Habituation, Adaptation and Prediction Processes in Neurodevelopmental Disorders: A Comprehensive Review
Abstract
:1. Introduction
2. From Behavioral Habituation to Neural Adaptation and Prediction Processes
2.1. Behavioral Habituation
2.2. Repetition Suppression (RS)
2.2.1. In Animals
2.2.2. In Humans
2.3. Repetition Enhancement (RE)
2.4. RS and RE Explained by Predictive Coding
3. Adaptation and Prediction through Typical Development
3.1. Behavioral Habituation and Familiarization
3.2. Neural Adaptation in Typical Development
3.3. Prediction in Typical Development
4. Adaptation and Prediction Are Altered in Neurodevelopmental and Psychiatric Disorders
4.1. Autism Spectrum Disorder (ASD)
| Study | Cortical/Subcortical | Modality | Population | Paradigm | Protocol | Stimuli Duration and SOA (ms) | Results |
|---|---|---|---|---|---|---|---|
| Martineau et al., 1992 [116] | Cortical | Auditory (pure tones) | 30 children 3–11 years | Oddball | 1 sequence of 60 tones | Stimuli: 100 |
|
| Guiraud et al., 2011 [155] | Cortical | Auditory (Pure tones) | 35 infants with high risk for ASD and 21 infants with low risk for ASD 9 months old | Oddball | 1 sequence (deviant always followed by two standards) | Stimuli: 100 SOA: 800 | High risk: less marked ↘ (less habituation) and less ↗ response to deviant (reduced discrimination) Low risk:
|
| Gonzalez-Gadea et al., 2015 [154] | Cortical | Auditory (pure tones) | 16 children 8–15 years | Oddball | 2 blocks of 220 sequences, 3 types of sequence:
| Stimuli: 50 SOA: 200 Inter sequence interval: between 700 and 1000 |
|
| Kolesnik et al., 2019 [162] | Cortical | Auditory (standard: pure tones Deviants: 1 white noise and 1 pure tone) | 116 children with high risk of autism (9.03 +/− 1.1 m—39.05 +/− 3.47 m) | Oddball | 1 sequence with standard and deviants (paradigm designed by Guiraud et al., 2011) [155] Measure the standard response after 1, 2 or 3 presentations | Stimuli: 100 Inter-trial interval: 700 |
|
| Font-Alaminos et al., 2020 [156] | Subcortical | Auditory (pure tones) | 17 children 9.1 +/− 1.7 years | Roving (8, 10 and 12 repetitions) | 1 sequence composed of 9 blocks, each block is composed of 30 trains of either 8, 10 and 12 repetitions | Stimuli: 100 SOA: 333 |
|
| Ruiz-Martínez et al., 2020 [57] | Cortical | Auditory (Electronic and human sounds) | 16 ASD children 7–10 years | Oddball | 8 blocks (4/sound type) (deviant separated by at least 2 standards; each block begins with 10 standard) | Stimuli: 85 SOA: 685–885 |
|
| Jamal et al., 2020 [121] | Cortical | Auditory (pure tones) and visual (radial checkerboard) | 13 children 7.4–12.8 years | Sequences of repeated stimulus | 2 sequences (1 visual and 1 auditory) with 300 repetitions of the same stimulus | SOA: 1,116 Stimuli duration: 116 |
|
| Cary et al., 2023 [153] | Cortical | Auditory (pure tones) | 13 children 12.81 +/− 2.63 years | Oddball | 1 sequence of 1000 trials (80% standard) | SOA: 600 Stimuli duration: 360 |
|
4.2. Schizophrenia
4.3. Attention-Deficit/Hyperactivity Disorder (ADHD)
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winkler, I.; Denham, S.L.; Nelken, I. Modeling the Auditory Scene: Predictive Regularity Representations and Perceptual Objects. Trends Cogn. Sci. 2009, 13, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Andrews, T.J.; Ewbank, M.P. Distinct Representations for Facial Identity and Changeable Aspects of Faces in the Human Temporal Lobe. Neuroimage 2004, 23, 905–913. [Google Scholar] [CrossRef]
- Summerfield, C.; Trittschuh, E.H.; Monti, J.M.; Mesulam, M.-M.; Egner, T. Neural Repetition Suppression Reflects Fulfilled Perceptual Expectations. Nat. Neurosci. 2008, 11, 1004–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldeweg, T.; Klugman, A.; Gruzelier, J.; Hirsch, S.R. Mismatch Negativity Potentials and Cognitive Impairment in Schizophrenia. Schizophr. Res. 2004, 69, 203–217. [Google Scholar] [CrossRef]
