High ECM2 Expression Predicts Poor Clinical Outcome and Promotes the Proliferation, Migration, and Invasiveness of Glioma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gene Expression Analysis in Gliomas
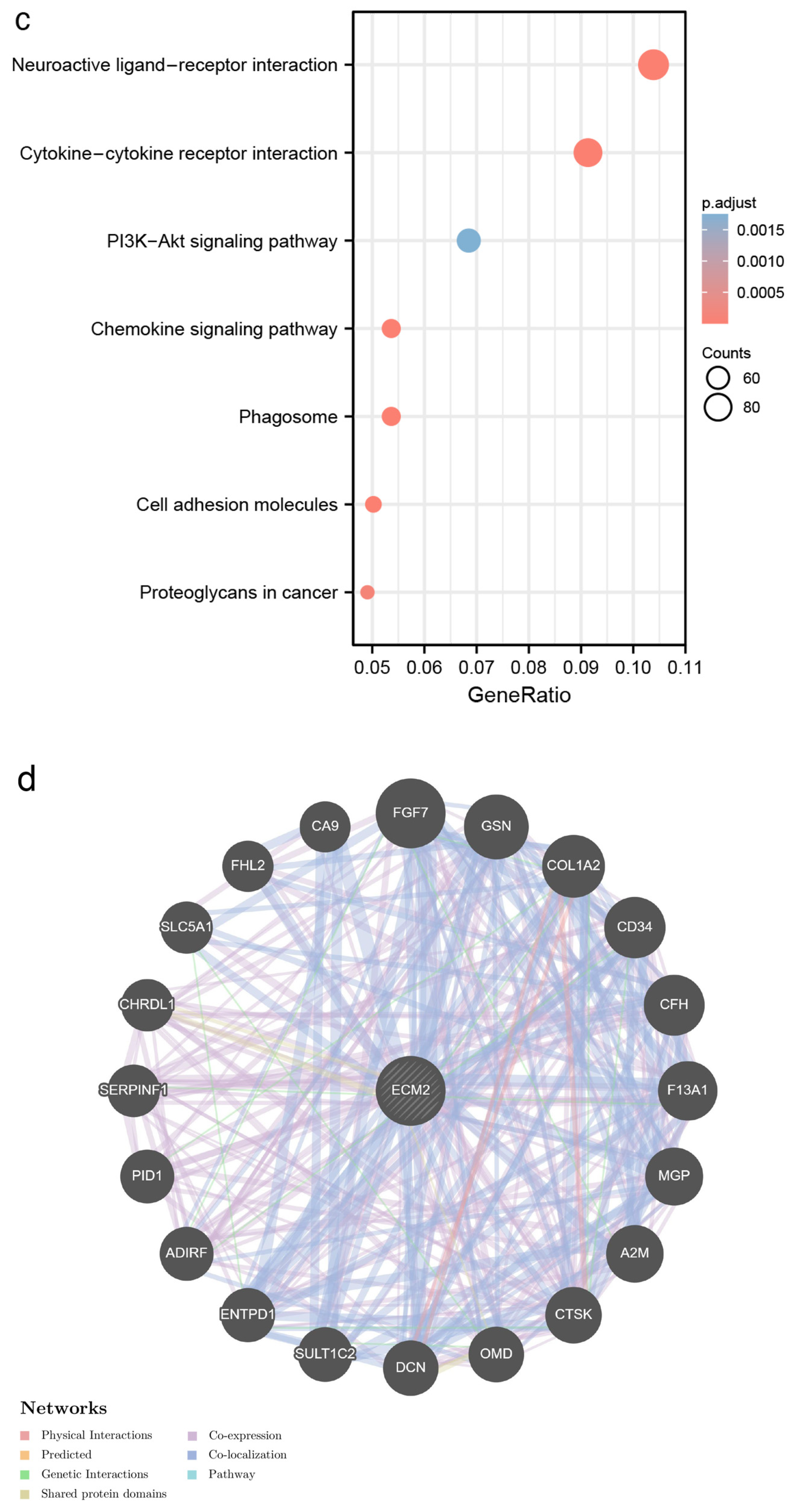
2.2. Differentially Expressed Gene (DEG), Genetic Interaction, and Functional Enrichment Analyses
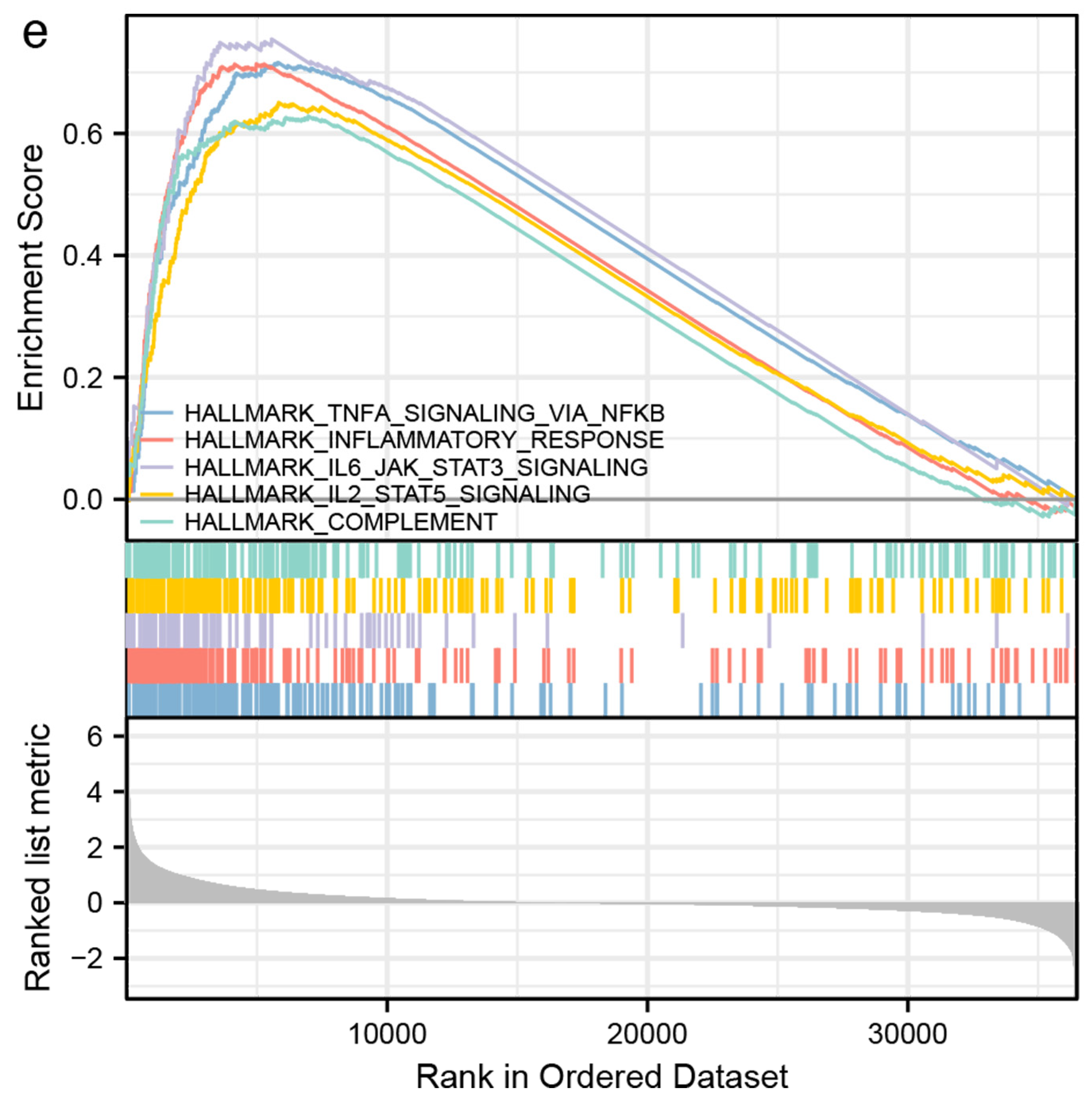
2.3. Immune-Related Analyses
2.4. Drug Sensitivity Analyses
2.5. Mutation Profiles in Different ECM2 Expression Levels
2.6. Development of Prognostic Model
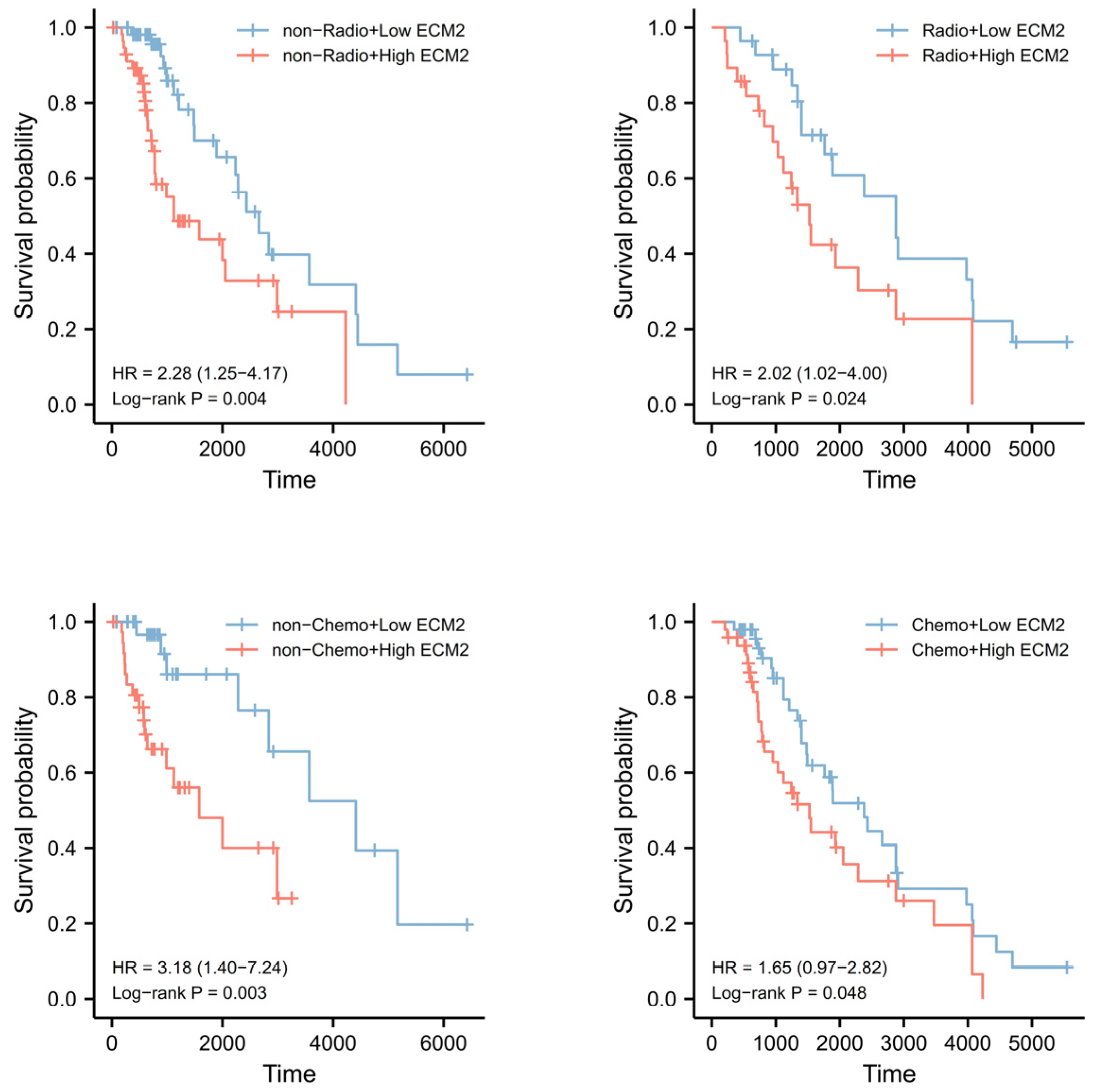
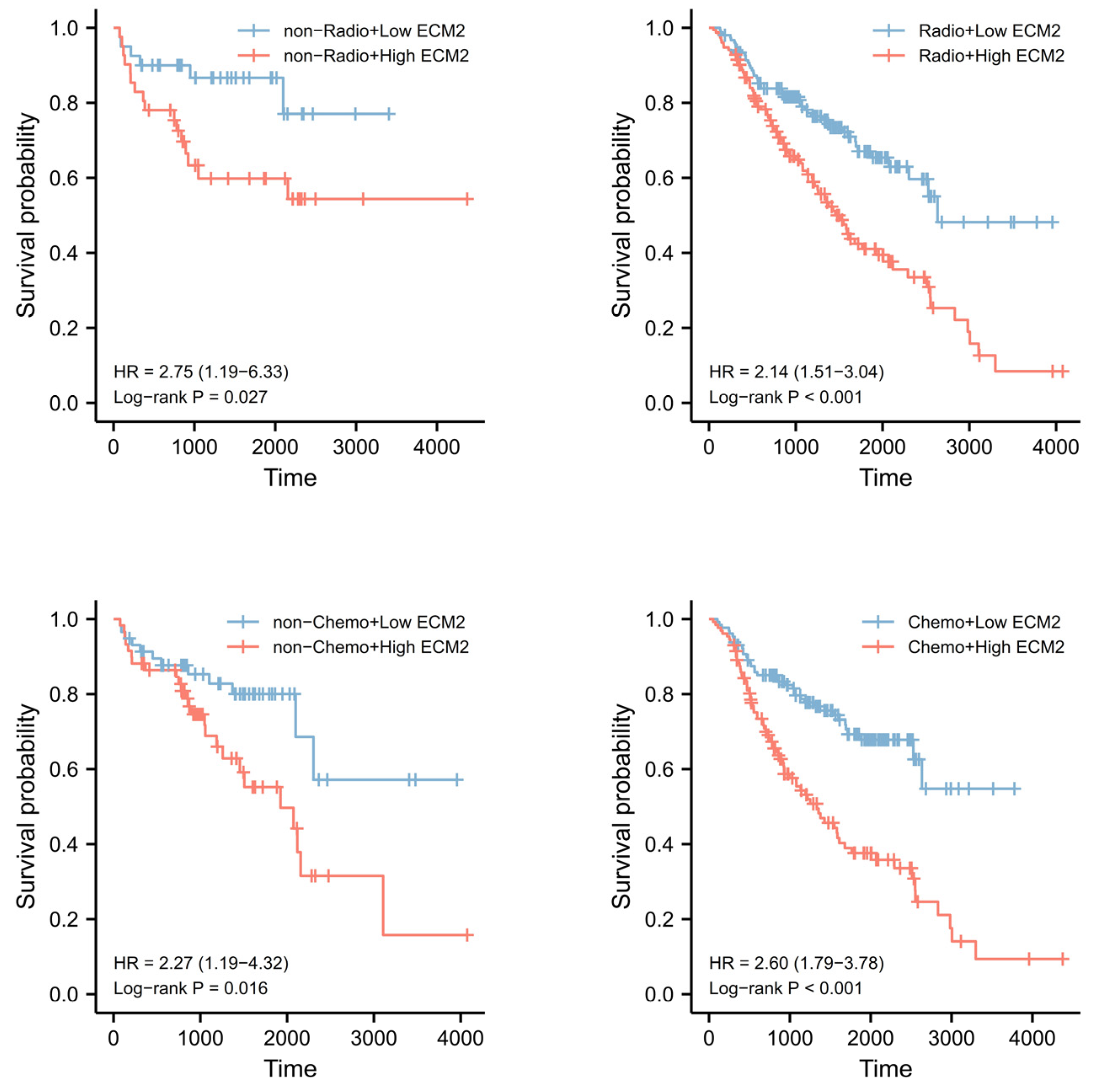
2.7. Survival Analysis Validation
2.8. Cell Culture and Transfection
2.9. qRT-PCR
2.10. Cell Counting Kit-8 Assay
2.11. Wound Healing Assay
2.12. Transwell Assay
2.13. Statistical Analyses
3. Results
3.1. ECM2 Expression Levels of Gliomas
3.2. Identification of DEGs and Functional Enrichment Analyses
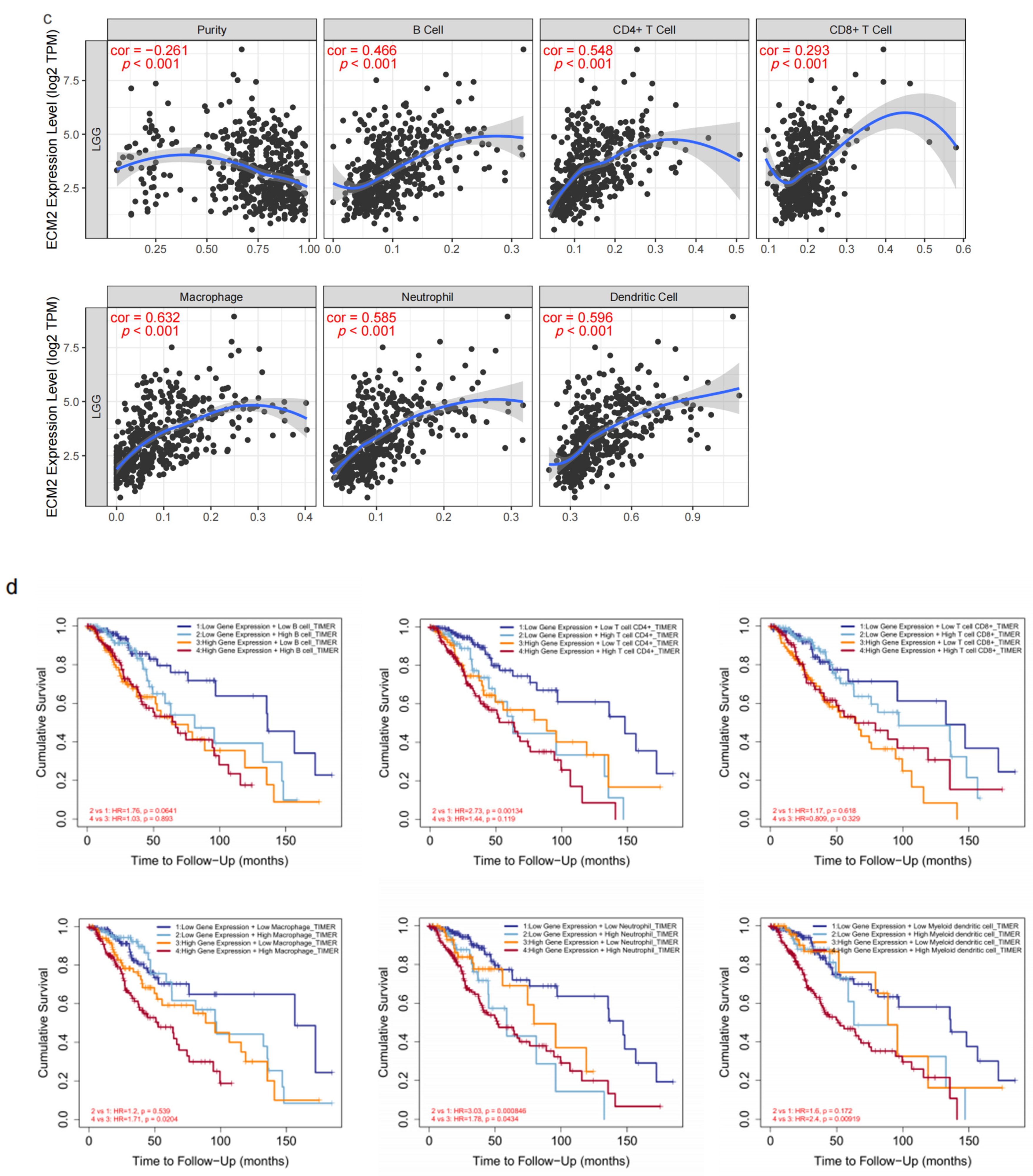
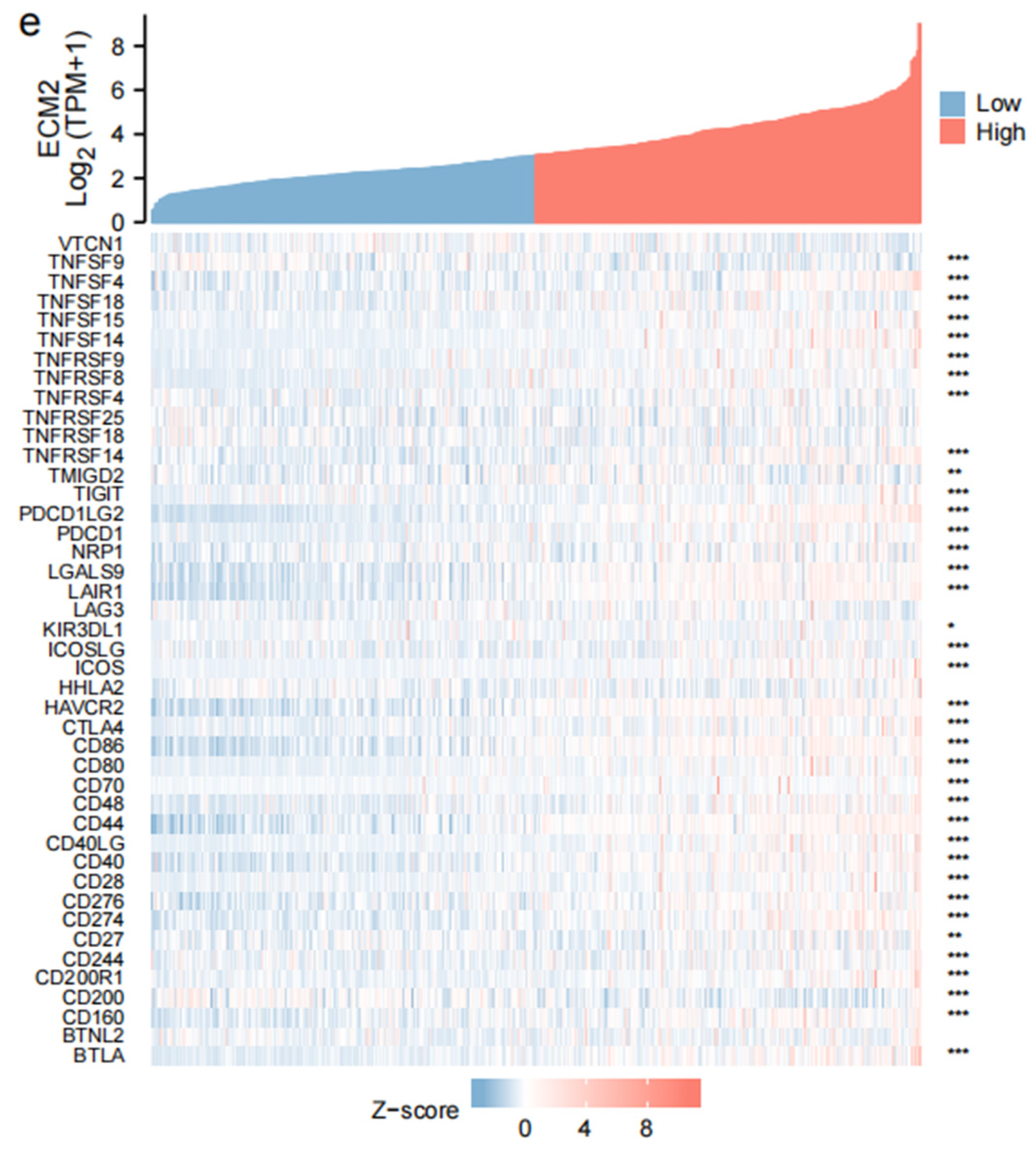
3.3. Immune-Related Analyses
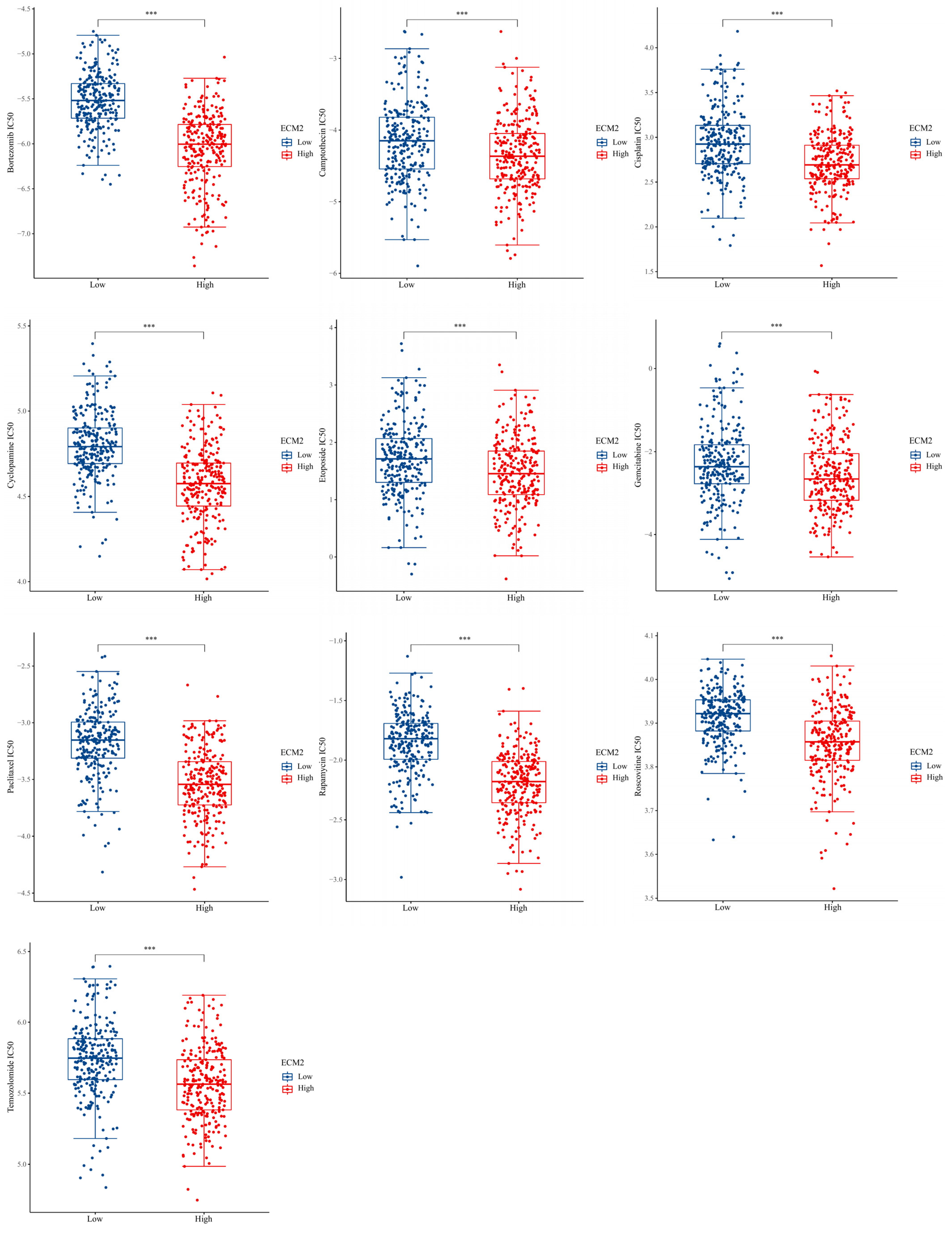
3.4. Drug Sensitivity Analyses
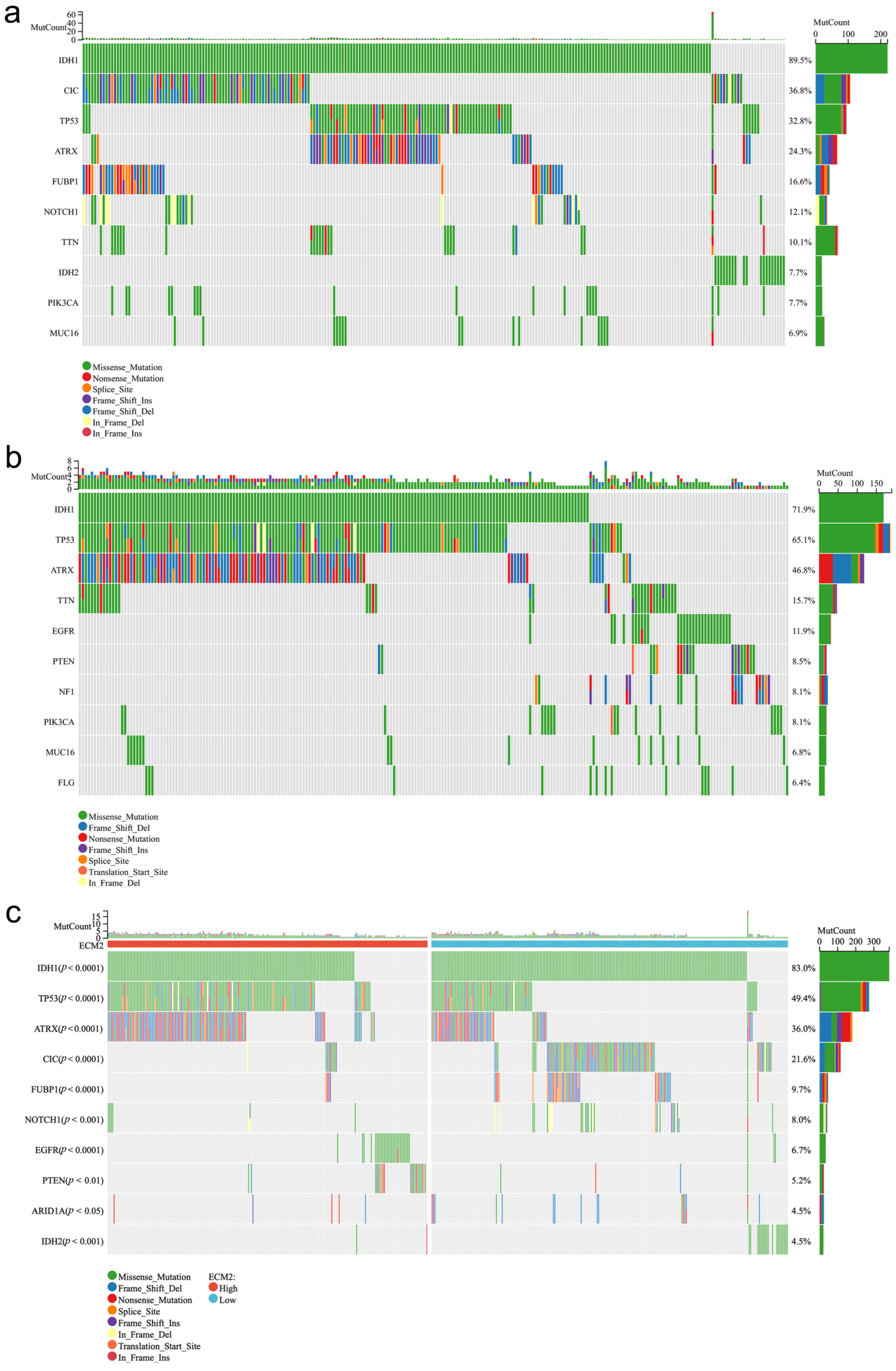
3.5. Mutation Profiles According to ECM2 Expression
3.6. Association between Clinical Features and ECM2 Expression
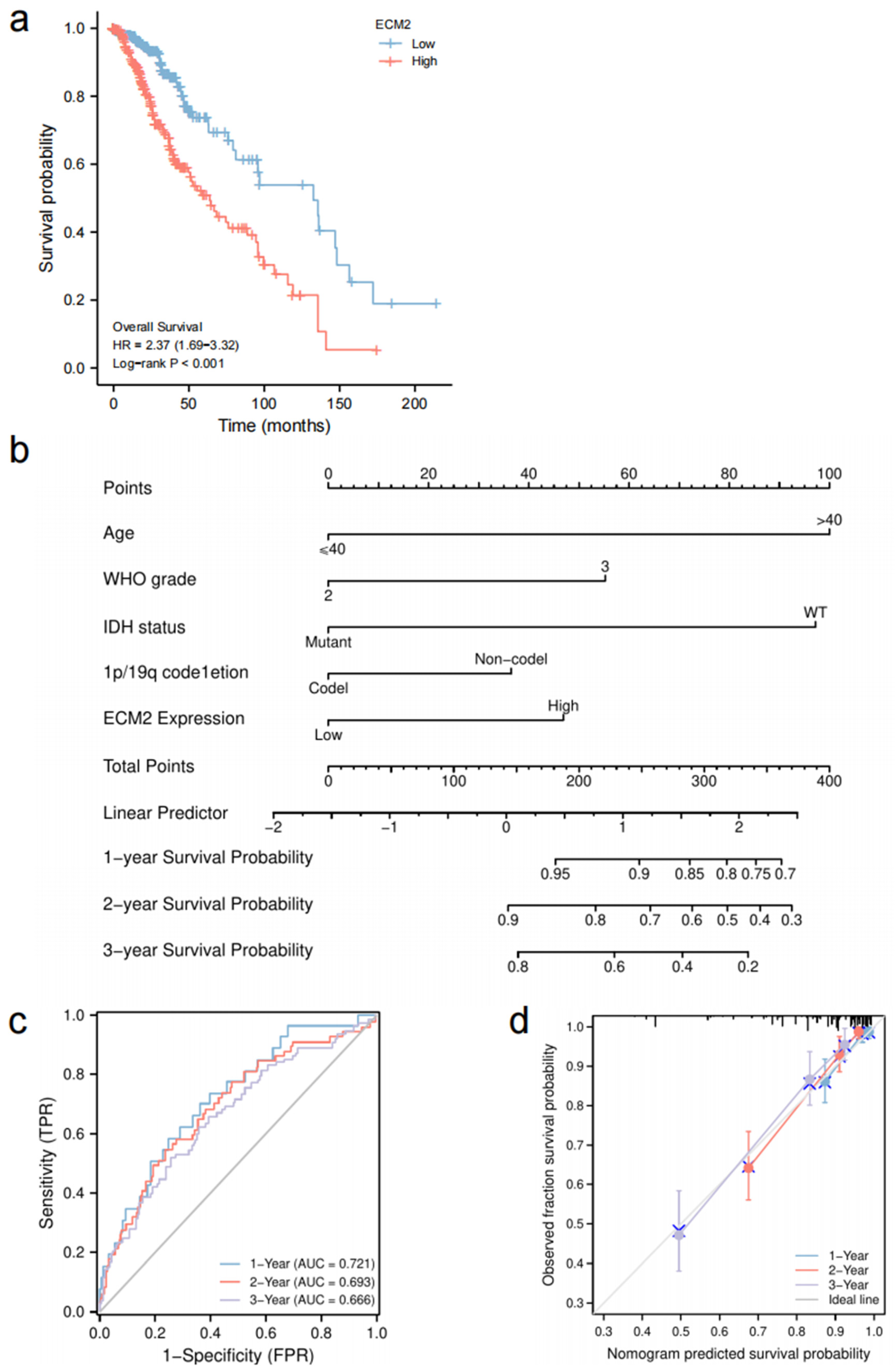
3.7. Prognostic Role of ECM2 and Nomogram Development
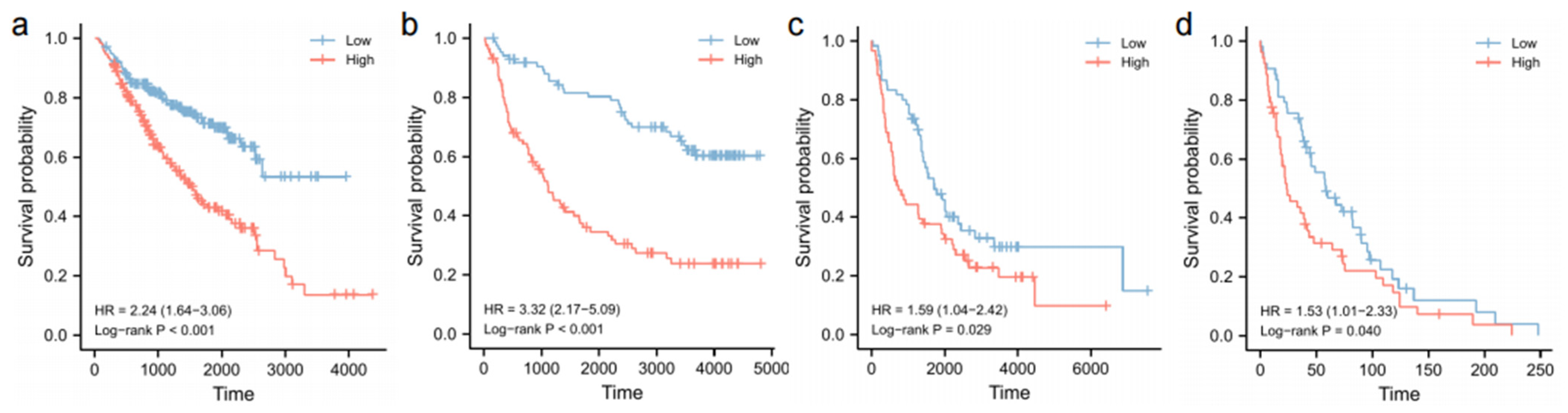
3.8. Validation of Survival Analyses
3.9. Knockdown of ECM2 Decreased the Proliferation, Migration, and Invasion In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Brat, D.J.; Verhaak, R.G.; Aldape, K.D.; Yung, W.K.; Salama, S.R.; Cooper, L.A.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; Morozova, O.; et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Li, J.; Tao, W.; Dong, W. DNA Methylation-Driven Genes for Developing Survival Nomogram for Low-Grade Glioma. Front. Oncol. 2021, 11, 629521. [Google Scholar] [CrossRef]
