The Relationship between Retained Primitive Reflexes and Hemispheric Connectivity in Autism Spectrum Disorders
Abstract
:1. Introduction
1.1. Quantitative EEG in Autism Spectrum Disorder
1.2. Retained Primitive Reflexes in Autism Spectrum Disorder
2. Materials and Methods
2.1. Participants
2.1.1. Inclusion Criteria
2.1.2. Exclusion Criteria
2.1.3. Informed Consent and Institutional Approval
2.2. Procedures
2.2.1. Reflex Testing
2.2.2. Cognitive Testing
2.2.3. Hearing and Vestibular Function
2.3. Quantitative Electroencephalography
2.3.1. EEG Pre-Processing
2.3.2. qEEG Spectral Analysis
2.3.3. Grouping of EEG Leads for Spectral Analysis
2.3.4. Computation of PSD and the Spectral Indices
2.3.5. Functional Connectivity Examined by qEEG
2.3.6. Statistical Analysis
3. Results
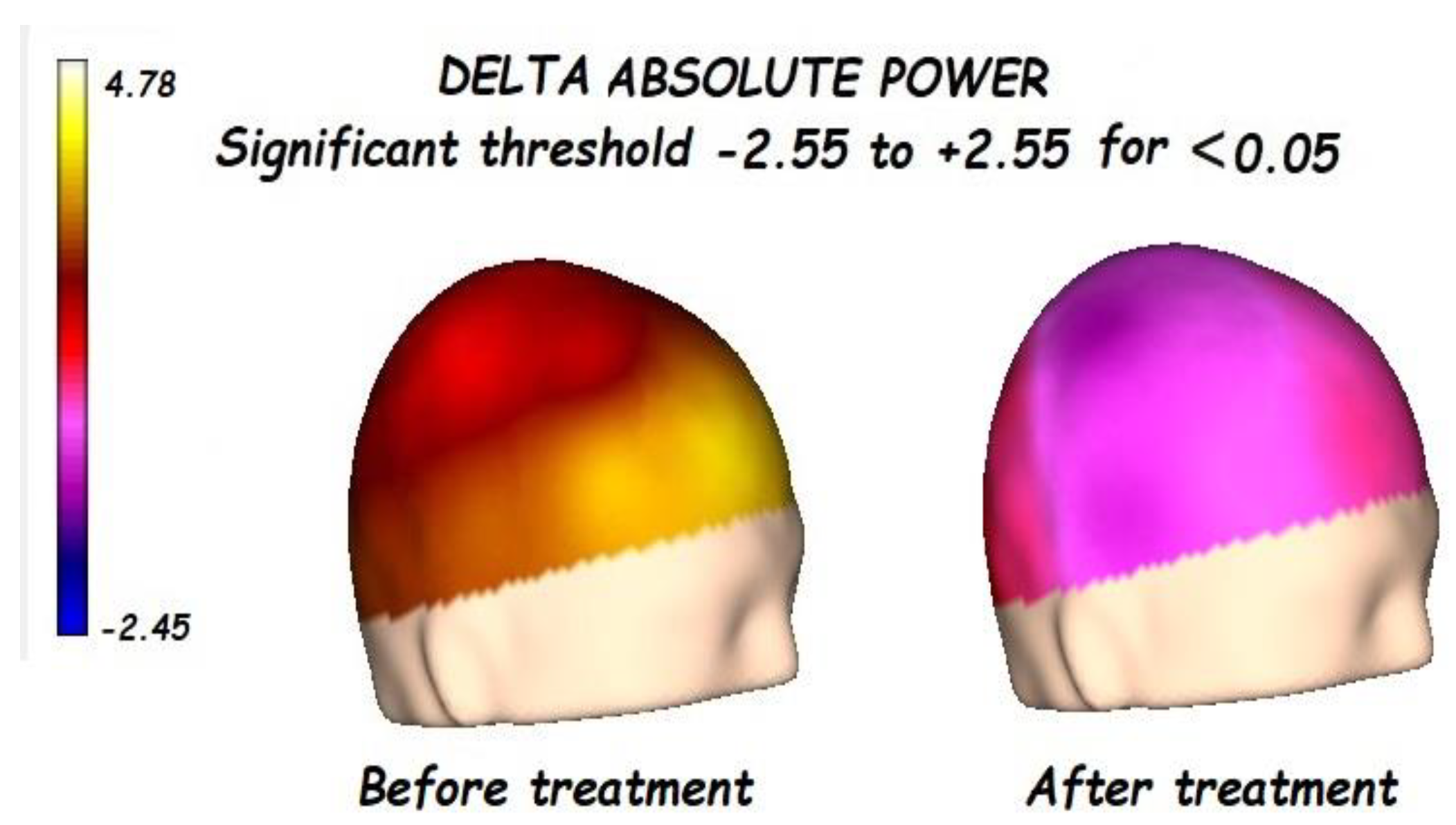
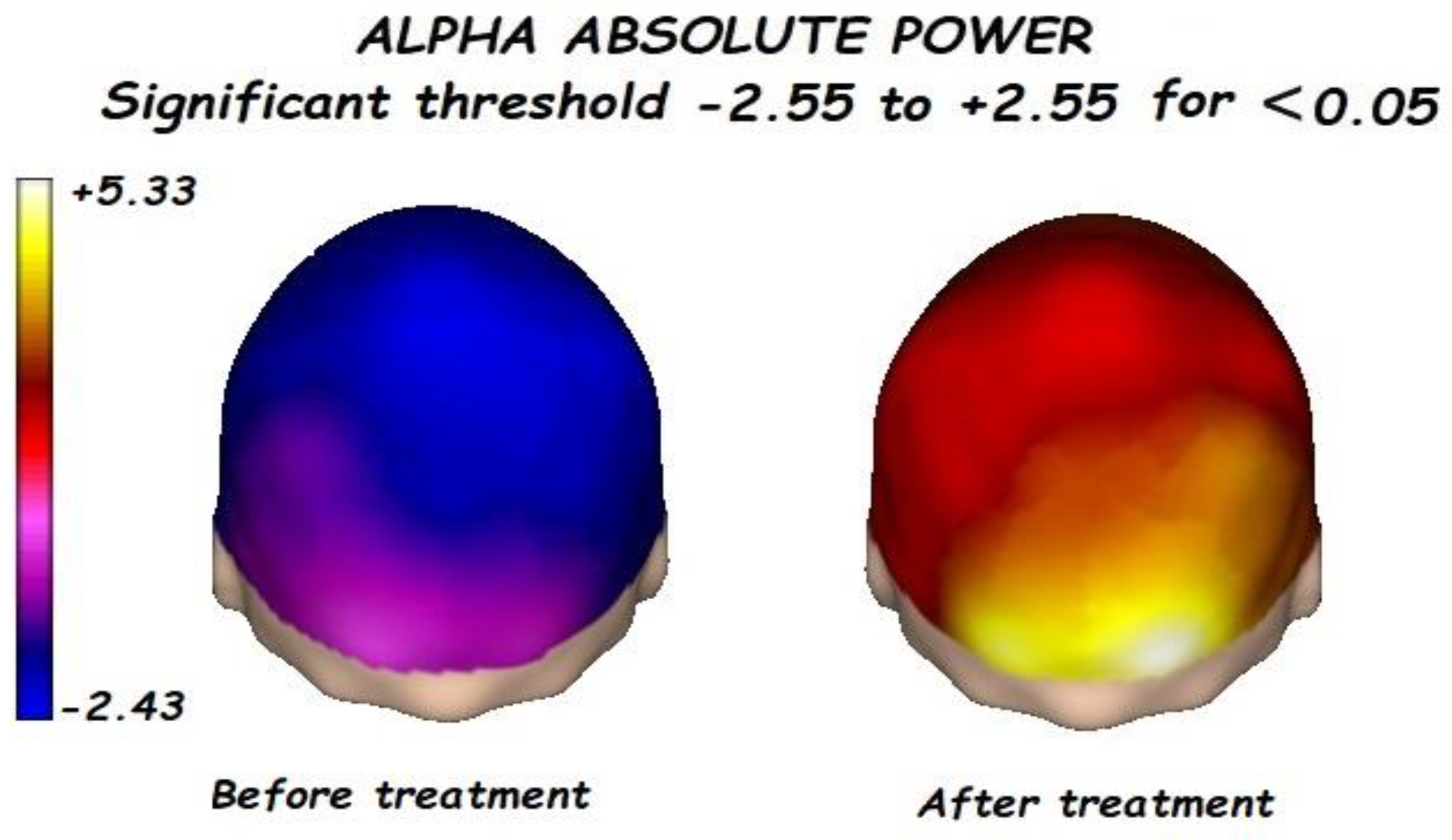
3.1. Effects of Treatment on Absolute Power
3.2. Functional Connectivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, J.; Zhou, Y.; Wang, K. Multiplex gene and phenotype network to characterize shared genetic pathways of epilepsy and autism. Sci. Rep. 2021, 11, 952. [Google Scholar] [CrossRef]
- Grossmann, T. Developmental Origins of the Pathway for Social Perception. Trends Cogn. Sci. 2021, 25, 546–547. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.; Volkmar, F.R. Autism and related disorders. Handb. Clin. Neurol. 