 377–388. [Google Scholar] [CrossRef]
- Froeliger, B.; Garland, E.L.; Kozink, R.V.; Modlin, L.A.; Chen, N.K.; McClernon, F.J.; Greeson, J.M.; Sobin, P. Meditation-State Functional Connectivity (msFC): Strengthening of the Dorsal Attention Network and Beyond. Evid.-Based Complement. Altern. Med. 2012, 2012, 680407. [Google Scholar] [CrossRef] [PubMed]
- Esterman, M.; Rosenberg, M.D.; Noonan, S.K. Intrinsic fluctuations in sustained attention and distractor processing. J. Neurosci. 2014, 34, 1724–1730. [Google Scholar] [CrossRef]
- Rohr, C.S.; Dimond, D.; Schuetze, M.; Cho, I.Y.K.; Lichtenstein-Vidne, L.; Okon-Singer, H.; Dewey, D.; Bray, S. Girls’ attentive traits associate with cerebellar to dorsal attention and default mode network connectivity. Neuropsychologia 2019, 127, 84–92. [Google Scholar] [CrossRef]
- Kaiser, R.H.; Andrews-Hanna, J.R.; Wager, T.D.; Pizzagalli, D.A. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry 2015, 72, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Pu, W.; Yao, S. Functional alterations of fronto-limbic circuit and default mode network systems in first-episode, drug-naive patients with major depressive disorder: A meta-analysis of resting-state fMRI data. J. Affect. Disord. 2016, 206, 280–286. [Google Scholar] [CrossRef]
- Rebello, K.; Moura, L.M.; Bueno, A.P.A.; Picon, F.A.; Pan, P.M.; Gadelha, A.; Miguel, E.C.; Bressan, R.A.; Rohde, L.A.; Sato, J.R. Associations between Family Functioning and Maternal Behavior on Default Mode Network Connectivity in School-Age Children. Int. J. Environ. Res. Public Health 2022, 19, 6055. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.M.; Pfeifer, J.H.; Fisher, P.A.; Carpenter, S.; Fair, D.A. Early life stress is associated with default system integrity and emotionality during infancy. J. Child Psychol. Psychiatry 2015, 56, 1212–1222. [Google Scholar] [CrossRef] [PubMed]

| Characteristics (Mean ± SD) | Adverse Parenting | Optimal Parenting | χ2/t | p | |
|---|---|---|---|---|---|
| n (%) | n (%) | ||||
| Gender | Female | 67 (78.8%) | 26 (65.0%) | 2.729 a | 0.099 a |
| Male | 18 (21.2%) | 14 (35.0%) | |||
| Education level | High school or | 6 (7.1%) | 4 (10%) | 0.339 a | 0.844 a |
| less | |||||
| Undergraduate | 65 (76.4%) | 30 (75%) | |||
| Postgraduate or | 14 (16.5%) | 6 (15%) | |||
| higher | |||||
| Medications | Drug-naïve | 50(58.8%) | 23(57.5%) | 0.020 a | 0.889 a |
| Age (years) | 25.6 ± 6.2 | 24.7 ± 5.4 | 0.744 b | 0.458 b | |
| Duration of illness (years) | 5.6 ± 4.3 | 4.8 ± 3.2 | 1.028 b | 0.306 b | |
| PHQ-9 | 15.92 ± 5.73 | 15.25 ± 5.42 | 0.619 b | 0.537 b | |
| HAMD-17 | 18.87 ± 7.22 | 18.23 ± 6.39 | 0.484 b | 0.630 b | |
| HAMA | 17.39 ± 7.75 | 15.75 ± 6.66 | 1.151 b | 0.252 b | |
| SHAPS | 32.63 ± 7.40 | 33.79 ± 5.85 | −0.854 b | 0.395 b | |
| DSST (n = 60) | 61.30 ± 14.08 | 61.75 ± 11.74 | −0.115 b | 0.909 b | |
| Maternal warmth | 7.09 ± 3.27 | 14.80 ± 2.74 | −12.912 b | <0.001 b | |
| Maternal authoritarianism | 7.28 ± 2.97 | 4.58 ± 2.67 | 4.896 b | <0.001 b | |
| Paternal warmth | 6.27 ± 4.46 | 11.4 ± 4.372 | −6.036 b | <0.001 b | |
| Paternal authoritarianism | 6.54 ± 3.07 | 4.13 ± 2.37 | 4.394 b | <0.001 b | |
| Cluster | Brain Area | L/R | Voxel | MNI Coordinates | T Values (Peak) | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Cluster 1 | medial superior frontal cortex | R | 13 | 6 | 57 | 3 | 4.762 |
| Cluster 2 | medial superior frontal cortex | R | 13 | 0 | 60 | 15 | 4.092 |
| Cluster 3 | medial superior frontal cortex | L | 19 | −9 | 51 | 45 | 4.069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, Z.; Xie, X.; Kang, L.; Wang, W.; Xu, S.; Chen, M.; Yao, L.; Gong, Q.; Zhou, E.; Li, M.; et al. A Cross-Sectional Study: Structural and Related Functional Connectivity Changes in the Brain: Stigmata of Adverse Parenting in Patients with Major Depressive Disorder? Brain Sci. 2023, 13, 694. https://doi.org/10.3390/brainsci13040694
Nie Z, Xie X, Kang L, Wang W, Xu S, Chen M, Yao L, Gong Q, Zhou E, Li M, et al. A Cross-Sectional Study: Structural and Related Functional Connectivity Changes in the Brain: Stigmata of Adverse Parenting in Patients with Major Depressive Disorder? Brain Sciences. 2023; 13(4):694. https://doi.org/10.3390/brainsci13040694
Chicago/Turabian StyleNie, Zhaowen, Xinhui Xie, Lijun Kang, Wei Wang, Shuxian Xu, Mianmian Chen, Lihua Yao, Qian Gong, Enqi Zhou, Meng Li, and et al. 2023. "A Cross-Sectional Study: Structural and Related Functional Connectivity Changes in the Brain: Stigmata of Adverse Parenting in Patients with Major Depressive Disorder?" Brain Sciences 13, no. 4: 694. https://doi.org/10.3390/brainsci13040694
APA StyleNie, Z., Xie, X., Kang, L., Wang, W., Xu, S., Chen, M., Yao, L., Gong, Q., Zhou, E., Li, M., Wang, H., Bu, L., & Liu, Z. (2023). A Cross-Sectional Study: Structural and Related Functional Connectivity Changes in the Brain: Stigmata of Adverse Parenting in Patients with Major Depressive Disorder? Brain Sciences, 13(4), 694. https://doi.org/10.3390/brainsci13040694