- Costa-Faidella, J.; Grimm, S.; Slabu, L.; Díaz-Santaella, F.; Escera, C. Multiple Time Scales of Adaptation in the Auditory System as Revealed by Human Evoked Potentials: Adaptation in the Human Auditory System. Psychophysiology 2011, 48, 774–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segaert, K.; Weber, K.; de Lange, F.P.; Petersson, K.M.; Hagoort, P. The Suppression of Repetition Enhancement: A Review of FMRI Studies. Neuropsychologia 2013, 51, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parras, G.G.; Nieto-Diego, J.; Carbajal, G.V.; Valdés-Baizabal, C.; Escera, C.; Malmierca, M.S. Neurons along the Auditory Pathway Exhibit a Hierarchical Organization of Prediction Error. Nat. Commun. 2017, 8, 2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avery, S.N.; Armstrong, K.; Blackford, J.U.; Woodward, N.D.; Cohen, N.; Heckers, S. Impaired Relational Memory in the Early Stage of Psychosis. Schizophr. Res. 2019, 212, 113–120. [Google Scholar] [CrossRef]
- Ramaswami, M. Network Plasticity in Adaptive Filtering and Behavioral Habituation. Neuron 2014, 82, 1216–1229. [Google Scholar] [CrossRef] [Green Version]
- Ewbank, M.P.; Pell, P.J.; Powell, T.E.; von dem Hagen, E.A.H.; Baron-Cohen, S.; Calder, A.J. Repetition Suppression and Memory for Faces Is Reduced in Adults with Autism Spectrum Conditions. Cereb. Cortex 2017, 27, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Pellicano, E.; Burr, D. When the World Becomes “Too Real”: A Bayesian Explanation of Autistic Perception. Trends Cogn. Sci. 2012, 16, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Groves, P.M.; Thompson, R.F. Habituation: A Dual-Process Theory. Psychol. Rev. 1970, 77, 419–450. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.F.; Spencer, W.A. Habituation: A Model Phenomenon for the Study of Neuronal Substrates of Behavior. Psychol. Rev. 1966, 73, 16–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hepper, P.G.; Leader, L.R. Fetal Habituation. Fet. Matern. Med. Rev. 1996, 8, 109–123. [Google Scholar] [CrossRef]
- Rankin, C.H.; Abrams, T.; Barry, R.J.; Bhatnagar, S.; Clayton, D.F.; Colombo, J.; Coppola, G.; Geyer, M.A.; Glanzman, D.L.; Marsland, S.; et al. Habituation Revisited: An Updated and Revised Description of the Behavioral Characteristics of Habituation. Neurobiol. Learn. Mem. 2009, 92, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Giles, A.C.; Rankin, C.H. Behavioral and Genetic Characterization of Habituation Using Caenorhabditis Elegans. Neurobiol. Learn. Mem. 2009, 92, 139–146. [Google Scholar] [CrossRef]
- Bronstein, P.M.; Neiman, H.; Wolkoff, F.D.; Levine, M.J. The Development of Habituation in the Rat. Anim. Learn. Behav. 1974, 2, 92–96. [Google Scholar] [CrossRef]
- Pinsker, H.; Kupfermann, I.; Castellucci, V.; Kandel, E. Habituation and Dishabituation of the Gill-Withdrawal Reflex in Aplysia. Science 1970, 167, 1740–1742. [Google Scholar] [CrossRef] [Green Version]
- Bell, R.; Röer, J.P.; Dentale, S.; Buchner, A. Habituation of the Irrelevant Sound Effect: Evidence for an Attentional Theory of Short-Term Memory Disruption. J. Exp. Psychol. Learn. Mem. Cogn. 2012, 38, 1542–1557. [Google Scholar] [CrossRef]
- Ornitz, E.M.; Guthrie, D. Long-Term Habituation and Sensitization of the Acoustic Startle Response in the Normal Adult Human. Psychophysiology 1989, 26, 166–173. [Google Scholar] [CrossRef]
- Pellegrino, R.; Sinding, C.; de Wijk, R.A.; Hummel, T. Habituation and Adaptation to Odors in Humans. Physiol. Behav. 2017, 177, 13–19. [Google Scholar] [CrossRef]
