Effect of Spontaneous Subarachnoid Hemorrhage on Cerebrospinal Fluid Indicators
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedure
2.3. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data
3.2. Characteristics of Routine Examination of CSF
3.3. Characteristics of Biochemical Examination of CSF
3.4. Comparison with Normal Reference Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SAH | Subarachnoid hemorrhage |
| CSF | Cerebrospinal fluid |
| CNS | Central nervous system |
| WBC | White blood cell count |
| RBC | Red blood cell |
References
- Ortiz, O.H.; García, H.G.; Ramírez, F.M.O.; Flórez, J.C.; Valencia, B.G.; Mantilla, S.M.; Ochoa, M.M.; Ochoa, J.S.; Jaimes, F. Development of a prediction rule for diagnosing postoperative meningitis: A cross-sectional study. J. Neurosurg. 2018, 128, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Huang, X.; Wang, Y.; Li, L.; Zhao, C.; Yao, Z.; Cui, W.; Zhang, G. Efficacy of intravenous plus intrathecal/intracerebral ventricle injection of polymyxin B for post-neurosurgical intracranial infections due to MDR/XDR Acinectobacter baumannii: A retrospective cohort study. Antimicrob. Resist. Infect. Control 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Remeš, F.; Tomáš, R.; Jindrák, V.; Vaniš, V.; Šetlík, M. Intraventricular and lumbar intrathecal administration of antibiotics in postneurosurgical patients with meningitis and/or ventriculitis in a serious clinical state. J. Neurosurg. 