2012, 106, 407–418. [Google Scholar]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9 (Suppl. S1), S55–S65. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.O.; Kruppa, J.A.; Fink, G.R.; Herpertz-Dahlmann, B.; Konrad, K.; Schulte-Rüther, M. Developmental Differences in Probabilistic Reversal Learning: A Computational Modeling Approach. Front. Neurosci. 2021, 14, 536596. [Google Scholar] [CrossRef] [PubMed]
- Wing, L.; Gould, J.; Gillberg, C. Autism spectrum disorders in the DSM-V: Better or worse than the DSM-IV? Res. Dev. Disabil. 2011, 32, 768–773. [Google Scholar] [CrossRef]
- Zappella, M. Reversible autism and intellectual disability in children. Am. J. Med. Genet. Part C Semin. Med. Genet. 2012, 160, 111–117. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar] [CrossRef]
- Tan, C.; Frewer, V.; Cox, G.; Williams, K.; Ure, A. Prevalence and Age of Onset of Regression in Children with Autism Spectrum Disorder: A Systematic Review and Meta-analytical Update. Autism Res. 2021, 14, 582–598. [Google Scholar] [CrossRef]
- Tanner, A.; Dounavi, K. The Emergence of Autism Symptoms Prior to 18 Months of Age: A Systematic Literature Review. J. Autism Dev. Disord. 2021, 51, 973–993. [Google Scholar] [CrossRef]