- Desimone, R. Neural Mechanisms for Visual Memory and Their Role in Attention. Proc. Natl. Acad. Sci. USA 1996, 93, 13494–13499. [Google Scholar] [CrossRef]
- Grill-Spector, K.; Henson, R.; Martin, A. Repetition and the Brain: Neural Models of Stimulus-Specific Effects. Trends Cogn. Sci. 2006, 10, 14–23. [Google Scholar] [CrossRef]
- Avidan, G.; Hasson, U.; Hendler, T.; Zohary, E.; Malach, R. Analysis of the Neuronal Selectivity Underlying Low FMRI Signals. Curr. Biol. 2002, 12, 964–972. [Google Scholar] [CrossRef] [Green Version]
- Grill-Spector, K.; Malach, R. FMR-Adaptation: A Tool for Studying the Functional Properties of Human Cortical Neurons. Acta Psychol. 2001, 107, 293–321. [Google Scholar] [CrossRef]
- Wiggs, C.L.; Martin, A. Properties and Mechanisms of Perceptual Priming. Curr. Opin. Neurobiol. 1998, 8, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, T.W.; Gauthier, I. Repetition-Induced Changes in BOLD Response Reflect Accumulation of Neural Activity. Hum. Brain Mapp. 2006, 27, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ulanovsky, N.; Las, L.; Farkas, D.; Nelken, I. Multiple time scales of adaptation in auditory cortex neurons. J. Neurosci. 2004, 24, 10440–10453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baylis, G.C.; Rolls, E.T. Responses of Neurons in the Inferior Temporal Cortex in Short Term and Serial Recognition Memory Tasks. Exp. Brain Res. 1987, 65, 614–622. [Google Scholar] [CrossRef] [PubMed]
- De Baene, W.; Vogels, R. Effects of Adaptation on the Stimulus Selectivity of Macaque Inferior Temporal Spiking Activity and Local Field Potentials. Cereb. Cortex 2010, 20, 2145–2165. [Google Scholar] [CrossRef] [Green Version]
- Dragoi, V.; Sharma, J.; Sur, M. Adaptation-Induced Plasticity of Orientation Tuning in Adult Visual Cortex. Neuron 2000, 28, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Kaliukhovich, D.A.; Vogels, R. Stimulus Repetition Probability Does Not Affect Repetition Suppression in Macaque Inferior Temporal Cortex. Cereb. Cortex 2011, 21, 1547–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaliukhovich, D.A.; Vogels, R. Stimulus Repetition Affects Both Strength and Synchrony of Macaque Inferior Temporal Cortical Activity. J. Neurophysiol. 2012, 107, 3509–3527. [Google Scholar] [CrossRef]
- Kaliukhovich, D.A.; Vogels, R. Neurons in Macaque Inferior Temporal Cortex Show No Surprise Response to Deviants in Visual Oddball Sequences. J. Neurosci. 2014, 34, 12801–12815. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Murray, S.O.; Jagadeesh, B. Time Course and Stimulus Dependence of Repetition-Induced Response Suppression in Inferotemporal Cortex. J. Neurophysiol. 2009, 101, 418–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, E.K.; Li, L.; Desimone, R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science 1991, 254, 1377–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, J.R.; Metha, A.B.; Krauskopf, J.; Lennie, P. Rapid Adaptation in Visual Cortex to the Structure of Images. Science 1999, 285, 1405–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringo, J.L. Stimulus Specific Adaptation in Inferior Temporal and Medial Temporal Cortex of the Monkey. Behav. Brain Res. 1996, 76, 191–197. [Google Scholar] [CrossRef]
- Sawamura, H.; Orban, G.A.; Vogels, R. Selectivity of Neuronal Adaptation Does Not Match Response Selectivity: A Single-Cell Study of the FMRI Adaptation Paradigm. Neuron 2006, 49, 307–318. [Google Scholar] [CrossRef] [Green Version]
- Vinken, K.; Vogels, R. Adaptation Can Explain Evidence for Encoding of Probabilistic Information in Macaque Inferior Temporal Cortex. Curr. Biol. 2017, 27, R1210–R1212. [Google Scholar] [CrossRef]