- Aquilanti, E.; Wen, P.Y. Current therapeutic options for glioblastoma and future perspectives. Expert Opin. Pharmacother. 2022, 23, 1629–1640. [Google Scholar] [CrossRef]
- Mahmoud, A.B.; Ajina, R.; Aref, S.; Darwish, M.; Alsayb, M.; Taher, M.; AlSharif, S.A.; Hashem, A.M.; Alkayyal, A.A. Advances in immunotherapy for glioblastoma multiforme. Front. Immunol. 2022, 13, 944452. [Google Scholar] [CrossRef]
- Asija, S.; Chatterjee, A.; Yadav, S.; Chekuri, G.; Karulkar, A.; Jaiswal, A.K.; Goda, J.S.; Purwar, R. Combinatorial approaches to effective therapy in glioblastoma (GBM): Current status and what the future holds. Int. Rev. Immunol. 2022, 41, 582–605. [Google Scholar] [CrossRef]
- Nishiu, J.; Tanaka, T.; Nakamura, Y. Identification of a novel gene (ECM2) encoding a putative extracellular matrix protein expressed predominantly in adipose and female-specific tissues and its chromosomal localization to 9q22.3. Genomics 1998, 52, 378–381. [Google Scholar] [CrossRef]
- Liu, C.; Tong, H.; Li, S.; Yan, Y. Effect of ECM2 expression on bovine skeletal muscle-derived satellite cell differentiation. Cell Biol. Int. 2018, 42, 525–532. [Google Scholar] [CrossRef]
- Wang, W.; Xu, C.; Ren, Y.; Wang, S.; Liao, C.; Fu, X.; Hu, H. A Novel Cancer Stemness-Related Signature for Predicting Prognosis in Patients with Colon Adenocarcinoma. Stem Cells Int. 2021, 2021, 7036059. [Google Scholar] [CrossRef]
- Kong, L.; Wang, J.; Cheng, J.; Zang, C.; Chen, F.; Wang, W.; Zhao, H.; Wang, Y.; Wang, D. Comprehensive Identification of the Human Secretome as Potential Indicators in Treatment Outcome of HPV-Positive and -Negative Cervical Cancer Patients. Gynecol. Obstet. Investig. 2020, 85, 405–415. [Google Scholar] [CrossRef]
