Maternal LPS Exposure Enhances the 5-HT Level in the Prefrontal Cortex of Autism-like Young Offspring
Abstract
:1. Introduction
2. Materials and Methods
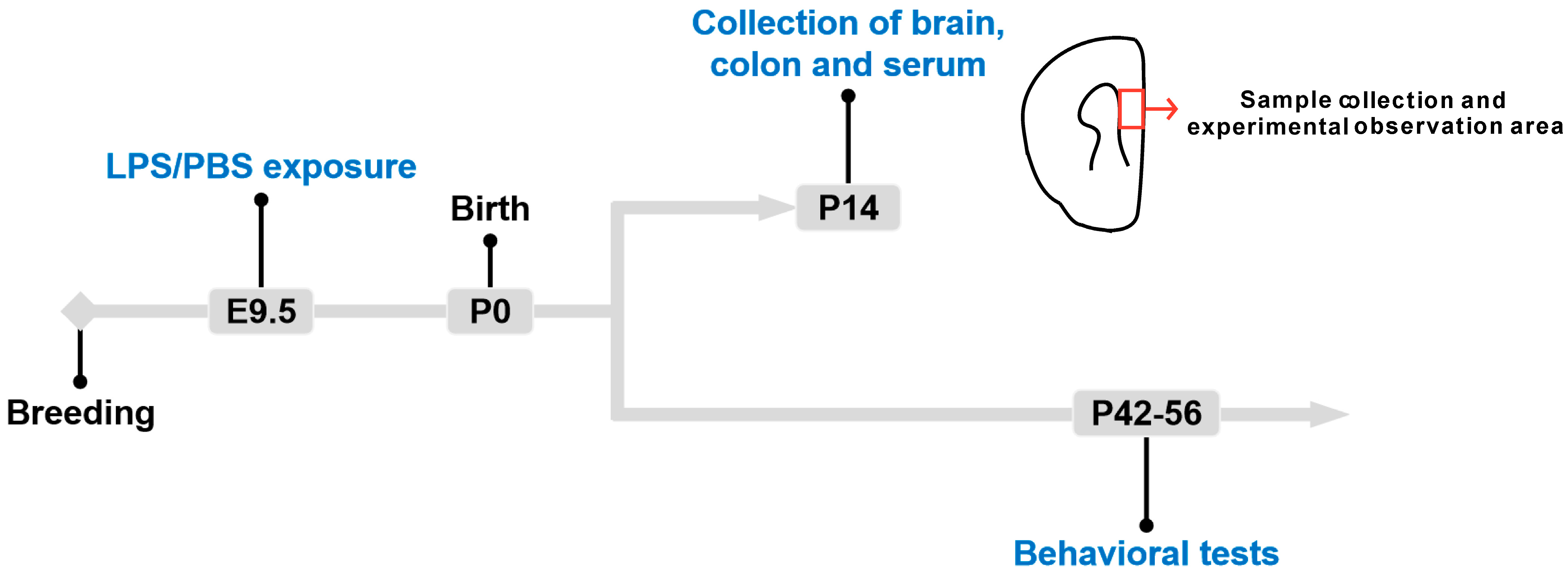
2.1. Animals and Drug Treatment
2.2. Behavioral Tests
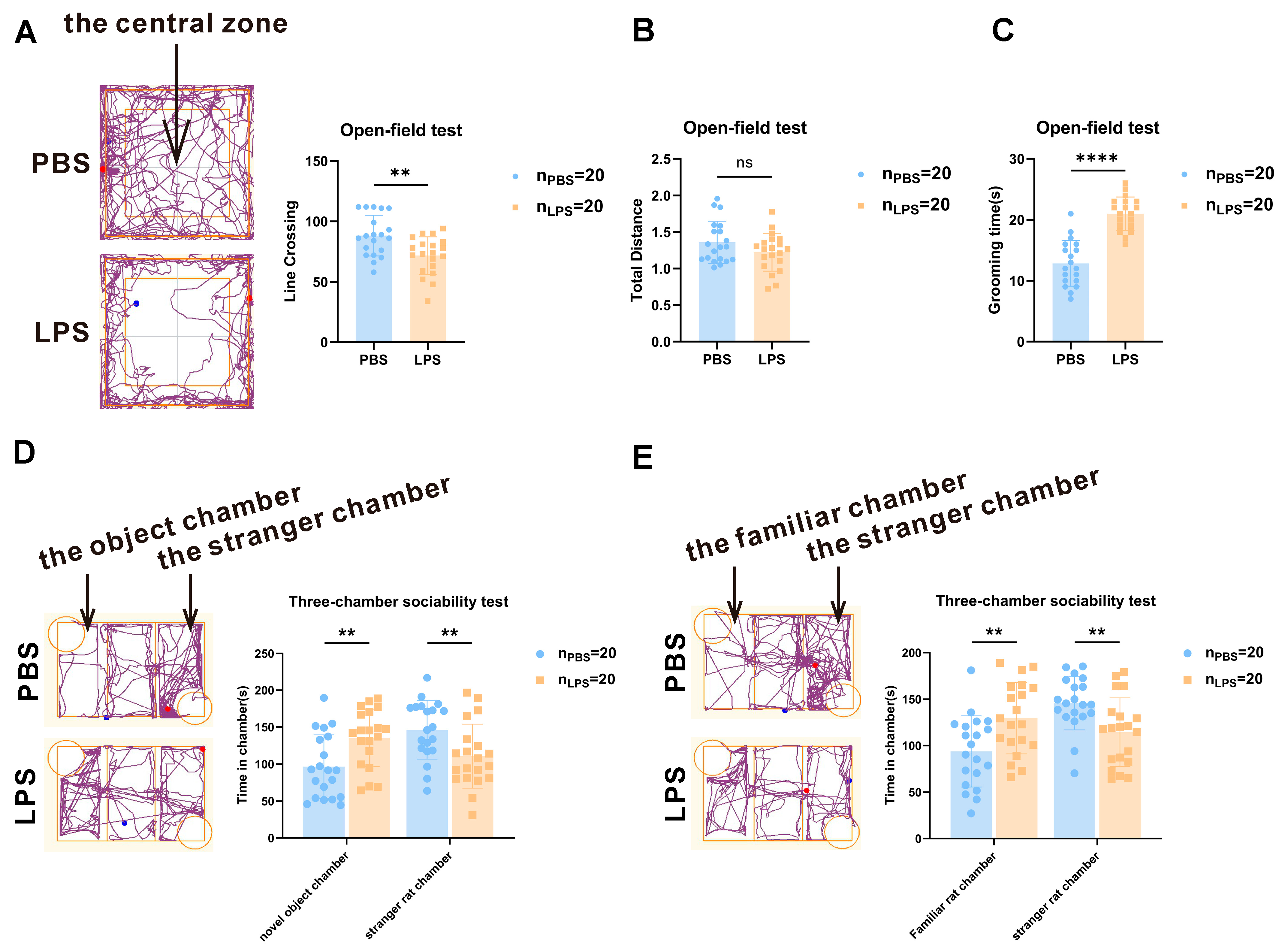
2.3. Open-Field Test
2.4. Three-Chamber Sociability Test
2.5. Animal Tissue Collection
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Immunofluorescence Staining
2.8. Golgi-Cox Staining
2.9. Quantitative Polymerase Chain Reaction
2.10. Protein Extraction and Western Blotting
2.11. RNA Sequencing Analysis
2.12. Tryptophan Target Metabolomics
2.13. Statistical Analysis
3. Results
3.1. Maternal LPS Treatment Resulted in ASD-like Behavioral Deficits in Offspring
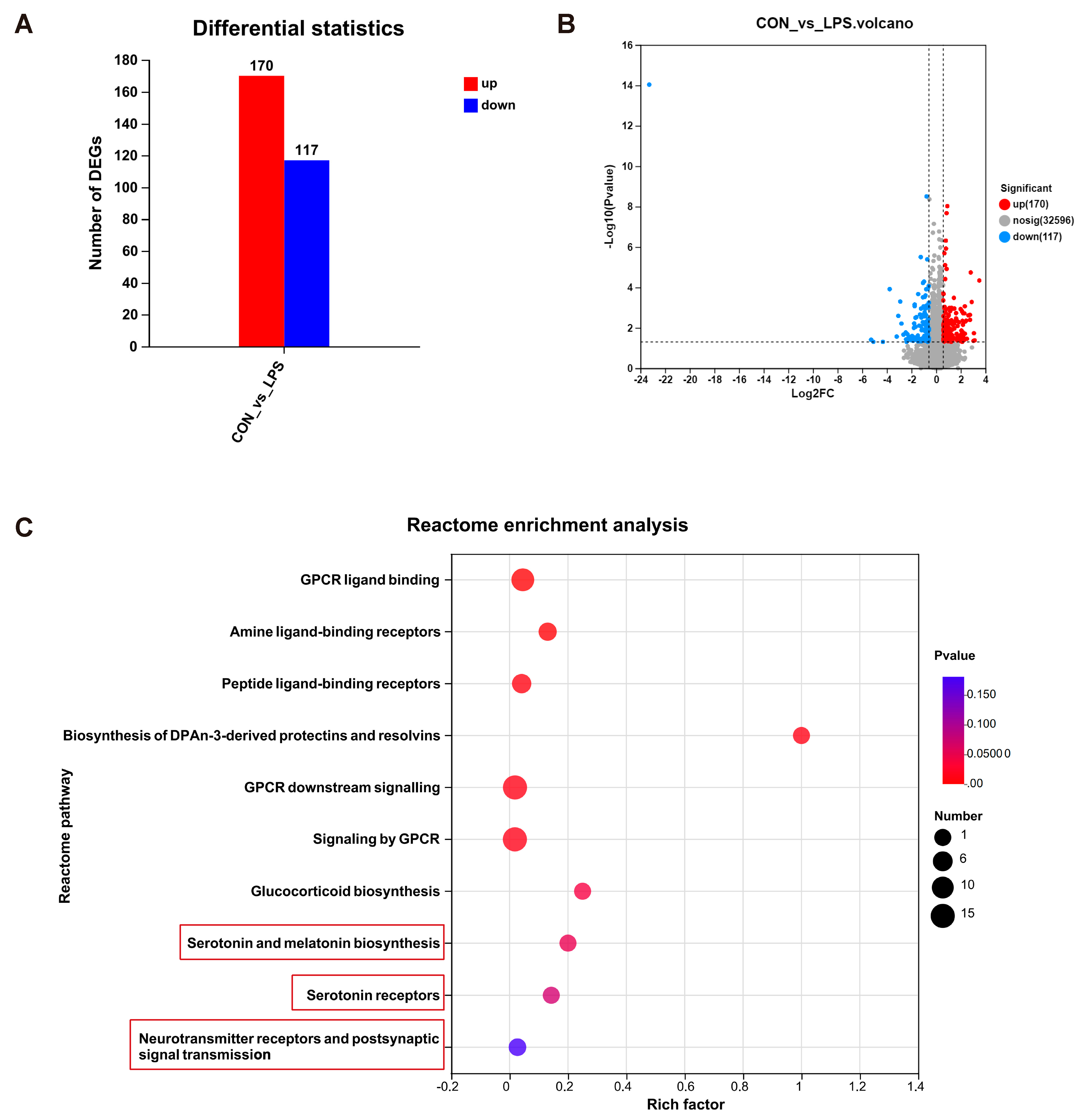
3.2. Maternal LPS Treatment Might Impact Serotonin Metabolism and the Neuronal System of the Offspring mPFC
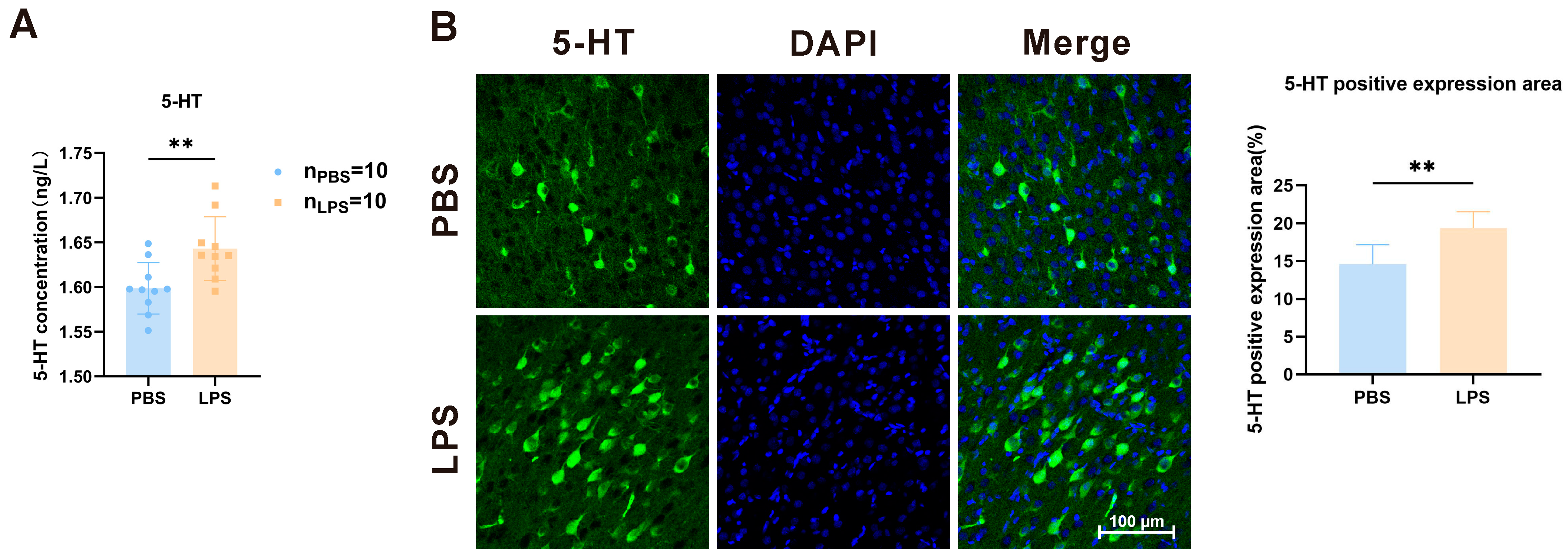
3.3. Maternal LPS Treatment Increased the 5-HT Level in the mPFC of Offspring
3.4. Maternal LPS Treatment Led to Abnormal Tryptophan Metabolism in the Offspring mPFC
3.5. The 5-HT Receptor Expression Level Was Induced and Neuronal Morphology Changed in the Offspring mPFC after Maternal LPS Treatment
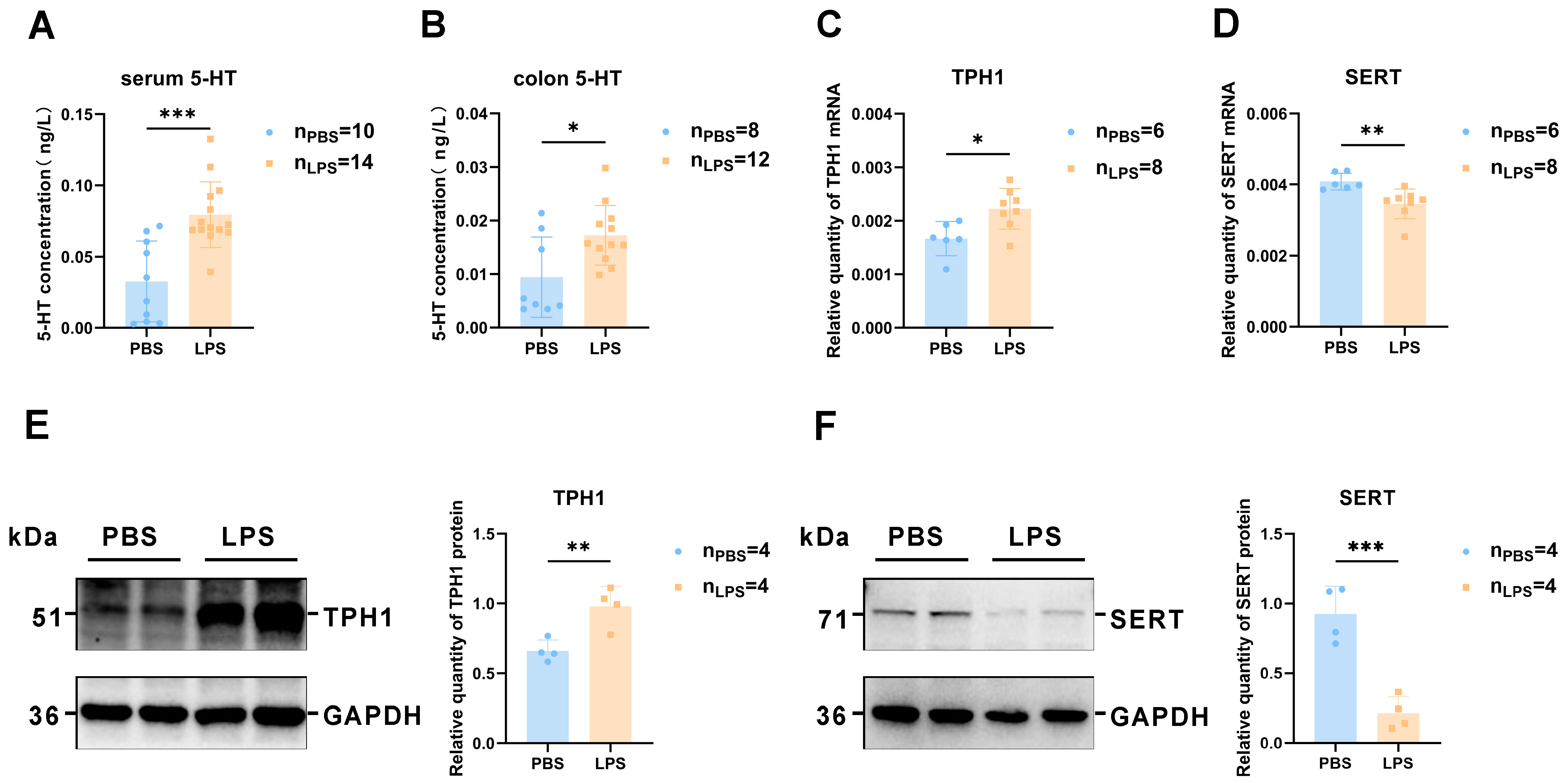
3.6. 5-HT Synthesis Was Induced and Reabsorption Was Reduced in the Offspring Colon after Maternal LPS Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism spectrum disorder. Nat. Rev. Dis. Prim. 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Girdler, S.; Marschik, P.B. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell. Mol. Life Sci. 2019, 76, 1275–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.; Choo, H.; Jeon, B. Serotonin Receptors as Therapeutic Targets for Autism Spectrum Disorder Treatment. Int. J. Mol. Sci. 2022, 23, 6515. [Google Scholar] [CrossRef]