- Kuravi, P.; Vogels, R. GABAergic and Cholinergic Modulation of Repetition Suppression in Inferior Temporal Cortex. Sci. Rep. 2018, 8, 13160. [Google Scholar] [CrossRef] [PubMed]
- Ulanovsky, N.; Las, L.; Nelken, I. Processing of Low-Probability Sounds by Cortical Neurons. Nat. Neurosci. 2003, 6, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Fishman, Y.I.; Steinschneider, M. Searching for the Mismatch Negativity in Primary Auditory Cortex of the Awake Monkey: Deviance Detection or Stimulus Specific Adaptation? J. Neurosci. 2012, 32, 15747–15758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelken, I.; Yaron, A.; Polterovich, A.; Hershenhoren, I. Stimulus-Specific Adaptation beyond Pure Tones. Adv. Exp. Med. Biol. 2013, 787, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Taaseh, N.; Yaron, A.; Nelken, I. Stimulus-Specific Adaptation and Deviance Detection in the Rat Auditory Cortex. PLoS ONE 2011, 6, e23369. [Google Scholar] [CrossRef]
- von der Behrens, W.; Bäuerle, P.; Kössl, M.; Gaese, B.H. Correlating Stimulus-Specific Adaptation of Cortical Neurons and Local Field Potentials in the Awake Rat. J. Neurosci. 2009, 29, 13837–13849. [Google Scholar] [CrossRef] [Green Version]
- Yaron, A.; Hershenhoren, I.; Nelken, I. Sensitivity to Complex Statistical Regularities in Rat Auditory Cortex. Neuron 2012, 76, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.A.; Malmierca, M.S. The Effect of Auditory Cortex Deactivation on Stimulus-Specific Adaptation in the Inferior Colliculus of the Rat. Eur. J. Neurosci. 2013, 37, 52–62. [Google Scholar] [CrossRef]
- Ayala, Y.A.; Malmierca, M.S. Stimulus-Specific Adaptation and Deviance Detection in the Inferior Colliculus. Front. Neural. Circuits 2012, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Pérez-González, D.; Malmierca, M.S.; Covey, E. Novelty Detector Neurons in the Mammalian Auditory Midbrain. Eur. J. Neurosci. 2005, 22, 2879–2885. [Google Scholar] [CrossRef]
- Pérez-González, D.; Malmierca, M.S. Variability of the Time Course of Stimulus-Specific Adaptation in the Inferior Colliculus. Front. Neural. Circuits 2012, 6, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, L.A.; Christianson, G.B.; Linden, J.F. Stimulus-Specific Adaptation Occurs in the Auditory Thalamus. J. Neurosci. 2009, 29, 7359–7363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes, F.M.; Nelken, I.; Covey, E.; Malmierca, M.S. Stimulus-Specific Adaptation in the Auditory Thalamus of the Anesthetized Rat. PLoS ONE 2010, 5, e14071. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.M.; Malmierca, M.S. Effect of Auditory Cortex Deactivation on Stimulus-Specific Adaptation in the Medial Geniculate Body. J. Neurosci. 2011, 31, 17306–17316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duque, D.; Malmierca, M.S.; Caspary, D.M. Modulation of Stimulus-Specific Adaptation by GABA(A) Receptor Activation or Blockade in the Medial Geniculate Body of the Anaesthetized Rat. J. Physiol. 2014, 592, 729–743. [Google Scholar] [CrossRef]
- Costa-Faidella, J.; Baldeweg, T.; Grimm, S.; Escera, C. Interactions between “What” and “When” in the Auditory System: Temporal Predictability Enhances Repetition Suppression. J. Neurosci. 2011, 31, 18590–18597. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Martínez, F.J.; Rodríguez-Martínez, E.I.; Wilson, C.E.; Yau, S.; Saldaña, D.; Gómez, C.M. Impaired P1 Habituation and Mismatch Negativity in Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2020, 50, 603–616. [Google Scholar] [CrossRef]
- Sayres, R.; Grill-Spector, K. Object-Selective Cortex Exhibits Performance-Independent Repetition Suppression. J. Neurophysiol. 2006, 95, 995–1007. [Google Scholar] [CrossRef]