- Nwosu, Z.C.; Megger, D.A.; Hammad, S.; Sitek, B.; Roessler, S.; Ebert, M.P.; Meyer, C.; Dooley, S. Identification of the Consistently Altered Metabolic Targets in Human Hepatocellular Carcinoma. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 303–323.e301. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Xu, L.; Dai, Y.; Yuan, Q.; Zhou, Z. ECM2 and GLT8D2 in human pulmonary artery hypertension: Fruits from weighted gene co-expression network analysis. J. Thorac. Dis. 2021, 13, 2242–2254. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Xia, S.; Yang, X.; Yu, J.; Guo, B.; Lin, Z.; Wei, R.; Mao, M.; Zhang, Z.; Zhao, G.; et al. Complex Involvement of the Extracellular Matrix, Immune Effect, and Lipid Metabolism in the Development of Idiopathic Pulmonary Fibrosis. Front. Mol. Biosci. 2021, 8, 800747. [Google Scholar] [CrossRef]
- Oritani, K.; Kanakura, Y.; Aoyama, K.; Yokota, T.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Tomiyama, Y.; Matsuzawa, Y.; Kincade, P.W. Matrix glycoprotein SC1/ECM2 augments B lymphopoiesis. Blood 1997, 90, 3404–3413. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef]
- Li, B.; Severson, E.; Pignon, J.C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Luo, H.; Wang, J.; Li, J.; Jiang, Y. Identification of a pyroptosis-related prognostic signature in breast cancer. BMC Cancer 2022, 22, 429. [Google Scholar] [CrossRef] [PubMed]
- Geeleher, P.; Cox, N.; Huang, R.S. pRRophetic: An R package for prediction of clinical chemotherapeutic response from tumor gene expression levels. PLoS ONE 2014, 9, e107468. [Google Scholar] [CrossRef]
- Liu, J.; Lichtenberg, T.; Hoadley, K.A.; Poisson, L.M.; Lazar, A.J.; Cherniack, A.D.; Kovatich, A.J.; Benz, C.C.; Levine, D.A.; Lee, A.V.; et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018, 173, 400–416.e411. [Google Scholar] [CrossRef] [PubMed]