- Bosl, W.; Tierney, A.; Tager-Flusberg, H.; Nelson, C. EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med. 2011, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Duffy, F.H.; Als, H. A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls—A large case control study. BMC Med. 2012, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Wantzen, P.; Clochon, P.; Doidy, F.; Wallois, F.; Mahmoudzadeh, M.; Desaunay, P.; Christian, M.; Guilé, J.M.; Guénolé, F.; Eustache, F.; et al. EEG resting-state functional connectivity: Evidence for an imbalance of external/internal information integration in autism. J. Neurodev. Disord. 2022, 14, 1–4. [Google Scholar]
- Gabard-Durnam, L.; Tierney, A.L.; Vogel-Farley, V.; Tager-Flusberg, H.; Nelson, C.A. Alpha Asymmetry in Infants at Risk for Autism Spectrum Disorders. J. Autism Dev. Disord. 2015, 45, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Cantor, D.S.; Thatcher, R.W.; Hrybyk, M.; Kaye, H. Computerized EEG analyses of autistic children. J. Autism Dev. Disord. 1986, 16, 169–187. [Google Scholar] [CrossRef]
- Cantor, D.S.; Chabot, R. QEEG Studies in the Assessment and Treatment of Childhood Disorders. Clin. EEG Neurosci. 2009, 40, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Gomez, G. The Development of Lateralized Brain Oscillations in Infancy: What We Can Learn from Autism. Master’s Thesis, McGill University, Montréal, QC, Canada, 2023. Available online: https://escholarship.mcgill.ca/downloads/8s45qg021) (accessed on 19 July 2023).