- Snyder, K.A.; Keil, A. Repetition Suppression of Induced Gamma Activity Predicts Enhanced Orienting toward a Novel Stimulus in 6-Month-Old Infants. J. Cogn. Neurosci. 2008, 20, 2137–2152. [Google Scholar] [CrossRef]
- Näätänen, R.; Gaillard, A.W.; Mäntysalo, S. Early Selective-Attention Effect on Evoked Potential Reinterpreted. Acta Psychol. 1978, 42, 313–329. [Google Scholar] [CrossRef]
- Näätänen, R.; Paavilainen, P.; Rinne, T.; Alho, K. The Mismatch Negativity (MMN) in Basic Research of Central Auditory Processing: A Review. Clin. Neurophysiol. 2007, 118, 2544–2590. [Google Scholar] [CrossRef]
- Winkler, I.; Schröger, E.; Cowan, N. The Role of Large-Scale Memory Organization in the Mismatch Negativity Event-Related Brain Potential. J. Cogn. Neurosci. 2001, 13, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, T.; Gliga, T.; Johnson, M.H.; Mareschal, D. The Neural Basis of Perceptual Category Learning in Human Infants. J. Cogn. Neurosci. 2009, 21, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Baldeweg, T.; Williams, J.D.; Gruzelier, J.H. Differential Changes in Frontal and Sub-Temporal Components of Mismatch Negativity. Int. J. Psychophysiol. 1999, 33, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N.; Winkler, I.; Teder, W.; Naatanen, R. Memory Prerequisites of Mismatch Negativity in the Auditory Event-Related Potential (ERP). J. Exp. Psychol. Learn. Mem. Cogn. 1993, 13, 909–921. [Google Scholar] [CrossRef]
- Haenschel, C.; Vernon, D.J.; Dwivedi, P.; Gruzelier, J.H.; Baldeweg, T. Event-Related Brain Potential Correlates of Human Auditory Sensory Memory-Trace Formation. J. Neurosci. 2005, 25, 10494–10501. [Google Scholar] [CrossRef] [Green Version]
- Recasens, M.; Leung, S.; Grimm, S.; Nowak, R.; Escera, C. Repetition Suppression and Repetition Enhancement Underlie Auditory Memory-Trace Formation in the Human Brain: An MEG Study. NeuroImage 2015, 108, 75–86. [Google Scholar] [CrossRef]
- Cooper, R.J.; Atkinson, R.J.; Clark, R.A.; Michie, P.T. Event-Related Potentials Reveal Modelling of Auditory Repetition in the Brain. Int. J. Psychophysiol. 2013, 88, 74–81. [Google Scholar] [CrossRef]
- Garrido, M.I.; Kilner, J.M.; Stephan, K.E.; Friston, K.J. The Mismatch Negativity: A Review of Underlying Mechanisms. Clin. Neurophysiol. 2009, 11, 453–463. [Google Scholar] [CrossRef] [Green Version]
- McCleery, A.; Mathalon, D.H.; Wynn, J.K.; Roach, B.J.; Hellemann, G.S.; Marder, S.R.; Green, M.F. Parsing Components of Auditory Predictive Coding in Schizophrenia Using a Roving Standard Mismatch Negativity Paradigm. Psychol. Med. 2019, 49, 1195–1206. [Google Scholar] [CrossRef]
- Heurteloup, C.; Merchie, A.; Roux, S.; Bonnet-Brilhault, F.; Escera, C.; Gomot, M. Neural Repetition Suppression to Vocal and Non-Vocal Sounds. Cortex 2022, 148, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, A.P.; Barros, C.; Vasconcelos, M.; Obermeier, C.; Kotz, S.A. Is Laughter a Better Vocal Change Detector than a Growl? Cortex 2017, 92, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Ylinen, S.; Huotilainen, M. Is There a Direct Neural Correlate for Memory-Trace Formation in Audition? NeuroReport 2007, 18, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Turk-Browne, N.; Yi, D.-J.; Leber, A.; Chun, M. Visual Quality Determines the Direction of Neural Repetition Effects. Cereb. Cortex 2007, 17, 425–433. [Google Scholar] [CrossRef]
- Doeller, C.F.; Opitz, B.; Mecklinger, A.; Krick, C.; Reith, W.; Schröger, E. Prefrontal Cortex Involvement in Preattentive Auditory Deviance Detection:: Neuroimaging and Electrophysiological Evidence. NeuroImage 2003, 20, 1270–1282. [Google Scholar] [CrossRef]