- Zerbo, O.; Qian, Y.; Yoshida, C.; Grether, J.K.; Van de Water, J.; Croen, L.A. Maternal Infection During Pregnancy and Autism Spectrum Disorders. J. Autism. Dev. Disord. 2015, 45, 4015–4025. [Google Scholar] [CrossRef] [Green Version]
- Zawadzka, A.; Cieslik, M.; Adamczyk, A. The Role of Maternal Immune Activation in the Pathogenesis of Autism: A Review of the Evidence, Proposed Mechanisms and Implications for Treatment. Int. J. Mol. Sci. 2021, 22, 11516. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, T.B.; Taricano, M.; Maiorka, P.C.; Palermo-Neto, J.; Bernardi, M.M. Prenatal lipopolysaccharide reduces social behavior in male offspring. Neuroimmunomodulation 2010, 17, 240–251. [Google Scholar] [CrossRef]
- Israelyan, N.; Margolis, K.G. Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol. Res. 2018, 132, 1–6. [Google Scholar] [CrossRef]
- Gaspar, P.; Cases, O.; Maroteaux, L. The developmental role of serotonin: News from mouse molecular genetics. Nat. Rev. Neurosci. 2003, 4, 1002–1012. [Google Scholar] [CrossRef]
- Chugani, D.C.; Muzik, O.; Rothermel, R.; Behen, M.; Chakraborty, P.; Mangner, T.; Da Silva, E.A.; Chugani, H.T. Altered Serotonin Synthesis in the Dentatothalamocortical Pathway in Autistic Boys. Ann. Neurol. 1997, 42, 666–669. [Google Scholar] [CrossRef]