- Oritani, K.; Kincade, P.W. Lymphopoiesis and matrix glycoprotein SC1/ECM2. Leuk. Lymphoma 1998, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tang, L.; Liu, Z.; Yang, K.; Cheng, Q. Bioinformatic Analyses Identify a Prognostic Autophagy-Related Long Non-coding RNA Signature Associated with Immune Microenvironment in Diffuse Gliomas. Front. Cell Dev. Biol. 2021, 9, 694633. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef]
- Offer, S.; Menard, J.A.; Pérez, J.E.; de Oliveira, K.G.; Chandran, V.I.; Johansson, M.C.; Bång-Rudenstam, A.; Siesjö, P.; Ebbesson, A.; Hedenfalk, I.; et al. Extracellular lipid loading augments hypoxic paracrine signaling and promotes glioma angiogenesis and macrophage infiltration. J. Exp. Clin. Cancer Res. CR 2019, 38, 241. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. A J. Virtual Libr. 2008, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yung, M.M.H.; Ngan, H.Y.S.; Chan, K.K.L.; Chan, D.W. The Impact of the Tumor Microenvironment on Macrophage Polarization in Cancer Metastatic Progression. Int. J. Mol. Sci. 2021, 22, 6560. [Google Scholar] [CrossRef] [PubMed]
- Leonard, F.; Curtis, L.T.; Ware, M.J.; Nosrat, T.; Liu, X.; Yokoi, K.; Frieboes, H.B.; Godin, B. Macrophage Polarization Contributes to the Anti-Tumoral Efficacy of Mesoporous Nanovectors Loaded with Albumin-Bound Paclitaxel. Front. Immunol. 2017, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; Kamp, V.M.; van Hoffen, E.; Visser, T.; Tak, T.; Lammers, J.W.; Ulfman, L.H.; Leenen, L.P.; Pickkers, P.; Koenderman, L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J. Clin. Investig. 2012, 122, 327–336. [Google Scholar] [CrossRef]