- Chan, A.S.; Leung, W.W.M. Differentiating autistic children with quantitative encephalography: A 3-month longitudinal study. J. Child Neurol. 2006, 21, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Balathay, D.; Narasimhan, U.; Belo, D.; Anandan, K. Quantitative assessment of cognitive profile and brain asymmetry in the characterization of autism spectrum in children: A task-based EEG study. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2023, 237, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Loth, E.; Spooren, W.; Ham, L.M.; Isaac, M.B.; Auriche-Benichou, C.; Banaschewski, T.; Baron-Cohen, S.; Broich, K.; Bölte, S.; Bourgeron, T.; et al. Identification and validation of biomarkers for autism spectrum disorders. Nat. Rev. Drug Discov. 2016, 15, 70. [Google Scholar] [CrossRef] [Green Version]
- Melillo, R.; Leisman, G. Neurobehavioral Disorders of Childhood: An Evolutionary Perspective; Springer: Cham, Switzerland, 2010. [Google Scholar]
- Fu, L.; Wang, Y.; Fang, H.; Xiao, X.; Xiao, T.; Li, Y.; Li, C.; Wu, Q.; Chu, K.; Xiao, C.; et al. Longitudinal Study of Brain Asymmetries in Autism and Developmental Delays Aged 2–5 Years. Neuroscience 2020, 432, 137–149. [Google Scholar] [CrossRef]
- Posar, A.; Visconti, P. Early Motor Signs in Autism Spectrum Disorder. Children 2022, 9, 294. [Google Scholar] [CrossRef]
- Chandradasa, M.; Rathnayake, L.C. Retained primitive reflexes in children, clinical implications and targeted home-based interventions. Nurs. Child. Young People 2023, 35. [Google Scholar] [CrossRef]
- Grigg, T.M.; Fox-Turnbull, W.; Culpan, I. Retained primitive reflexes: Perceptions of parents who have used Rhythmic Movement Training with their children. J. Child Health Care 2018, 22, 406–418. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.K.; Wolpaw, J.R. Targeted neuroplasticity for rehabilitation. Prog. Brain Res. 2015, 218, 157–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Meer, A.; van der Weel, F.R. Motor Development: Biological Aspects of Brain and Behavior. In Oxford Research Encyclopedia of Psychology; Oxford University Press: Oxford, UK, 2022. [Google Scholar]
- Melillo, R.; Leisman, G.; Mualem, R.; Ornai, A.; Carmeli, E. Persistent Childhood Primitive Reflex Reduction Effects on Cognitive, Sensorimotor, and Academic Performance in ADHD. Front. Public Health 2020, 8, 684. [Google Scholar] [CrossRef]
- Melillo, R.; Leisman, G.; Machado-Ferrer, Y.; Chinchilla, M.; Machado, C.; Sheridan, S.; Melillo, T.; Carmeli, E. Cognitive effects of retained primitive reflexes in autistic spectrum disorder. Front. Neurol. 2022, 13, 922322. [Google Scholar] [CrossRef] [PubMed]
- Sigafoos, J.; Roche, L.; O’Reilly, M.F.; Lancioni, G.E. Persistence of primitive reflexes in developmental disorders. Curr Dev Disord Rep 2021, 8, 98–105. [Google Scholar] [CrossRef]
- Melillo, R.; Leisman, G.; Machado CMachado-Ferrer, Y.; Chinchilla-Acosta, M.; Melillo, T.; Carmeli, E. Identification and reduction of retained primitive reflexes in autism spectrum disorder: Effects on qEEG networks and cognitive function. BMJ-CR, 2023; in press. [Google Scholar]
- Gadow, K.D.; Drabick, D.A. Symptoms of autism and schizophrenia spectrum disorders in clinically referred youth with oppositional defiant disorder. Res. Dev. Disabil. 2012, 33, 1157–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitsika, V.; Sharpley, C.F. Neurobiological Concomitants of Autism as Informers of Clinical Practice: A Status Review. Adv. Neurodev. Disord. 2023, 1–13. [Google Scholar] [CrossRef]