- Opitz, B.; Rinne, T.; Mecklinger, A.; von Cramon, D.Y.; Schröger, E. Differential Contribution of Frontal and Temporal Cortices to Auditory Change Detection: FMRI and ERP Results. NeuroImage 2002, 15, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Opitz, B.; Schröger, E.; Cramon, D.Y.V. Sensory and Cognitive Mechanisms for Preattentive Change Detection in Auditory Cortex. Eur. J. Neurosci. 2005, 21, 531–535. [Google Scholar] [CrossRef]
- Eger, E.; Henson, R.N.A.; Driver, J.; Dolan, R.J. BOLD Repetition Decreases in Object-Responsive Ventral Visual Areas Depend on Spatial Attention. J. Neurophysiol. 2004, 92, 1241–1247. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.O.; Wojciulik, E. Attention Increases Neural Selectivity in the Human Lateral Occipital Complex. Nat. Neurosci. 2003, 7, 70–74. [Google Scholar] [CrossRef]
- Gomot, M.; Bernard, F.A.; Davis, M.H.; Belmonte, M.K.; Ashwin, C.; Bullmore, E.T.; Baron-Cohen, S. Change Detection in Children with Autism: An Auditory Event-Related FMRI Study. NeuroImage 2006, 29, 475–484. [Google Scholar] [CrossRef] [Green Version]
- Sabri, M.; Kareken, D.A.; Dzemidzic, M.; Lowe, M.J.; Melara, R.D. Neural Correlates of Auditory Sensory Memory and Automatic Change Detection. NeuroImage 2004, 21, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Schall, U.; Johnston, P.; Todd, J.; Ward, P.B.; Michie, P.T. Functional Neuroanatomy of Auditory Mismatch Processing: An Event-Related FMRI Study of Duration-Deviant Oddballs. NeuroImage 2003, 20, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Cacciaglia, R.; Costa-Faidella, J.; Zarnowiec, K.; Grimm, S.; Escera, C. Auditory Predictions Shape the Neural Rsponses to Stimulus Repetition and Sensory Change. Neuroimage 2019, 186, 200–210. [Google Scholar] [CrossRef]
- Sokolov, E.N.; Sokolov, Y.N.; Sokolov, E.N.; Waydenfeld, S.W.; Worters, R.; Clarke, A.D.B. Perception and the Conditioned Reflex; Pergamon Press: Oxford, UK, 1963. [Google Scholar]
- Vogels, R. Sources of Adaptation of Inferior Temporal Cortical Responses. Cortex 2016, 80, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Peel, H.J.; Chouinard, P.A. FMRI Form Adaptation and Size Repetition Enhancement in Different Subdivisions of the Lateral Occipital Complex. Cortex 2022, 154, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Henson, R.; Shallice, T.; Dolan, R. Neuroimaging Evidence for Dissociable Forms of Repetition Priming. Science 2000, 287, 1269–1272. [Google Scholar] [CrossRef] [Green Version]
- Ishai, A.; Bikle, P.C.; Ungerleider, L.G. Temporal Dynamics of Face Repetition Suppression. Brain Res. Bull. 2006, 70, 289–295. [Google Scholar] [CrossRef]
- Todorovic, A.; de Lange, F.P. Repetition Suppression and Expectation Suppression Are Dissociable in Time in Early Auditory Evoked Fields. J. Neurosci. 2012, 32, 13389–13395. [Google Scholar] [CrossRef] [Green Version]
- van Turennout, M. Modulation of Neural Activity during Object Naming: Effects of Time and Practice. Cereb. Cortex 2003, 13, 381–391. [Google Scholar] [CrossRef] [Green Version]
- van Turennout, M.; Ellmore, T.; Martin, A. Long-Lasting Cortical Plasticity in the Object Naming System. Nat. Neurosci. 2000, 3, 1329–1334. [Google Scholar] [CrossRef] [Green Version]
- Lange, K. The Reduced N1 to Self-Generated Tones: An Effect of Temporal Predictability?: N1 Reduction and Temporal Predictability. Psychophysiology 2011, 48, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. A Theory of Cortical Responses. Phil. Trans. R. Soc. 2005, 360, 815–836. [Google Scholar] [CrossRef]
- Grotheer, M.; Kovács, G. Can Predictive Coding Explain Repetition Suppression? Cortex 2016, 80, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Wacongne, C.; Labyt, E.; van Wassenhove, V.; Bekinschtein, T.; Naccache, L.; Dehaene, S. Evidence for a Hierarchy of Predictions and Prediction Errors in Human Cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 20754–20759. [Google Scholar] [CrossRef] [PubMed]
