Abnormal Brain Structure Is Associated with Social and Communication Deficits in Children with Autism Spectrum Disorder: A Voxel-Based Morphometry Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and MRI Data Collection
2.2. MRI Data Processing
2.3. Statistical Analysis
3. Results
3.1. Demographic Data
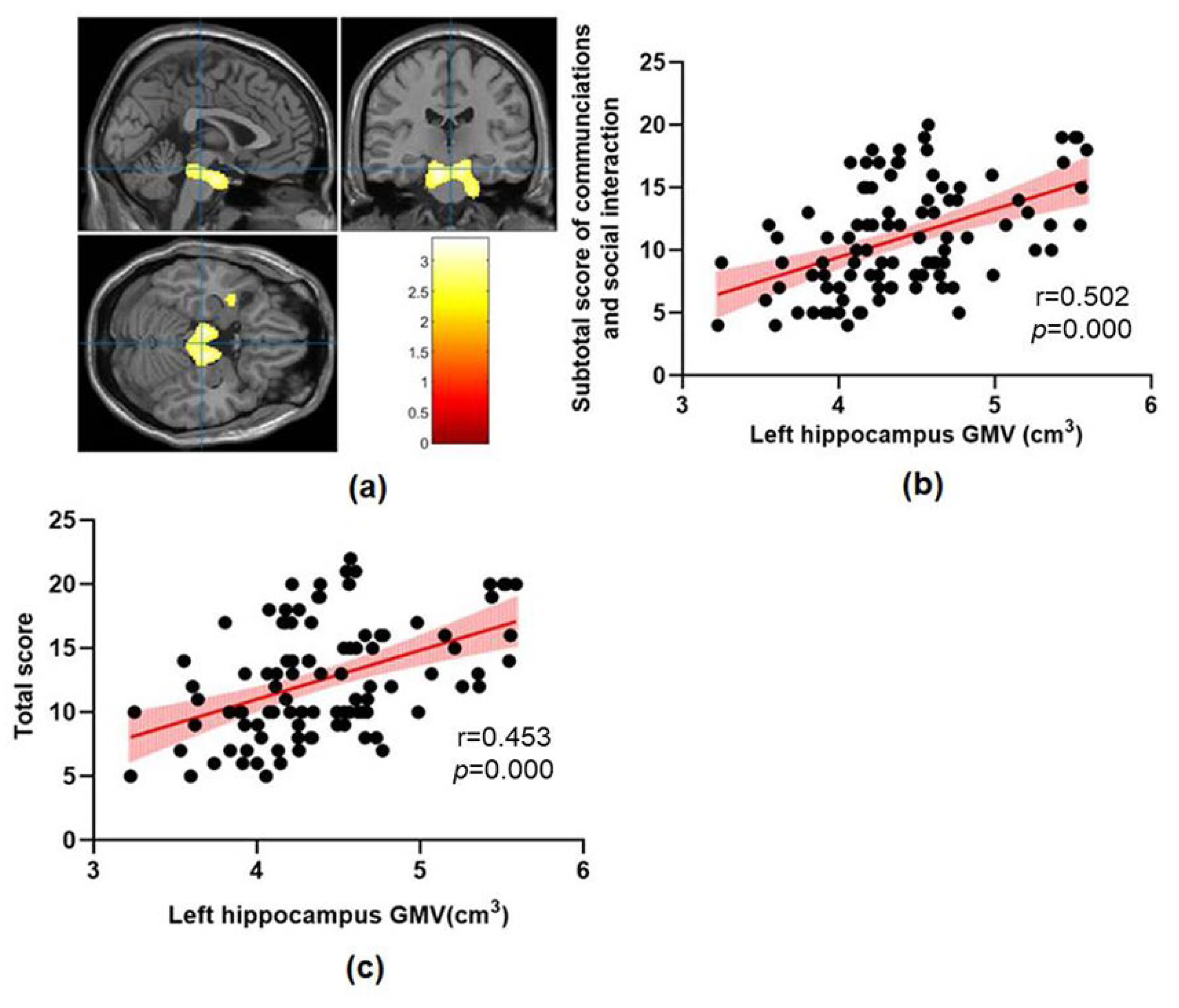
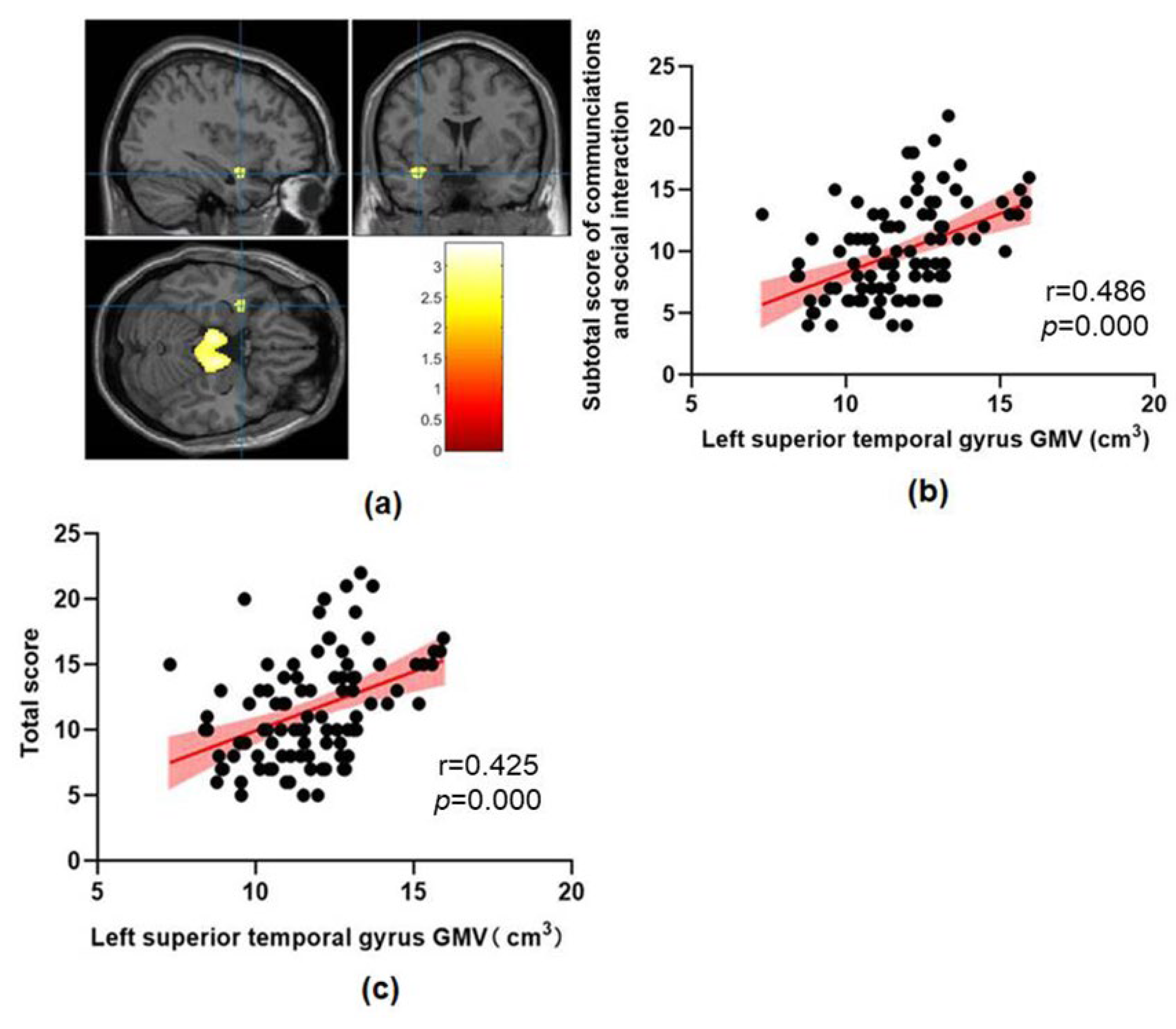
3.2. MRI Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blazquez Hinojosa, A.; Lazaro Garcia, L.; Puig Navarro, O.; Varela Bondelle, E.; Calvo Escalona, R. Sensitivity and specificity of DSM-5 diagnostic criteria for autism spectrum disorder in a child and adolescent sample. Rev. Psiquiatr. Salud Ment. 2021, 14, 202–211. [Google Scholar] [CrossRef]
- Ferrara, R.; Nappo, R.; Ansermet, F.; Ricci, P.; Massoni, F.; Carbone, G.; Sparaci, A.; Nonnis, E.; Ricci, L.; Ricci, S. The Impact of DSM-5 on the Diagnosis of Autism Spectrum Disorder. Psychiatr. Ann. 2021, 51, 38–46. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Z.H.; Xu, L.Z.; Yang, L.; Ji, Z.Z.; Tang, X.Z.; Liu, J.R.; Li, X.; Cao, Q.J.; Liu, J. Developmental brain structural atypicalities in autism: A voxel-based morphometry analysis. Child Adolesc. Psychiatry Ment. Health 2022, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Pappaianni, E.; Siugzdaite, R.; Vettori, S.; Venuti, P.; Job, R.; Grecucci, A. Three shades of grey: Detecting brain abnormalities in children with autism using source-, voxel- and surface-based morphometry. Eur. J. Neurosci. 2018, 47, 690–700. [Google Scholar] [CrossRef]
- Manoli, D.S.; State, M.W. Autism Spectrum Disorder Genetics and the Search for Pathological Mechanisms. Am. J. Psychiatry 2021, 178, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Guo, R.; Xu, W.; Guo, Q.; Hao, C.; Ni, X.; Li, W. Biological implications of genetic variations in autism spectrum disorders from genomics studies. Biosci. Rep. 2021, 41, BSR20210593. [Google Scholar] [CrossRef]
- Ke, X.; Hong, S.; Tang, T.; Zou, B.; Li, H.; Hang, Y.; Zhou, Z.; Ruan, Z.; Lu, Z.; Tao, G.; et al. Voxel-based morphometry study on brain structure in children with high-functioning autism. Neuroreport 2008, 19, 921–925. [Google Scholar] [CrossRef]
- Yang, X.; Si, T.; Gong, Q.; Qiu, L.; Jia, Z.; Zhou, M.; Zhao, Y.; Hue, X.; Wu, M.; Zhu, H. Brain gray matter alterations and associated demographic profiles in adults with autism spectrum disorder: A meta-analysis of voxel-based morphometry studies. Aust. New Zealand J. Psychiatry 2016, 50, 741–753. [Google Scholar] [CrossRef]
- Yu, Y.; Ren, Z.; Ward, J.; Jiang, Q. Atypical Brain Structures as a Function of Gray Matter Volume (GMV) and Gray Matter Density (GMD) in Young Adults Relating to Autism Spectrum Traits. Front. Psychol. 2020, 11, 523. [Google Scholar] [CrossRef]