- Silver, W.G.; Rapin, I. Neurobiological Basis of Autism. Pediatr. Clin. N. Am. 2012, 59, 45–61. [Google Scholar] [CrossRef]
- Charman, T.; Pickles, A.; Simonoff, E.; Chandler, S.; Loucas, T.; Baird, G. IQ in children with autism spectrum disorders: Data from the Special Needs and Autism Project (SNAP). Psychol. Med. 2011, 41, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Charman, T.; Jones, C.; Pickles, A.; Simonoff, E.; Baird, G.; Happé, F. Defining the cognitive phenotype of autism. Brain Res. 2011, 1380, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Canivez, G.L. Incremental criterion validity of WAIS–IV factor index scores: Relationships with WIAT–II and WIAT–III subtest and composite scores. Psychol. Assess. 2013, 25, 484. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, A.S.; Flanagan, D.P.; Alfonso, V.C.; Mascolo, J.T. Test Review: Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV). J. Psychoeduc. Assess. 2006, 24, 278–295. [Google Scholar] [CrossRef]
- Brown, H.M.; Oram-Cardy, J.; Johnson, A. A Meta-Analysis of the Reading Comprehension Skills of Individuals on the Autism Spectrum. J. Autism Dev. Disord. 2013, 43, 932–955. [Google Scholar] [CrossRef] [PubMed]
- Mayes, S.D.; Calhoun, S.L.; Murray, M.J.; Morrow, J.D.; Yurich, K.K.; Mahr, F.; Cothren, S.; Purichia, H.; Bouder, J.N.; Petersen, C. Comparison of scores on the Checklist for Autism Spectrum Disorder, Childhood Autism Rating Scale, and Gilliam Asperger’s Disorder Scale for children with low functioning autism, high functioning autism, Asperger’s disorder, ADHD, and typical development. J. Autism Dev. Disord. 2009, 39, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, V.; Magyar, C.I.; Dill, C.A. Constructs Assessed by the GARS-2: Factor Analysis of Data from the Standardization Sample. J. Autism Dev. Disord. 2010, 40, 1118–1130. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Wang, C.-H.; Jung, T.-P.; Wu, T.-L.; Jeng, S.-K.; Duann, J.-R.; Chen, J.-H. EEG-Based Emotion Recognition in Music Listening. IEEE Trans. Biomed. Eng. 2010, 57, 1798–1806. [Google Scholar] [CrossRef]
- Aoude, A.A.; Motto, A.L.; Galiana, H.L.; Brown, K.A.; Kearney, R.E. Power-Based Segmentation of Respiratory Signals Using Forward-Backward Bank Filtering. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 4631–4634. [Google Scholar] [CrossRef]
- Lázár, A.S.; Lázár, Z.I.; Bíró, A.; Győri, M.; Tárnok, Z.; Prekop, C.; Keszei, A.; Stefanik, K.; Gádoros, J.; Halász, P. Reduced fronto-cortical brain connectivity during NREM sleep in Asperger syndrome: An EEG spectral and phase coherence study. Clin. Neurophysiol. 2010, 121, 1844–1854. [Google Scholar] [CrossRef]
- Bogéa Ribeiro, L.; da Silva Filho, M. Systematic review on eeg analysis to diagnose and treat autism by evaluating functional connectivity and spectral power. Neuropsychiat Dis. Treat. 2023, 19, 415–424. [Google Scholar] [CrossRef]
- Machado, C.; Estévez, M.; Leisman, G.; Melillo, R.; Rodríguez, R.; DeFina, P.; Hernández, A.; Pérez-Nellar, J.; Naranjo, R.; Chinchilla, M.; et al. EEG coherence assessment of autistic children in three different experimental conditions. J. Autism Dev. Disabil. 2015, 45, 406–424. [Google Scholar] [CrossRef] [Green Version]