- Tassinari, H.; Hudson, T.E.; Landy, M.S. Combining Priors and Noisy Visual Cues in a Rapid Pointing Task. J. Neurosci. 2006, 26, 10154–10163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, J.; Ngo, V.; Goldreich, D. Tactile Length Contraction as Bayesian Inference. J. Neurophysiol. 2016, 116, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, A. Whatever next? Predictive Brains, Situated Agents, and the Future of Cognitive Science. Behav. Brain Sci. 2013, 36, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. The Free-Energy Principle: A Unified Brain Theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Feldman, H.; Friston, K.J. Attention, Uncertainty, and Free-Energy. Front. Hum. Neurosci. 2010, 4, 215. [Google Scholar] [CrossRef] [Green Version]
- Bendixen, A.; SanMiguel, I.; Schröger, E. Early Electrophysiological Indicators for Predictive Processing in Audition: A Review. Int. J. Psychophysiol. 2012, 83, 120–131. [Google Scholar] [CrossRef]
- Winkler, I. Interpreting the Mismatch Negativity. J. Psychophysiol. 2007, 21, 147–163. [Google Scholar] [CrossRef]
- Winkler, I.; Czigler, I. Mismatch Negativity: Deviance Detection or the Maintenance of the “Standard”. Neuroreport 1998, 9, 3809–3813. [Google Scholar] [CrossRef] [PubMed]
- Mindell, J.A.; Williamson, A.A. Benefits of a Bedtime Routine in Young Children: Sleep, Development, and Beyond. Sleep Med. Rev. 2018, 40, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Rodger, S.; Umaibalan, V. The Routines and Rituals of Families of Typically Developing Children Compared with Families of Children with Autism Spectrum Disorder: An Exploratory Study. Br. J. Occup. Ther. 2011, 74, 20–26. [Google Scholar] [CrossRef]
- Nakano, T.; Watanabe, H.; Homae, F.; Taga, G. Prefrontal Cortical Involvement in Young Infants’ Analysis of Novelty. Cereb. Cortex 2009, 19, 455–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turk-Browne, N.B.; Scholl, B.J.; Chun, M.M. Babies and Brains: Habituation in Infant Cognition and Functional Neuroimaging. Front. Hum. Neurosci. 2008, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Kulig, J.W.; Tighe, T.J. Habituation in Children within a Behavior Suppression Paradigm. J. Exp. Child Psychol. 1981, 32, 425–442. [Google Scholar] [CrossRef]
- Nguyen, L.Y.; Spehar, B. Visual Adaptation to Natural Scene Statistics and Visual Preference. Vis. Res. 2021, 180, 87–95. [Google Scholar] [CrossRef]
- Fantz, R.L. visual experience in infants: Decreased attention to familiar patterns relative to novel ones. Science 1964, 146, 668–670. [Google Scholar] [CrossRef]
- Nordt, M.; Hoehl, S.; Weigelt, S. The Use of Repetition Suppression Paradigms in Developmental Cognitive Neuroscience. Cortex 2016, 80, 61–75. [Google Scholar] [CrossRef]
- Fagan, J.F., 3rd. Infants’ Recognition Memory for Faces. J. Exp. Child Psychol. 1972, 14, 453–476. [Google Scholar] [CrossRef]
- Hunter, M.A.; Ames, E.W. A Multifactor Model of Infant Preferences for Novel and Familiar Stimuli. In Advances in Infancy Research; Ablex Publishing: Westport, CT, USA, 1988; Volume 5, pp. 69–95. ISBN 0-89391-378-2. [Google Scholar]
- Dehaene-Lambertz, G.; Dehaene, S.; Anton, J.-L.; Campagne, A.; Ciuciu, P.; Dehaene, G.P.; Denghien, I.; Jobert, A.; LeBihan, D.; Sigman, M.; et al. Functional Segregation of Cortical Language Areas by Sentence Repetition. Hum. Brain Mapp. 2006, 27, 360–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchon, C.; Nazzi, T.; Gervain, J. Hemispheric Asymmetries in Repetition Enhancement and Suppression Effects in the Newborn Brain. PLoS ONE 2015, 10, e0140160. [Google Scholar] [CrossRef] [Green Version]