- Naoko, N.; Michiko, K.; Mami, T.; Kaoru, M.; Masaaki, N.; Nobuo, O. Increased Monoamine Concentration in the Brain and Blood of Fetal Thalidomide- and Valproic Acid-Exposed Rat: Putative Animal Models for Autism. Pediatr. Res. 2002, 52, 576–579. [Google Scholar] [CrossRef] [Green Version]
- Tsujino, N.; Nakatani, Y.; Seki, Y.; Nakasato, A.; Nakamura, M.; Sugawara, M.; Arita, H. Abnormality of circadian rhythm accompanied by an increase in frontal cortex serotonin in animal model of autism. Neurosci. Res. 2007, 57, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.P.; Commons, K.G. Serotonin neuron abnormalities in the BTBR mouse model of autism. Autism. Res. 2017, 10, 66–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.; Zhu, J.; Yang, T.; Lai, X.; Liu, X.; Liu, J.; Chen, J.; Li, T. Vitamin A improves the symptoms of autism spectrum disorders and decreases 5-hydroxytryptamine (5-HT): A pilot study. Brain Res. Bull. 2018, 137, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hua, X.; Yang, T.; Guo, M.; Li, Q.; Xiao, L.; Li, L.; Chen, J.; Li, T. Alterations in Gut Vitamin and Amino Acid Metabolism are Associated with Symptoms and Neurodevelopment in Children with Autism Spectrum Disorder. J. Autism. Dev. Disord. 2022, 52, 3116–3128. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M. Housing, husbandry and handling of rodents for behavioral experiments. Nat. Protoc. 2006, 1, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Rein, B.; Ma, K.; Yan, Z. A standardized social preference protocol for measuring social deficits in mouse models of autism. Nat. Protoc. 2020, 15, 3464–3477. [Google Scholar] [CrossRef]