- Perlstein, W.M.; Carter, C.S.; Noll, D.C.; Cohen, J.D. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am. J. Psychiatry 2001, 158, 1105–1113. [Google Scholar] [CrossRef]
- Tanaka, H.; Shou, Q.; Kiyonari, T.; Matsuda, T.; Sakagami, M.; Takagishi, H. Right dorsolateral prefrontal cortex regulates default prosociality preference. Cereb. Cortex 2022, 33, 5420–5425. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Salmeron, B.J.; Gu, H.; Adinoff, B.; Stein, E.A.; Yang, Y. Functional connectivity of dorsolateral prefrontal cortex predicts cocaine relapse: Implications for neuromodulation treatment. Brain Commun. 2021, 3, fcab120. [Google Scholar] [CrossRef] [PubMed]
- Carper, R.A.; Courchesne, E. Inverse correlation between frontal lobe and cerebellum sizes in children with autism. Brain 2000, 123, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Abell, F.; Krams, M.; Ashburner, J.; Passingham, R.; Friston, K.J.; Frackowiak, R.; Happe, F.; Frith, C.; Frith, U. The neuroanatomy of autism: A voxel-based whole brain analysis of structural scans. Neuroreport 1999, 10, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Waiter, G.D.; Williams, J.H.G.; Murray, A.D.; Gilchrist, A.; Perrett, D.I.; Whiten, A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage 2004, 22, 619–625. [Google Scholar] [CrossRef]
- Neeley, E.S.; Bigler, E.D.; Krasny, L.; Ozonoff, S.; McMahon, W.; Lainhart, J.E. Quantitative temporal lobe differences: Autism distinguished from controls using classification and regression tree analysis. Brain Dev. 2007, 29, 389–399. [Google Scholar] [CrossRef]
- Garcia-Penas, J.J. Autism, epilepsy and temporal lobe pathology. Rev. Neurol. 2009, 48 (Suppl. S2), S35–S45. [Google Scholar]
- Kobayashi, A.; Yokota, S.; Takeuchi, H.; Asano, K.; Asano, M.; Sassa, Y.; Taki, Y.; Kawashima, R. Increased grey matter volume of the right superior temporal gyrus in healthy children with autistic cognitive style: A VBM study. Brain Cogn. 2020, 139, 105514. [Google Scholar] [CrossRef]
- Lukito, S.; Norman, L.; Carlisi, C.; Radua, J.; Hart, H.; Simonoff, E.; Rubia, K. Comparative meta-analyses of brain structural and functional abnormalities during cognitive control in attention-deficit/hyperactivity disorder and autism spectrum disorder. Psychol. Med. 2020, 50, 894–919. [Google Scholar] [CrossRef]
- Bigler, E.D.; Tate, D.F.; Neeley, E.S.; Wolfson, L.J.; Miller, M.J.; Rice, S.A.; Cleavinger, H.; Anderson, C.; Coon, H.; Ozonoff, S.; et al. Temporal lobe, autism, and macrocephaly. Am. J. Neuroradiol. 2003, 24, 2066–2076. [Google Scholar]