2013, 119, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Young, N.; Thomas, M. Meningitis in adults: Diagnosis and management. Intern. Med. J. 2018, 48, 1294–1307. [Google Scholar] [CrossRef] [PubMed]
- Tunkel, A.R.; Rodrigo, H.; Adarsh, B.; Karin, B.; Kaplan, S.L.; Michael, S.W.; Diederik, V.D.B.; Bleck, T.P.; Garton, H.J.L.; Zunt, J.R. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin. Infect. Dis. 2017, 64, 701–706. [Google Scholar] [CrossRef]
- Lamagni, T.; Elgohari, S.; Wloch, C.; Charlett, A.; Harrington, P.; Johnson, A.P. National Surgical Site Infection (SSI) surveillance: Response to Jenks. J. Hosp. Infect. 2017, 97, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Chanunya, C.; Castelblanco, R.L.; Salazar, L.; Wootton, S.H.; Aguilera, E.; Ostrosky-Zeichner, L.; Sandberg, D.I.; Choi, H.A.; Lee, K.; Kitigawa, R. Clinical Characteristics and Predictors of Adverse Outcome in Adult and Pediatric Patients with Healthcare-Associated Ventriculitis and Meningitis. Open Forum Infect. Dis. 2016, 3, ofw077. [Google Scholar]