- Guo, C.; Pirozzi, C.J.; Lopez, G.Y.; Yan, H. Isocitrate dehydrogenase mutations in gliomas: Mechanisms, biomarkers and therapeutic target. Curr. Opin. Neurol. 2011, 24, 648–652. [Google Scholar] [CrossRef]
- Vigneswaran, K.; Neill, S.; Hadjipanayis, C.G. Beyond the World Health Organization grading of infiltrating gliomas: Advances in the molecular genetics of glioma classification. Ann. Transl. Med. 2015, 3, 95. [Google Scholar] [CrossRef]
- Dunn, G.P.; Rinne, M.L.; Wykosky, J.; Genovese, G.; Quayle, S.N.; Dunn, I.F.; Agarwalla, P.K.; Chheda, M.G.; Campos, B.; Wang, A.; et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012, 26, 756–784. [Google Scholar] [CrossRef]
- Martin, S.; Janouskova, H.; Dontenwill, M. Integrins and p53 pathways in glioblastoma resistance to temozolomide. Front. Oncol. 2012, 2, 157. [Google Scholar] [CrossRef]
- Wang, P.; Ye, J.A.; Hou, C.X.; Zhou, D.; Zhan, S.Q. Combination of lentivirus-mediated silencing of PPM1D and temozolomide chemotherapy eradicates malignant glioma through cell apoptosis and cell cycle arrest. Oncol. Rep. 2016, 36, 2544–2552. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Das, N.; Sarkar, M.; Chatterjee, U.; Chatterjee, S.; Ghosh, M.K. Exosome-mediated delivery of the intrinsic C-terminus domain of PTEN protects it from proteasomal degradation and ablates tumorigenesis. Mol. Ther. J. Am. Soc. Gene Ther. 2015, 23, 255–269. [Google Scholar] [CrossRef]
- Bazzichetto, C.; Conciatori, F.; Pallocca, M.; Falcone, I.; Fanciulli, M.; Cognetti, F.; Milella, M.; Ciuffreda, L. PTEN as a Prognostic/Predictive Biomarker in Cancer: An Unfulfilled Promise? Cancers 2019, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Ymer, S.I.; Greenall, S.A.; Cvrljevic, A.; Cao, D.X.; Donoghue, J.F.; Epa, V.C.; Scott, A.M.; Adams, T.E.; Johns, T.G. Glioma Specific Extracellular Missense Mutations in the First Cysteine Rich Region of Epidermal Growth Factor Receptor (EGFR) Initiate Ligand Independent Activation. Cancers 2011, 3, 2032–2049. [Google Scholar] [CrossRef] [PubMed]