- Knyazev, G.G. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci. Biobehav. Rev. 2012, 36, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Costa-Faidella, J.; Dacewicz, A.; Escera, C.; Szelag, E. Altered event-related potentials and theta oscillations index auditory working memory deficits in healthy aging. Neurobiol. Aging 2021, 108, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. Waves of prediction. PLoS Biol. 2019, 17, e3000426. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Karwowski, W.; Fafrowicz, M.; Hancock, P.A. Neuroergonomics applications of electroencephalography in physical activities: A systematic review. Front. Hum. Neurosci. 2019, 13, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strüber, D.; Herrmann, C.S. Gamma Activity in Sensory and Cognitive Processing. In The Oxford Handbook of EEG Frequency; Oxford University Press: Oxford, UK, 2022; p. 145. [Google Scholar]
- Kang, J.; Song, J.; Casanova, M.F.; Sokhadze, E.M.; Li, X. Effects of repetitive transcranial magnetic stimulation on children with low-function autism. CNS Neurosci. Ther. 2019, 25, 1254–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milovanovic, M.; Grujicic, R. Electroencephalography in Assessment of Autism Spectrum Disorders: A Review. Front. Psychiatry 2021, 12, 686021. [Google Scholar] [CrossRef]
- Buchanan, G.F. Slow Down and Seize: Seizures Triggered by Slow Wave Oscillations in a GABAergic Model of Dravet Syndrome. Epilepsy Curr. 2023, 15357597231174111. [Google Scholar] [CrossRef]
- Sears, S.M.; Hewett, S.J. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp. Biol. Med. 2021, 246, 1069–1083. [Google Scholar] [CrossRef]
- Fontes-Dutra, M.; Righes Marafiga, J.; Santos-Terra, J.; Deckmann, I.; Brum Schwingel, G.; Rabelo, B.; Kazmierzak de Moraes, R.; Rockenbach, M.; Vendramin Pasquetti, M.; Gottfried, C.; et al. GABAergic synaptic transmission and cortical oscillation patterns in the primary somatosensory area of a valproic acid rat model of autism spectrum disorder. Eur. J. Neurosci. 2023, 57, 527–546. [Google Scholar] [CrossRef]
- Adaikkan, C.; Tsai, L.-H. Gamma Entrainment: Impact on Neurocircuits, Glia, and Therapeutic Opportunities. Trends Neurosci. 2020, 43, 24–41. [Google Scholar] [CrossRef]
- Leisman, G.; Machado, C. Many paths to consciousness or just one?: Life in a bounded continuum. J. Conscious. Stud. 2021, 28, 83–96. [Google Scholar]
- Zikopoulos, B.; Barbas, H. Changes in Prefrontal Axons May Disrupt the Network in Autism. J. Neurosci. 2010, 30, 14595–14609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S.D.; Jha, N.K.; Ojha, S.; Sadek, B. mTOR Signaling Disruption and Its Association with the Development of Autism Spectrum Disorder. Molecules 2023, 28, 1889. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melillo, R.; Leisman, G.; Machado, C.; Machado-Ferrer, Y.; Chinchilla-Acosta, M.; Melillo, T.; Carmeli, E. The Relationship between Retained Primitive Reflexes and Hemispheric Connectivity in Autism Spectrum Disorders. Brain Sci. 2023, 13, 1147. https://doi.org/10.3390/brainsci13081147
Melillo R, Leisman G, Machado C, Machado-Ferrer Y, Chinchilla-Acosta M, Melillo T, Carmeli E. The Relationship between Retained Primitive Reflexes and Hemispheric Connectivity in Autism Spectrum Disorders. Brain Sciences. 2023; 13(8):1147. https://doi.org/10.3390/brainsci13081147
Chicago/Turabian StyleMelillo, Robert, Gerry Leisman, Calixto Machado, Yanin Machado-Ferrer, Mauricio Chinchilla-Acosta, Ty Melillo, and Eli Carmeli. 2023. "The Relationship between Retained Primitive Reflexes and Hemispheric Connectivity in Autism Spectrum Disorders" Brain Sciences 13, no. 8: 1147. https://doi.org/10.3390/brainsci13081147
APA StyleMelillo, R., Leisman, G., Machado, C., Machado-Ferrer, Y., Chinchilla-Acosta, M., Melillo, T., & Carmeli, E. (2023). The Relationship between Retained Primitive Reflexes and Hemispheric Connectivity in Autism Spectrum Disorders. Brain Sciences, 13(8), 1147. https://doi.org/10.3390/brainsci13081147