- Lyons, T.W.; Cruz, A.T.; Freedman, S.B.; Neuman, M.I.; Fran, B.; Mistry, R.D.; Prashant, M.; Aronson, P.L.; Thomson, J.E.; Pruitt, C.M. Interpretation of Cerebrospinal Fluid White Blood Cell Counts in Young Infants with a Traumatic Lumbar Puncture. Ann. Emerg. Med. 2018, 5, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Montes, K.; Jenkinson, H.; Habib, O.B.; Esquenazi, Y.; Hasbun, R. Corrected white blood cell count, cell index, and validation of a clinical model for the diagnosis of health care-associated ventriculitis and meningitis in adults with intracranial hemorrhage—ScienceDirect. Clin. Neurol. Neurosurg. 2019, 178, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Hoen, B.; Varon, E.; Debroucker, T.D.; Fantin, B.; Duval, X. Management of acute community-acquired bacterial meningitis (excluding newborns). Short text. Med. Mal. Infect. 2019, 49, 367–404. [Google Scholar] [CrossRef]
- Lapenna, P.A.; Roos, K.L. Bacterial Infections of the Central Nervous System. Semin. Neurol. 2019, 39, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Carlos, G.-M.J. Healthcare-Acquired Meningitis and Ventriculitis. CNS Infect. A Clin. Approach 2014, 3, 29–44. [Google Scholar]
- Mcgill, F.; Heyderman, R.S.; Panagiotou, S.; Tunkel, A.R.; Solomon, T. Acute bacterial meningitis in adults. Lancet 2016, 388, 3036–3047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, P.; Zhang, H.C.; Wu, H.L.; Zhang, W.H. Clinical application and evaluation of metagenomic next-generation sequencing in suspected adult central nervous system infection. J. Transl. Med. 2020, 18, 199. [Google Scholar] [CrossRef] [PubMed]