- Golding, S.E.; Morgan, R.N.; Adams, B.R.; Hawkins, A.J.; Povirk, L.F.; Valerie, K. Pro-survival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol. Ther. 2009, 8, 730–738. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. CB 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef]
- Choi, J.; Gyamfi, J.; Jang, H.; Koo, J.S. The role of tumor-associated macrophage in breast cancer biology. Histol. Histopathol. 2018, 33, 133–145. [Google Scholar] [CrossRef]






| Characteristic | Low Expression of ECM2 | High Expression of ECM2 | p Value |
|---|---|---|---|
| Age, n (%) | 0.931 | ||
| ≤40 | 133 (50.4%) | 131 (49.6%) | |
| >40 | 131 (49.6%) | 133 (50.4%) | |
| Gender, n (%) | 0.162 | ||
| Female | 128 (48.5%) | 111 (42%) | |
| Male | 136 (51.5%) | 153 (58%) | |
| WHO grade, n (%) | <0.001 * | ||
| 2 | 165 (31.2%) | 120 (22.7%) | |
| 3 | 99 (18.8%) | 144 (61.3%) | |
| IDH status, n (%) | <0.001 * | ||
| Wild type | 14 (5.3%) | 86 (16.3%) | |
| Mutant | 250 (94.7%) | 178 (68.2%) | |
| 1p/19q codeletion, n (%) | <0.001 * | ||
| Noncodel | 110 (41.7%) | 247 (93.6%) | |
| Codel | 154 (58.3%) | 17 (6.4%) |
| Characteristic | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95%CI) | p Value | Hazard Ratio (95%CI) | p Value | |
| Age | ||||
| ≤40 | Reference | Reference | ||
| >40 | 2.889 (2.009–4.155) | <0.001 * | 2.931 (1.989–4.320) | <0.001 * |
| Gender | ||||
| Female | Reference | |||
| Male | 1.124 (0.800–1.580) | 0.499 | ||
| WHO grade | ||||
| 2 | Reference | Reference | ||
| 3 | 2.354 (1.664–3.332) | <0.001 * | 1.812 (1.266–2.593) | 0.001 * |
| IDH status | ||||