- Li, D.Y.; Karnath, H.O.; Xu, X. Candidate Biomarkers in Children with Autism Spectrum Disorder: A Review of MRI Studies. Neurosci. Bull. 2017, 33, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.M.; Zhao, W.D.; Luo, C.; Liu, X.J.; Jiang, H.; Tang, Y.Q.; Liu, C.; Yao, D.Z. Identifying and Predicting Autism Spectrum Disorder Based on Multi-Site Structural MRI With Machine Learning. Front. Hum. Neurosci. 2022, 15, 765517. [Google Scholar] [CrossRef] [PubMed]
- Riva, D.; Annunziata, S.; Contarino, V.; Erbetta, A.; Aquino, D.; Bulgheroni, S. Gray Matter Reduction in the Vermis and CRUS-II Is Associated with Social and Interaction Deficits in Low-Functioning Children with Autistic Spectrum Disorders: A VBM-DARTEL Study. Cerebellum 2013, 12, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Maximo, J.O.; Cadena, E.J.; Kana, R.K. The Implications of Brain Connectivity in the Neuropsychology of Autism. Neuropsychol. Rev. 2014, 24, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.R.; Reiss, A.L.; Tatusko, D.H.; Ikuta, I.; Kazmerski, D.B.; Botti, J.A.C.; Burnette, C.P.; Kates, W.R. Neuroanatomic Alterations and Social and Communication Deficits in Monozygotic Twins Discordant for Autism Disorder. Am. J. Psychiatry 2009, 166, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Yan, C.G.; Li, Q.; Denio, E.; Castellanos, F.X.; Alaerts, K.; Anderson, J.S.; Assaf, M.; Bookheimer, S.Y.; Dapretto, M.; et al. The autism brain imaging data exchange: Towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry 2014, 19, 659–667. [Google Scholar] [CrossRef]
- Banker, S.M.; Gu, X.S.; Schiller, D.; Foss-Feig, J.H. Hippocampal contributions to social and cognitive deficits in autism spectrum disorder. Trends Neurosci. 2021, 44, 793–807. [Google Scholar] [CrossRef]
- Maier, S.; van Elst, L.T.; Beier, D.; Ebert, D.; Fangmeier, T.; Radtke, M.; Perlov, E.; Riedel, A. Increased hippocampal volumes in adults with high functioning autism spectrum disorder and an IQ > 100: A manual morphometric study. Psychiatry Res. Neuroimaging 2015, 234, 152–155. [Google Scholar] [CrossRef]
- Liu, C.X.; Li, D.Y.; Yang, H.W.; Li, H.P.; Xu, Q.; Zhou, B.R.; Hu, C.C.; Li, C.Y.; Wang, Y.; Qiao, Z.W.; et al. Altered striatum centered brain structures in SHANK3 deficient Chinese children with genotype and phenotype profiling. Prog. Neurobiol. 2021, 200, 101985. [Google Scholar] [CrossRef]
- Geng, F.; Xu, W.; Riggins, T. Interactions between the hippocampus and fronto-parietal regions during memory encoding in early childhood. Hippocampus 2022, 32, 108–120. [Google Scholar] [CrossRef]