- Davis, L. Acute Bacterial Meningitis. Continuum 2018, 24, 1264–1283. [Google Scholar] [CrossRef] [PubMed]
- Russa, R.L.; Maiese, A.; Fazio, N.D.; Morano, A.; Bonaventura, C.D.; Matteis, A.D.; Fazio, V.; Frati, P.; Fineschi, V. Post-Traumatic Meningitis Is a Diagnostic Challenging Time: A Systematic Review Focusing on Clinical and Pathological Features. Int. J. Mol. Sci. 2020, 21, 4148. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.S.; Greenwood, B.; Brandtzaeg, P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 2007, 369, 2196–2210. [Google Scholar] [CrossRef] [PubMed]
- Thurnher, M.M.; Sundgren, P.C. Intracranial Infection and Inflammation. In Diseases of the Brain, Head and Neck, Spine; IDKD Springer Series; Diagnostic Imaging; Springer: Berlin/Heidelberg, Germany, 2020; Chapter 6. [Google Scholar]
- Swinburne, N.C.; Bansal, A.G.; Aggarwal, A.; Doshi, A.H. Neuroimaging in Central Nervous System Infections. Curr. Neurol. Neurosci. Rep. 2017, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.C.; Diederik, V. Epidemiology, diagnosis, and treatment of brain abscesses. Curr. Opin. Infect. Dis. 2017, 30, 129–134. [Google Scholar] [CrossRef] [PubMed]
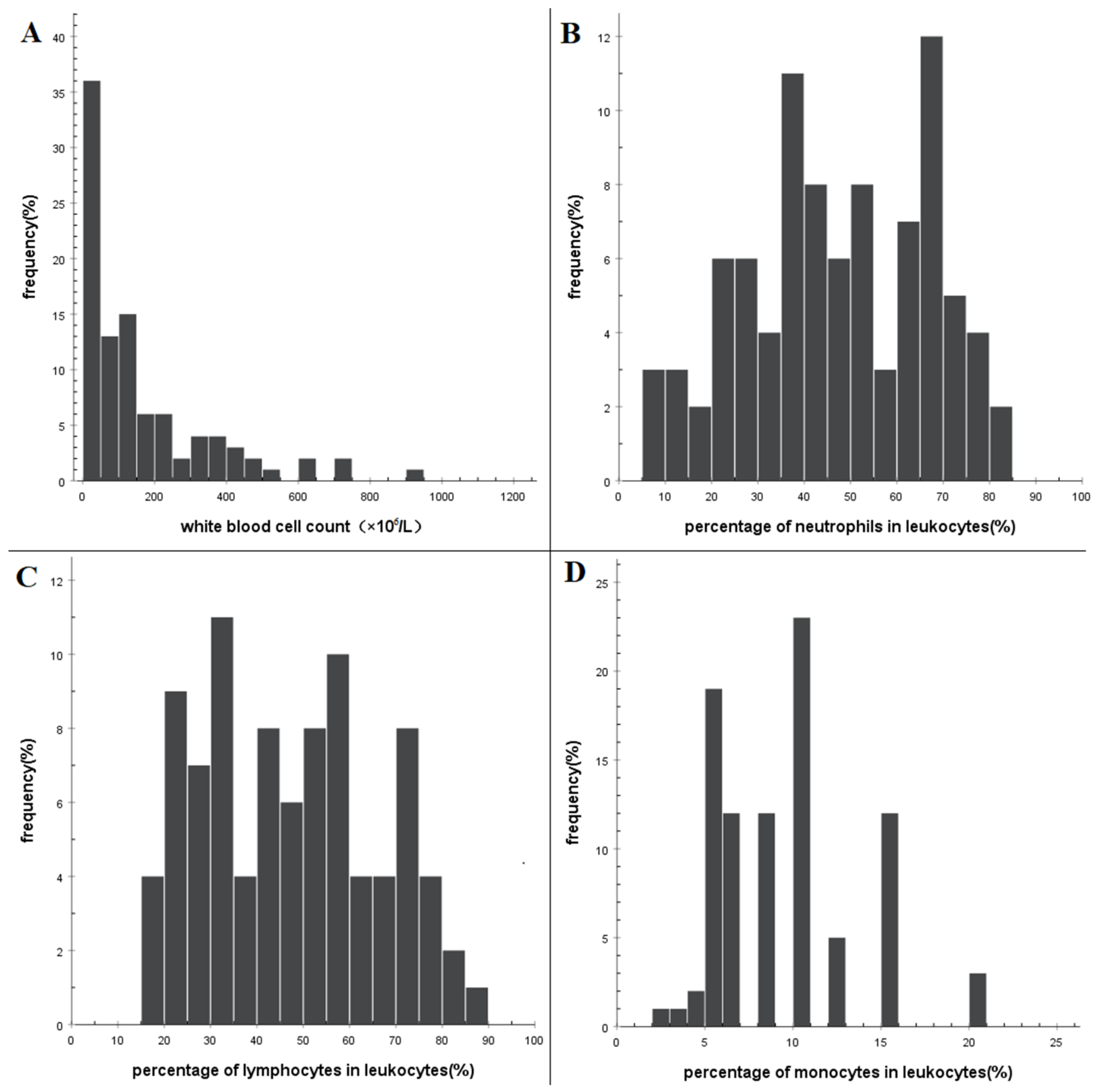
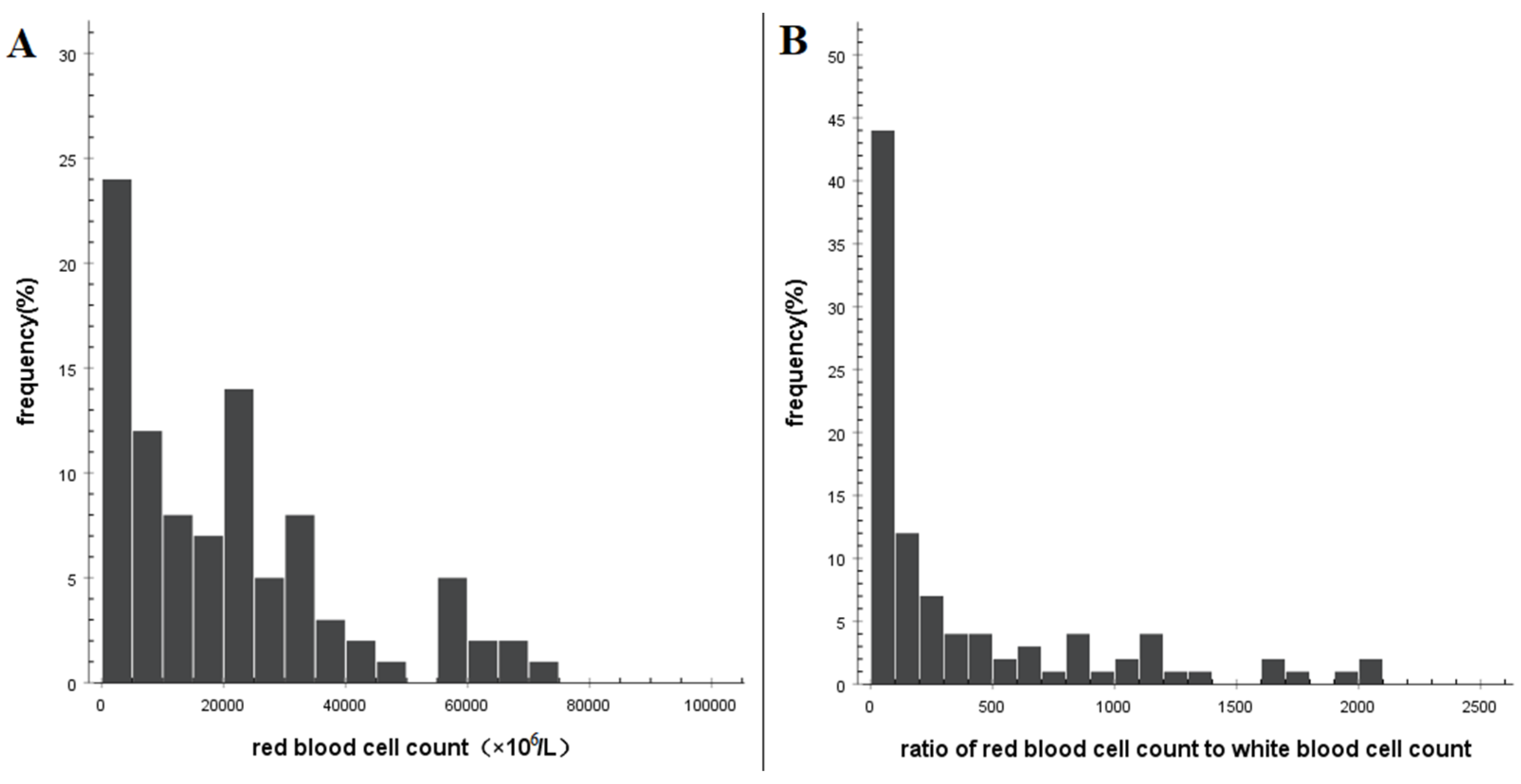