| Wild type | Reference | Reference | ||
| Mutant | 0.206 (0.145–0.292) | <0.001 * | 0.351 (0.236–0.523) | <0.001 * |
| 1p/19q codeletion | ||||
| Noncodel | Reference | Reference | ||
| Codel | 0.401 (0.256–0.629) | <0.001 * | 0.676 (0.380–1.199) | 0.181 |
| Radiotherapy | ||||
| No | Reference | |||
| Yes | 0.950 (0.610–1.480) | 0.820 | ||
| Chemotherapy | ||||
| No | Reference | |||
| Yes | 1.415 (0.877–2.284) | 0.155 | ||
| ECM2 expression | ||||
| Low | Reference | Reference | ||
| High | 2.449 (1.698–3.534) | <0.001 * | 1.656 (1.055–2.600) | 0.028 * |
| Characteristic | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95%CI) | p Value | Hazard Ratio (95%CI) | p Value | |
| Age | ||||
| ≤40 | Reference | |||
| >40 | 1.208 (0.882–1.655) | 0.239 | ||
| Gender | ||||
| Female | Reference | |||
| Male | 1.007 (0.734–1.381) | 0.965 | ||
| WHO grade | ||||
| 2 | Reference | Reference | ||
| 3 | 2.544 (1.780–3.635) | <0.001 * | 2.673 (1.787–4.000) | <0.001 * |
| IDH status | ||||
| Wild type | Reference | Reference | ||
| Mutant | 0.459 (0.325–0.647) | <0.001 * | 0.581 (0.390–0.867) | 0.008 * |
| 1p/19q codeletion | ||||
| Noncodel | Reference | Reference | ||
| Codel | 0.353 (0.231–0.541) | <0.001 * | 0.562 (0.347–0.911) | 0.019 * |
| Radiotherapy | ||||
| No | Reference | Reference | ||
| Yes | 1.592 (1.012–2.505) | 0.044 * | 1.352 (0.818–2.233) | 0.239 |
| Chemotherapy | ||||
| No | Reference | |||
| Yes | 1.290 (0.889–1.873) | 0.180 | ||
| ECM2 expression | ||||
| Low | Reference | Reference | ||
| High | 2.408 (1.734–3.343) | <0.001 * | 2.119 (1.427–3.147) | <0.001 * |
| Characteristic | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95%CI) | p Value | Hazard Ratio (95%CI) | p Value | |
| Age | ||||
| ≤40 | Reference | |||
| >40 | 1.348 (0.891–2.039) | 0.157 | ||
| Gender | ||||