- Tanaka, M.; Toth, F.; Polyak, H.; Szabo, A.; Mandi, Y.; Vecsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hortnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of Serotonin by a Second Tryptophan Hydroxylase Isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef] [PubMed]
- Orabona, G.; Griesi-Oliveira, K.; Vadasz, E.; Bulcão, V.; Takahashi, V.; Moreira, E.; Furia-Silva, M.; Ros-Melo, A.; Dourado, F.; Matioli, R.; et al. HTR1B and HTR2C in autism spectrum disorders in Brazilian families. Brain Res. 2009, 1250, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Khatri, N.; Simpson, K.L.; Lin, R.C.; Paul, I.A. Lasting neurobehavioral abnormalities in rats after neonatal activation of serotonin 1A and 1B receptors: Possible mechanisms for serotonin dysfunction in autistic spectrum disorders. Psychopharmacology 2014, 231, 1191–1200. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol. 2012, 72, 1272–1276. [Google Scholar] [CrossRef] [Green Version]
- Instanes, J.T.; Halmoy, A.; Engeland, A.; Haavik, J.; Furu, K.; Klungsoyr, K. Attention-Deficit/Hyperactivity Disorder in Offspring of Mothers With Inflammatory and Immune System Diseases. Biol. Psychiatry 2017, 81, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Muller, C.L.; Anacker, A.M.J.; Veenstra-Vander Weele, J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 2016, 321, 24–41. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.J.; Christoffel, D.J.; Heifets, B.D.; Ben-Dor, G.A.; Selimbeyoglu, A.; Hung, L.W.; Deisseroth, K.; Malenka, R.C. 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature 2018, 560, 589–594. [Google Scholar] [CrossRef]