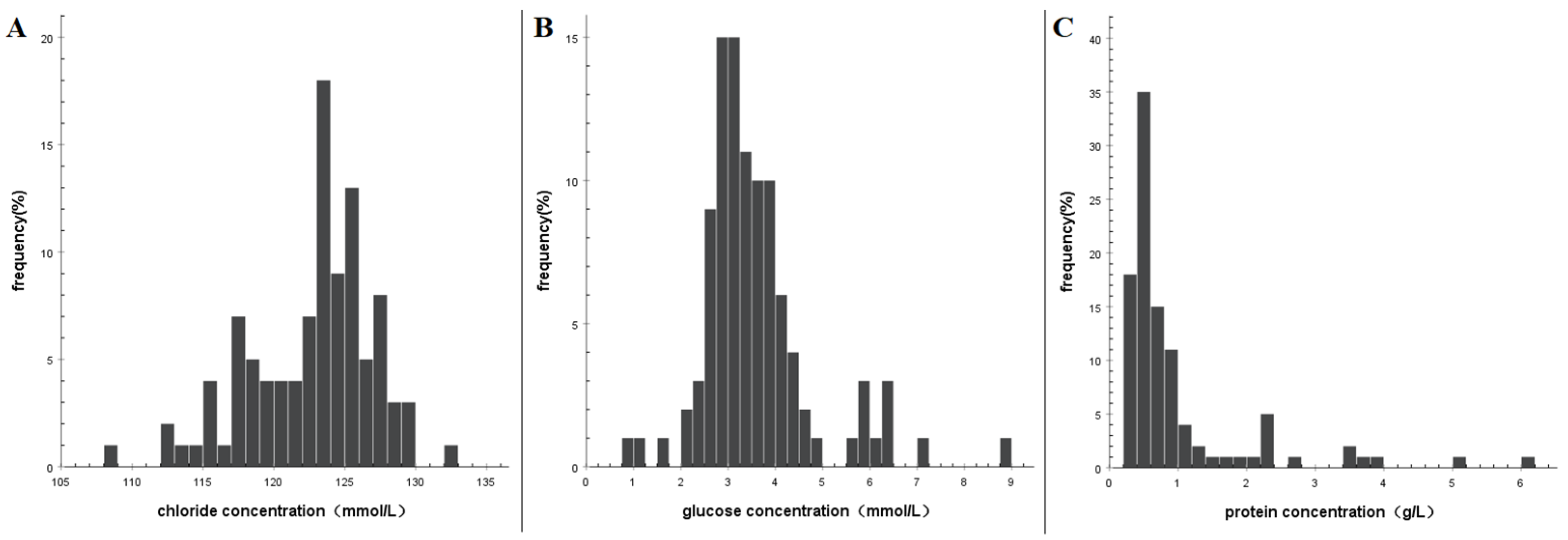
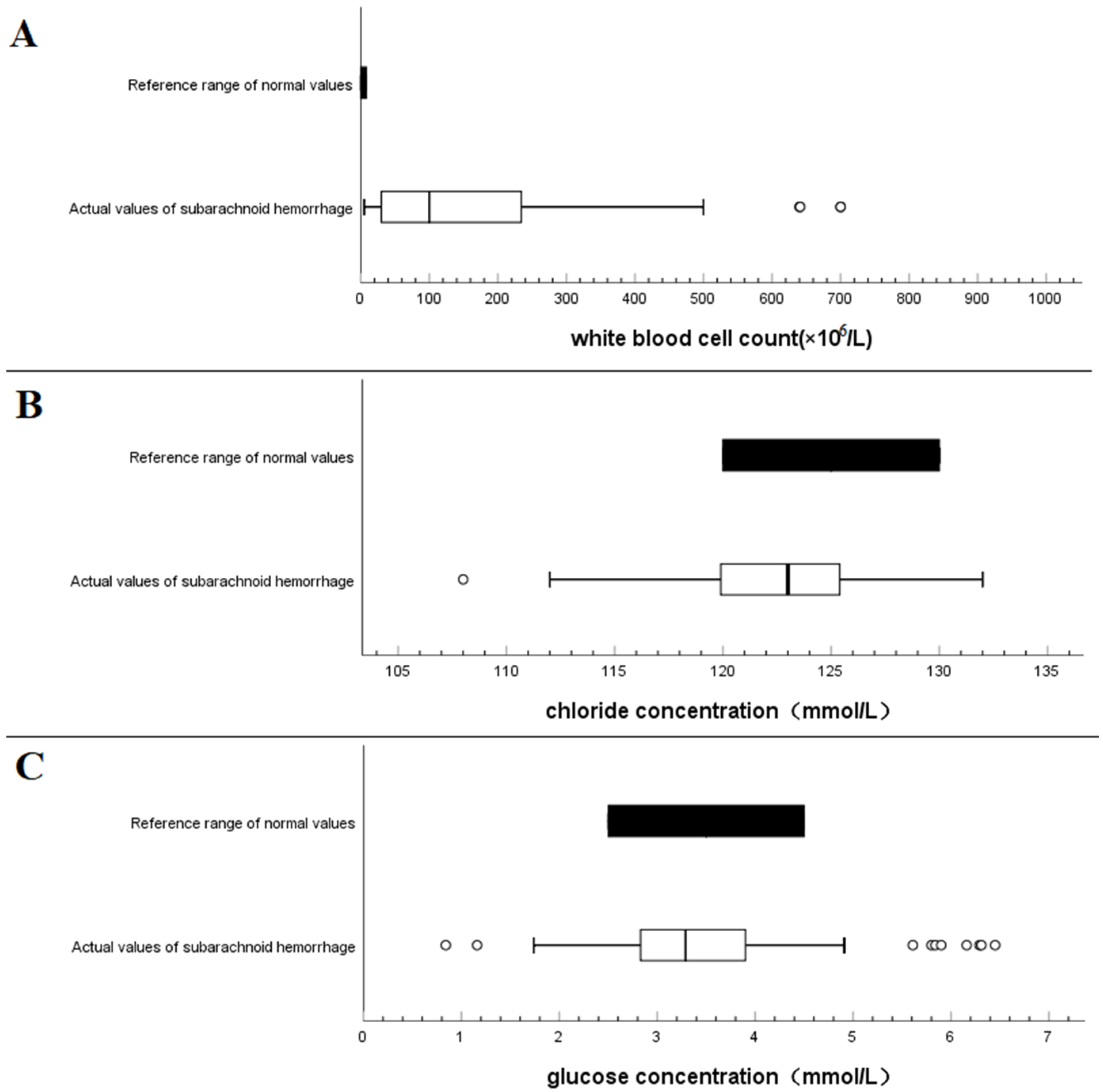
| Factors | n (%) | WBC (×106/L) | p Value | Chloride (mmol/L) | p Value | Glucose (mmol/L) | p Value | |
|---|---|---|---|---|---|---|---|---|
| Gender | Female | 67 (66.3) | 110 (40–310) | 0.038 | 125.8 (123.4–125.8) | 0.059 | 3.4 (3.0–3.9) | 0.274 |
| Male | 34 (33.7) | 38 (20–192) | 125.1 (122.1–125.1) | 3.0 (2.7–3.9) | ||||
| Age (years) | <60 | 61 (60.4) | 100 (22–306) | 0.532 | 124 (122.1–125.8) | <0.001 | 3.3 (2.8–3.9) | 0.274 |
| ≥60 | 40 (39.6) | 100 (42–212) | 120.7 (117.0–123.7) | 3.4 (3.0–4.2) | ||||
| treatment method | conservative | 15 (14.9) | 48 (16–100) | 0.04 | 120.5 (118.0–124.0) | 0.089 | 4.4 (3.6–5.9) | 0.002 |
| endovascular embolization | 86 (85.1) | 110 (30–310) | 123.4 (120.8–125.5) | 3.2 (2.8–3.8) | ||||
| CSF collection time (days) | ≤3 | 25 (24.8) | 120 (35–550) | 0.328 | 125.8 (122.5–127.1) | 0.001 | 3.7 (3.1–4.6) | 0.052 |
| 4–7 | 41 (40.6) | 70 (28–153) | 123.2 (121.5–125.2) | 3.1 (2.8–3.8) | ||||
| >7 | 35 (34.6) | 110 (30–234) | 121 (117.0–123.8) | 3.3 (2.9–4.0) | ||||
| RBC (×106/L) | WBC (×106/L) | Leukocyte | Chloride (mmol/L) | Glucose (mmol/L) | Protein (g/L) | |||
|---|---|---|---|---|---|---|---|---|
| Neutrophil (%) | Monocyte (%) | Lymphocyte (%) | ||||||
| Average | 64,995 | 241 | 45.7 | 9.0 | 45.7 | 122.4 | 3.6 | 0.9 |