| Female | Reference | Reference | ||
| Male | 0.635 (0.420–0.960) | 0.031 * | 0.624 (0.401–0.968) | 0.036 * |
| WHO grade | ||||
| 2 | Reference | Reference | ||
| 3 | 3.579 (2.335–5.485) | <0.001 * | 3.265 (1.975–5.397) | <0.001 * |
| IDH status | ||||
| Wild type | Reference | Reference | ||
| Mutant | 0.379 (0.244–0.587) | <0.001 * | 0.869 (0.532–1.420) | 0.576 |
| 1p/19q codeletion | ||||
| Noncodel | Reference | Reference | ||
| Codel | 0.156 (0.082–0.294) | <0.001 * | 0.233 (0.110–0.492) | <0.001 * |
| Radiotherapy | ||||
| No | Reference | Reference | ||
| Yes | 0.564 (0.339–0.938) | 0.027 * | 0.773 (0.445–1.340) | 0.359 |
| Chemotherapy | ||||
| No | Reference | Reference | ||
| Yes | 1.610 (1.039–2.495) | 0.033 * | 1.101 (0.673–1.803) | 0.702 |
| ECM2 expression | ||||
| Low | Reference | Reference | ||
| High | 3.451 (2.211–5.385) | <0.001 * | 1.769 (1.049–2.986) | 0.033 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, S.; He, Q.; Lin, F.; Tao, C.; Ding, Y.; Wang, J.; Zhao, J.; Wang, W. High ECM2 Expression Predicts Poor Clinical Outcome and Promotes the Proliferation, Migration, and Invasiveness of Glioma. Brain Sci. 2023, 13, 851. https://doi.org/10.3390/brainsci13060851
Li J, Wang S, He Q, Lin F, Tao C, Ding Y, Wang J, Zhao J, Wang W. High ECM2 Expression Predicts Poor Clinical Outcome and Promotes the Proliferation, Migration, and Invasiveness of Glioma. Brain Sciences. 2023; 13(6):851. https://doi.org/10.3390/brainsci13060851
Chicago/Turabian StyleLi, Junsheng, Siyu Wang, Qiheng He, Fa Lin, Chuming Tao, Yaowei Ding, Jia Wang, Jizong Zhao, and Wen Wang. 2023. "High ECM2 Expression Predicts Poor Clinical Outcome and Promotes the Proliferation, Migration, and Invasiveness of Glioma" Brain Sciences 13, no. 6: 851. https://doi.org/10.3390/brainsci13060851