- Luigi, B.; Chin-Fu, C.; Ayla, R.P.; Cindy, D.S.; Heather, J.M.; Kelly, J.; Bochner, B.R.; Stevenson, R.E.; Schwartz, C.E. Decreased tryptophan metabolism in patients with autism spectrum disorders. Mol. Autism. 2013, 4, 16. [Google Scholar]
- Tanaka, M.; Sato, A.; Kasai, S.; Hagino, Y.; Kotajima-Murakami, H.; Kashii, H.; Takamatsu, Y.; Nishito, Y.; Inagaki, M.; Mizuguchi, M.; et al. Brain hyperserotonemia causes autism-relevant social deficits in mice. Mol. Autism. 2018, 9, 60. [Google Scholar] [CrossRef]
- Gabriele, S.; Sacco, R.; Persico, A.M. Blood serotonin levels in autism spectrum disorder: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2014, 24, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.S.; Daniel, X.F. Studies on 5-bydroxyindole metabolism in autistic and other mentally retarded children. J. Pediatr. 1961, 58, 315–320. [Google Scholar]
- Henry, G.H.; Stephen, M.S.; Daniel, X.F. Hyperserotonemia and Amine Metabolites in Autistic and Retarded Children. Arch. Gen. Phychiatry 1977, 34, 521–531. [Google Scholar]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Benabou, M.; Rolland, T.; Leblond, C.S.; Millot, G.A.; Huguet, G.; Delorme, R.; Leboyer, M.; Pagan, C.; Callebert, J.; Maronde, E.; et al. Heritability of the melatonin synthesis variability in autism spectrum disorders. Sci. Rep. 2017, 7, 17746. [Google Scholar] [CrossRef] [Green Version]
- Bortolato, M.; Godar, S.C.; Alzghoul, L.; Zhang, J.; Darling, R.D.; Simpson, K.L.; Bini, V.; Chen, K.; Wellman, C.L.; Lin, R.C.S.; et al. Monoamine oxidase A and A/B knockout mice display autistic-like features. Int. J. Neuropsychopharmacol. 2013, 16, 869–888. [Google Scholar] [CrossRef] [Green Version]