- Quiroga, R.Q. How Are Memories Stored in the Human Hippocampus? Trends Cogn. Sci. 2021, 25, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.Z. Heterogeneous representations in the hippocampus. Neurosci. Res. 2021, 165, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tehrani-Doost, M.; Salmanian, M.; Motlagh, G.M. Delayed face recognition in children with autism spectrum disorders. Eur. Child Adolesc. Psychiatry 2011, 20, S149–S150. [Google Scholar]
- Weigelt, S.; Koldewyn, K.; Kanwisher, N. Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neurosci. Biobehav. Rev. 2012, 36, 1060–1084. [Google Scholar] [CrossRef] [PubMed]
- Gev, T.; Avital, H.; Rosenan, R.; Aronson, L.O.; Golan, O. Socio emotional competence in young children with ASD during interaction with their typically developing peers. Res. Autism Spectr. Disord. 2021, 86, 101818. [Google Scholar] [CrossRef]
- Kang, J.; Kang, W.; Lee, S.-H. Stronger memory representation after memory reinstatement during retrieval in the human hippocampus. Neuroimage 2022, 260, 119493. [Google Scholar] [CrossRef]
- Liu, E.S.; Hou, M.; Koen, J.D.; Rugg, M.D. Effects of age on the neural correlates of encoding source and item information: An fMRI study. Neuropsychologia 2022, 177, 108415. [Google Scholar] [CrossRef]
- Setton, R.; Mwilambwe-Tshilobo, L.; Sheldon, S.; Turner, G.R.; Spreng, R.N. Hippocampus and temporal pole functional connectivity is associated with age and individual differences in autobiographical memory. Proc. Natl. Acad. Sci. USA 2022, 119, e2203039119. [Google Scholar] [CrossRef]
- Cooper, R.A.; Richter, F.R.; Bays, P.M.; Plaisted-Grant, K.C.; Baron-Cohen, S.; Simons, J.S. Reduced Hippocampal Functional Connectivity During Episodic Memory Retrieval in Autism. Cereb. Cortex 2017, 27, 888–902. [Google Scholar] [CrossRef]
- Groen, W.; Teluij, M.; Buitelaar, J.; Tendolkar, I. Amygdala and Hippocampus Enlargement During Adolescence in Autism. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 552–560. [Google Scholar] [CrossRef]
- Saitoh, O.; Karns, C.M.; Courchesne, E. Development of the hippocampal formation from 2 to 42 years—MRI evidence of smaller area dentata in autism. Brain 2001, 124, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Li, G.N.; Chen, M.H.; Li, G.; Wu, D.; Sun, Q.S.; Shen, D.G.; Wang, L. A Preliminary Volumetric Mri Study of Amygdala and Hippocampal Subfields in Autism during Infancy. In Proceedings of the 16th IEEE International Symposium on Biomedical Imaging (ISBI), Venice, Italy, 8–11 April 2019; pp. 1052–1056. [Google Scholar]