| Standard deviation | 215,168 | 500 | 19.7 | 4.0 | 18.8 | 4.4 | 1.2 | 1.0 |
| p-value of normality test | <0.001 | <0.001 | 0.009 | <0.001 | 0.005 | 0.007 | <0.001 | <0.001 |
| 5th percentile | 290 | 8 | 10.0 | 4.6 | 18.2 | 114.9 | 2.2 | 0.2 |
| 10th percentile | 900 | 10 | 20.0 | 5.0 | 20.0 | 116.8 | 2.6 | 0.3 |
| median | 19,000 | 100 | 46.0 | 8.0 | 45.0 | 123.0 | 3.3 | 0.6 |
| 90th percentile | 66,162 | 496 | 70.0 | 10.0 | 73.8 | 127.2 | 5.5 | 2.3 |
| 95th percentile | 355,642 | 880 | 75.4 | 15.0 | 75.0 | 128.9 | 6.3 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, H.; Li, W.; Chen, Q. Effect of Spontaneous Subarachnoid Hemorrhage on Cerebrospinal Fluid Indicators. Brain Sci. 2023, 13, 778. https://doi.org/10.3390/brainsci13050778
You H, Li W, Chen Q. Effect of Spontaneous Subarachnoid Hemorrhage on Cerebrospinal Fluid Indicators. Brain Sciences. 2023; 13(5):778. https://doi.org/10.3390/brainsci13050778
Chicago/Turabian StyleYou, Huichao, Wenqi Li, and Qianxue Chen. 2023. "Effect of Spontaneous Subarachnoid Hemorrhage on Cerebrospinal Fluid Indicators" Brain Sciences 13, no. 5: 778. https://doi.org/10.3390/brainsci13050778
APA StyleYou, H., Li, W., & Chen, Q. (2023). Effect of Spontaneous Subarachnoid Hemorrhage on Cerebrospinal Fluid Indicators. Brain Sciences, 13(5), 778. https://doi.org/10.3390/brainsci13050778