- Whitaker-Azmitia, P.M.; Zhang, X.; Clarke, C. Effects of gestational exposure to monoamine oxidase inhibitors in rats: Preliminary behavioral and neurochemical studies. Neuropsychopharmacology 1994, 11, 125–132. [Google Scholar] [CrossRef]
- Kane, M.J.; Angoa-Peréz, M.; Briggs, D.; Sykes, C.E.; Francescutti, D.M.; Rosenberg, D.R.; Kuhn, D.M. Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: Possible relevance to autism. PLoS ONE 2012, 7, e48975. [Google Scholar] [CrossRef]
- Coon, H.; Dunn, D.; Lainhart, J.; Miller, J.; Hamil, C.; Battaglia, A.; Tancredi, R.; Leppert, M.F.; Weiss, R.; McMahon, W. Possible association between autism and variants in the brain-expressed tryptophan hydroxylase gene (TPH2). Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 135B, 42–46. [Google Scholar] [CrossRef]
- Lesch, K.P.; Waider, J. Serotonin in the modulation of neural plasticity and networks: Implications for neurodevelopmental disorders. Neuron 2012, 76, 175–191. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, S.P.; Gaspar, P. Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacology 2012, 62, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Daws, L.C.; Gould, G.G. Ontogeny and regulation of the serotonin transporter: Providing insights into human disorders. Pharmacol. Ther. 2011, 131, 61–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef]
- Abdulamir, H.A.; Abdul-Rasheed, O.F.; Abdulghani, E.A. Serotonin and serotonin transporter levels in autistic children. Saudi Med. J. 2018, 39, 487–494. [Google Scholar] [CrossRef]