- Hasan, K.M.; Walimuni, I.S.; Frye, R.E. Global Cerebral and Regional Multimodal Neuroimaging Markers of the Neurobiology of Autism: Development and Cognition. J. Child Neurol. 2013, 28, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.F.; Zuo, C.Y.; Liao, S.R.; Long, Y.; Wang, Y.P. Abnormal development pattern of the amygdala and hippocampus from childhood to adulthood with autism. J. Clin. Neurosci. 2020, 78, 327–332. [Google Scholar] [CrossRef]
- Barnea-Goraly, N.; Frazier, T.W.; Piacenza, L.; Minshew, N.J.; Keshavan, M.S.; Reiss, A.L.; Hardan, A.Y. A preliminary longitudinal volumetric MRI study of amygdala and hippocampal volumes in autism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Hu, X.; Guo, K.F.; Yang, P.Y.; Situ, M.J.; Huang, Y. Increased Left Inferior Temporal Gyrus Was Found in Both Low Function Autism and High Function Autism. Front. Psychiatry 2018, 9, 542. [Google Scholar] [CrossRef] [PubMed]
- Toal, F.; Daly, E.M.; Page, L.; Deeley, Q.; Hallahan, B.; Bloemen, O.; Cutter, W.J.; Brammer, M.J.; Curran, S.; Robertson, D.; et al. Clinical and anatomical heterogeneity in autistic spectrum disorder: A structural MRI study. Psychol. Med. 2010, 40, 1171–1181. [Google Scholar] [CrossRef]
- Harkins, C.M.; Handen, B.L.; Mazurek, M.O. The Impact of the Comorbidity of ASD and ADHD on Social Impairment. J. Autism Dev. Disord. 2022, 52, 2512–2522. [Google Scholar] [CrossRef]
- Okcun-Akcamus, M.C. Social Communication Skills and Language Development of Children with Autism Spectrum Disorders. Ank. Univ. Egit. Bilim. Fak. Ozel Egit. Derg. Ank. Univ. Fac. Educ. Sci. J. Spec. Educ. 2016, 17, 163–192. [Google Scholar] [CrossRef]
- Stagg, S.D.; Linnell, K.J.; Heaton, P. Investigating eye movement patterns, language, and social ability in children with autism spectrum disorder. Dev. Psychopathol. 2014, 26, 529–537. [Google Scholar] [CrossRef]
- Ji, Y.T.; Xu, M.Y.; Liu, X.; Dai, Y.; Zhou, L.; Li, F.; Zhang, L.L. Temporopolar volumes are associated with the severity of social impairment and language development in children with autism spectrum disorder with developmental delay. Front. Psychiatry 2022, 13, 1072272. [Google Scholar] [CrossRef]
- Pereira, A.M.; Campos, B.M.; Coan, A.C.; Pegoraro, L.F.; de Rezende, T.J.R.; Obeso, I.; Dalgalarrondo, P.; da Costa, J.C.; Dreher, J.C.; Cendes, F. Differences in Cortical Structure and Functional MRI Connectivity in High Functioning Autism. Front. Neurol. 2018, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Chua, S.E.; Cheung, V.; Khong, P.L.; Tai, K.S.; Wong, T.K.W.; Ho, T.P.; McAlonan, G.M. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J. Child Psychol. Psychiatry 2009, 50, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Adornetti, I. Broca’s and Wernicke’s Aphasias in the Light of Contemporary Neuroscience. Riv. Internazionale Filos. Psicol. 2019, 10, 295–312. [Google Scholar] [CrossRef]