- Gothert, M. Serotonin discovery and stepwise disclosure of 5-HT receptor complexity over four decades. Part I. General background and discovery of serotonin as a basis for 5-HT receptor identification. Pharmacol. Rep. 2013, 65, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Lawson, S.K.; Gray, A.C.; Woehrle, N.S. Effects of oxytocin on serotonin 1B agonist-induced autism-like behavior in mice. Behav. Brain Res. 2016, 314, 52–64. [Google Scholar] [CrossRef]
- Wirth, A.; Holst, K.; Ponimaskin, E. How serotonin receptors regulate morphogenic signalling in neurons. Prog. Neurobiol. 2017, 151, 35–56. [Google Scholar] [CrossRef]
- Homberg, J.R.; Kolk, S.M.; Schubert, D. Editorial perspective of the Research Topic “Deciphering serotonin’s role in neurodevelopment”. Front. Cell Neurosci. 2013, 7, 212. [Google Scholar] [CrossRef] [Green Version]
- Baharnoori, M.; Brake, W.G.; Srivastava, L.K. Prenatal immune challenge induces developmental changes in the morphology of pyramidal neurons of the prefrontal cortex and hippocampus in rats. Schizophr. Res. 2009, 107, 99–109. [Google Scholar] [CrossRef] [Green Version]
- Whitaker-Azmitia, P.M. Behavioral and cellular consequences of increasing serotonergic activity during brain development: A role in autism? Int. J. Dev. Neurosci. 2005, 23, 75–83. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, B.; Zhou, D.; Xing, J.; Li, H.; Li, J.; Zhang, Z.; Zhang, B.; Li, P. Supplementation of Diet With Different n-3/n-6 PUFA Ratios Ameliorates Autistic Behavior, Reduces Serotonin, and Improves Intestinal Barrier Impairments in a Valproic Acid Rat Model of Autism. Front. Psychiatry 2020, 11, 552345. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yan, J.; Yang, T.; Zhu, J.; Li, T.; Wei, H.; Chen, J. Fecal Microbiome Transplantation from Children with Autism Spectrum Disorder Modulates Tryptophan and Serotonergic Synapse Metabolism and Induces Altered Behaviors in Germ-Free Mice. mSystems 2021, 6, e01343-20. [Google Scholar] [CrossRef] [PubMed]



| Name | Sequence |
|---|---|
| TPH1 | CCATCTTCCGAGAGCTAAACAAA |
| TCTTCCCGATAGCCACAGTATT | |
| TPH2 | GTGACCCTGAATCCGCCTG |
| GGTGCCGTACATGAGGACT | |
| SERT | GGCGGAGATGAGGAATGAAGATGTG |
| TGGATGCTGGCATGTTGGCTATTG | |
| 5-HT1BR | CACCCTTCTTCTGGCGTCAAGC |
| CCGTGGAGTAGACCGTGTAGAGG | |
| MAOA | GACACGCTCAGGAATGGGACAAG |
| ACAGGAACCACAGGGCAGATACC | |
| AANAT | GGTTCACTTTGGGACAAGGAGAGAC |
| GAAGGTATCGCCACAGCAGGAC | |
| ASMT | AGGGAGAGACGTTGGAATCAGAGG |
| CTTGCTTGAGGGTGCCACTTCTG | |
| GAPDH | ACGGCAAGTTCAACGGCACAG |
| CGACATACTCAGCACCAGCATCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, F.; Wang, X.; Luo, R.; Yuan, B.; Ye, S.; Yang, T.; Xiao, L.; Chen, J. Maternal LPS Exposure Enhances the 5-HT Level in the Prefrontal Cortex of Autism-like Young Offspring. Brain Sci. 2023, 13, 958. https://doi.org/10.3390/brainsci13060958
Lin F, Wang X, Luo R, Yuan B, Ye S, Yang T, Xiao L, Chen J. Maternal LPS Exposure Enhances the 5-HT Level in the Prefrontal Cortex of Autism-like Young Offspring. Brain Sciences. 2023; 13(6):958. https://doi.org/10.3390/brainsci13060958
Chicago/Turabian StyleLin, Fang, Xinyuan Wang, Ruifang Luo, Binlin Yuan, Shasha Ye, Ting Yang, Lu Xiao, and Jie Chen. 2023. "Maternal LPS Exposure Enhances the 5-HT Level in the Prefrontal Cortex of Autism-like Young Offspring" Brain Sciences 13, no. 6: 958. https://doi.org/10.3390/brainsci13060958