- Jancke, L.; Liem, F.; Merillat, S. Are language skills related to structural features in Broca’s and Wernicke’s area? Eur. J. Neurosci. 2021, 53, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.J.; Chabris, C.F.; Clark, J.; Urban, T.; Aharon, I.; Steele, S.; McGrath, L.; Condouris, K.; Tager-Flusberg, H. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006, 61, 54–68. [Google Scholar] [CrossRef]
- Ci, H.; van Graan, A.; Gonzalvez, G.; Thompson, P.; Hill, A.; Duncan, J.S. Mandarin functional MRI Language paradigms. Brain Behav. 2016, 6, e00525. [Google Scholar] [CrossRef]
- Ubellacker, D.M.; Hillis, A.E. The neural underpinnings of word comprehension and production: The critical roles of the temporal lobes. Handb. Clin. Neurol. 2022, 187, 211–220. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Li, W.B.; Song, Y.W.; Zhao, Z.B.; Yan, Y.N.; Yang, Y.H.; Lv, P.Y.; Yin, Y. Correlation between focal lesion sites and language deficits in the acute phase of post-stroke aphasia. Folia Neuropathol. 2022, 60, 60–68. [Google Scholar] [CrossRef]
- Drane, D.L.; Pedersen, N.P. Knowledge of language function and underlying neural networks gained from focal seizures and epilepsy surgery. Brain Lang. 2019, 189, 20–33. [Google Scholar] [CrossRef]
- Roger, E.; Pichat, C.; Torlay, L.; David, O.; Renard, F.; Banjac, S.; Attye, A.; Minotti, L.; Lamalle, L.; Kahane, P.; et al. Hubs disruption in mesial temporal lobe epilepsy. A resting-state fMRI study on a language-and-memory network. Hum. Brain Mapp. 2020, 41, 779–796. [Google Scholar] [CrossRef]
- Ainsworth, M.; Sallet, J.; Joly, O.; Kyriazis, D.; Kriegeskorte, N.; Duncan, J.; Schuffelgen, U.; Rushworth, M.F.S.; Bell, A.H. Viewing ambiguous social interactions increases functional connectivity between frontal and temporal nodes of the social brain. J. Neurosci. 2021, 41, 6070–6086. [Google Scholar] [CrossRef] [PubMed]
- Zovetti, N.; Rossetti, M.G.; Perlini, C.; Brambilla, P.; Bellani, M. Neuroimaging studies exploring the neural basis of social isolation. Epidemiol. Psychiatr. Sci. 2021, 30, e29. [Google Scholar] [CrossRef] [PubMed]
- Balgova, E.; Diveica, V.; Walbrin, J.; Binney, R.J. The role of the ventrolateral anterior temporal lobes in social cognition. Hum. Brain Mapp. 2022, 43, 4589–4608. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Borras, J.; Vuilleumier, P. Amygdala function in emotion, cognition, and behavior. Handb. Clin. Neurol. 2022, 187, 359–380. [Google Scholar] [CrossRef]
- Zhang, Y.-j.; Hu, H.-x.; Wang, L.-l.; Wang, X.; Wang, Y.; Huang, J.; Wang, Y.; Lui, S.S.Y.; Hui, L.; Chan, R.C.K. Decoupling between hub-connected functional connectivity of the social brain network and real-world social network in individuals with social anhedonia. Psychiatry Res. Neuroimaging 2022, 326, 111528. [Google Scholar] [CrossRef]
- Dickstein, D.P.; Pescosolido, M.F.; Reidy, B.L.; Galvan, T.; Kim, K.L.; Seymour, K.E.; Laird, A.R.; Di Martino, A.; Barrett, R.P. Developmental Meta-Analysis of the Functional Neural Correlates of Autism Spectrum Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 279–289. [Google Scholar] [CrossRef]
- Liu, J.K.; Yao, L.; Zhang, W.J.; Xiao, Y.; Liu, L.; Gao, X.; Shah, C.; Li, S.Y.; Tao, B.; Gong, Q.Y.; et al. Gray matter abnormalities in pediatric autism spectrum disorder: A meta-analysis with signed differential mapping. Eur. Child Adolesc. Psychiatry 2017, 26, 933–945. [Google Scholar] [CrossRef]
- Katuwal, G.J.; Baum, S.A.; Cahill, N.D.; Dougherty, C.C.; Evans, E.; Evans, D.W.; Moore, G.J.; Michael, A.M. Inter-Method Discrepancies in Brain Volume Estimation May Drive Inconsistent Findings in Autism. Front. Neurosci. 2016, 10, 439. [Google Scholar] [CrossRef]
- Bedford, S.A.; Park, M.T.M.; Devenyi, G.A.; Tullo, S.; Germann, J.; Patel, R.; Anagnostou, E.; Baron-Cohen, S.; Bullmore, E.T.; Chura, L.R.; et al. Large-scale analyses of the relationship between sex, age and intelligence quotient heterogeneity and cortical morphometry in autism spectrum disorder. Mol. Psychiatry 2020, 25, 614–628. [Google Scholar] [CrossRef]
- Riddle, K.; Cascio, C.J.; Woodward, N.D. Brain structure in autism: A voxel-based morphometry analysis of the Autism Brain Imaging Database Exchange (ABIDE). Brain Imaging Behav. 2017, 11, 541–551. [Google Scholar] [CrossRef]
- Dmello, A.M.; Crocetti, D.; Mostofsky, S.H.; Stoodley, C.J. Cerebellar gray matter and lobular volumes correlate with core autism symptoms. Neuroimage—Clin. 2015, 7, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J.; Schmahmann, J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 2010, 46, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Strick, P.L.; Dum, R.P.; Fiez, J.A. Cerebellum and Nonmotor Function. Annu. Rev. Neurosci. 2009, 32, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Prigge, M.B.D.; Lange, N.; Bigler, E.D.; King, J.B.; Dean, D.C.; Adluru, N.; Alexander, A.L.; Lainhart, J.E.; Zielinski, B.A. A 16-year study of longitudinal volumetric brain development in males with autism. Neuroimage 2021, 236, 118067. [Google Scholar] [CrossRef] [PubMed]
- Caspi, Y.; Brouwer, R.M.; Schnack, H.G.; van de Nieuwenhuijzen, M.E.; Cahn, W.; Kahn, R.S.; Niessen, W.J.; van der Lugt, A.; Pol, H.H. Changes in the intracranial volume from early adulthood to the sixth decade of life: A longitudinal study. Neuroimage 2020, 220, 116842. [Google Scholar] [CrossRef]
- Jockwitz, C.; Merillat, S.; Liem, F.; Oschwald, J.; Amunts, K.; Caspers, S.; Jancke, L. Generalizing age effects on brain structure and cognition: A two-study comparison approach. Hum. Brain Mapp. 2019, 40, 2305–2319. [Google Scholar] [CrossRef]
- Freitag, C.M.; Luders, E.; Hulst, H.E.; Narr, K.L.; Thompson, P.M.; Toga, A.W.; Krick, C.; Konrad, C. Total Brain Volume and Corpus Callosum Size in Medication-Naive Adolescents and Young Adults with Autism Spectrum Disorder. Biol. Psychiatry 2009, 66, 316–319. [Google Scholar] [CrossRef]

| ASD (n = 98) Mean ± SD | TD (n = 105) Mean ± SD | t/χ2 | p | |
|---|---|---|---|---|
| Age | 10.17 ± 1.23 | 10.06 ± 1.34 | 0.54 | 0.49 |
| Gender (male/ female) | 67/31 | 64/41 | 4.48 | 0.06 |
| Full IQ | 111.75 ± 15.32 | 113.82 ± 12.58 | −1.46 | 0.38 |
| ADOS scores | ||||
| Communication score | 3.54 ± 1.45 | |||
| Social score | 7.75 ± 2.68 | |||
| Stereotypic behavior score | 2.41 ± 1.65 | |||
| Subtotal score of communications and social interaction Total score | 11.29 + 3.82 13.70 ± 4.21 | |||
| Clusters | Peak MNI | Voxel Number | Regions | t | p | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| ASD > TD | |||||||
| Cluster1 | −9 | −18 | −25.5 | 5299 | Midbrain, Pontine Bilateral hippocampus, Left parahippocampal gyrus | 3.28 | 0.001 |
| Cluster2 | −39 | 3 | −19.5 | 170 | Left superior temporal gyrus, Left temporal pole | 2.57 | 0.005 |
| Cluster3 | −31.5 | −52.5 | 6 | 544 | Left middle temporal gyrus, Left superior occipital gyrus | 2.88 | 0.002 |
| ASD < TD | No significant results | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.-X.; Ju, X.-D. Abnormal Brain Structure Is Associated with Social and Communication Deficits in Children with Autism Spectrum Disorder: A Voxel-Based Morphometry Analysis. Brain Sci. 2023, 13, 779. https://doi.org/10.3390/brainsci13050779
Xu M-X, Ju X-D. Abnormal Brain Structure Is Associated with Social and Communication Deficits in Children with Autism Spectrum Disorder: A Voxel-Based Morphometry Analysis. Brain Sciences. 2023; 13(5):779. https://doi.org/10.3390/brainsci13050779
Chicago/Turabian StyleXu, Ming-Xiang, and Xing-Da Ju. 2023. "Abnormal Brain Structure Is Associated with Social and Communication Deficits in Children with Autism Spectrum Disorder: A Voxel-Based Morphometry Analysis" Brain Sciences 13, no. 5: 779. https://doi.org/10.3390/brainsci13050779
APA StyleXu, M.-X., & Ju, X.-D. (2023). Abnormal Brain Structure Is Associated with Social and Communication Deficits in Children with Autism Spectrum Disorder: A Voxel-Based Morphometry Analysis. Brain Sciences, 13(5), 779. https://doi.org/10.3390/brainsci13050779