- Martineau, J.; Roux, S.; Garreau, B.; Adrien, J.L.; Lelord, G. Unimodal and Crossmodal Reactivity in Autism: Presence of Auditory Evoked Responses and Effect of the Repetition of Auditory Stimuli. Biol. Psychiatry 1992, 31, 1190–1203. [Google Scholar] [CrossRef]
- von Koss Torkildsen, J.; Hansen, H.F.; Svangstu, J.M.; Smith, L.; Simonsen, H.G.; Moen, I.; Lindgren, M. Brain Dynamics of Word Familiarization in 20-Month-Olds: Effects of Productive Vocabulary Size. Brain Lang. 2009, 108, 73–88. [Google Scholar] [CrossRef]
- Garrido, M.I.; Kilner, J.M.; Kiebel, S.J.; Stephan, K.E.; Baldeweg, T.; Friston, K.J. Repetition Suppression and Plasticity in the Human Brain. Neuroimage 2009, 48, 269–279. [Google Scholar] [CrossRef] [Green Version]
- Gorina-Careta, N.; Zarnowiec, K.; Costa-Faidella, J.; Escera, C. Timing Predictability Enhances Regularity Encoding in the Human Subcortical Auditory Pathway. Sci. Rep. 2016, 6, 37405. [Google Scholar] [CrossRef] [PubMed]
- Fryer, S.L.; Roach, B.J.; Hamilton, H.K.; Bachman, P.; Belger, A.; Carrión, R.E.; Duncan, E.; Johannesen, J.; Light, G.A.; Niznikiewicz, M.; et al. Deficits in Auditory Predictive Coding in Individuals with the Psychosis Risk Syndrome: Prediction of Conversion to Psychosis. J. Abnorm. Psychol. 2020, 129, 599–611. [Google Scholar] [CrossRef]
- Jamal, W.; Cardinaux, A.; Haskins, A.J.; Kjelgaard, M.; Sinha, P. Reduced Sensory Habituation in Autism and Its Correlation with Behavioral Measures. J. Autism Dev. Disord. 2020, 51, 3153–3164. [Google Scholar] [CrossRef] [PubMed]
- Feuerriegel, D.; Yook, J.; Quek, G.L.; Hogendoorn, H.; Bode, S. Visual Mismatch Responses Index Surprise Signalling but Not Expectation Suppression. Cortex 2021, 134, 16–29. [Google Scholar] [CrossRef]
- Alho, K.; Sainio, K.; Sajaniemi, N.; Reinikainen, K.; Näätänen, R. Event-Related Brain Potential of Human Newborns to Pitch Change of an Acoustic Stimulus. Electroencephalogr. Clin. Neurophysiol. 1990, 77, 151–155. [Google Scholar] [CrossRef]
- Dehaene-Lambertz, G.; Dehaene, S. Speed and Cerebral Correlates of Syllable Discrimination in Infants. Nature 1994, 370, 292–295. [Google Scholar] [CrossRef]
- Cheour, M.; Alho, K.; Ceponiené, R.; Reinikainen, K.; Sainio, K.; Pohjavuori, M.; Aaltonen, O.; Näätänen, R. Maturation of Mismatch Negativity in Infants. Int. J. Psychophysiol. 1998, 29, 217–226. [Google Scholar] [CrossRef]
- Rapaport, H.; Seymour, R.A.; Benikos, N.; He, W.; Pellicano, E.; Brock, J.; Sowman, P.F. Investigating Predictive Coding in Younger and Older Children Using MEG and a Multi-Feature Auditory Oddball Paradigm. Cereb. Cortex 2023, 33, 7489–7499. [Google Scholar] [CrossRef]
- Gomot, M.; Giard, M.-H.; Roux, S.; Barthélémy, C.; Bruneau, N. Maturation of Frontal and Temporal Components of Mismatch Negativity (MMN) in Children. NeuroReport 2000, 11, 3109–3112. [Google Scholar] [CrossRef] [PubMed]
- Cheour, M.; Leppänen, P.H.; Kraus, N. Mismatch Negativity (MMN) as a Tool for Investigating Auditory Discrimination and Sensory Memory in Infants and Children. Clin. Neurophysiol. 2000, 111, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, J.; Kovarski, K.; Houy-Durand, E.; Malvy, J.; Saby, A.; Bonnet-Brilhault, F.; Latinus, M.; Gomot, M. Emotional Prosodic Change Detection in Autism Spectrum Disorder: An Electrophysiological Investigation in Children and Adults. J. Neurodev. Disord. 2018, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Charpentier, J.; Kovarski, K.; Roux, S.; Houy-Durand, E.; Saby, A.; Bonnet-Brilhault, F.; Latinus, M.; Gomot, M. Brain Mechanisms Involved in Angry Prosody Change Detection in School-Age Children and Adults, Revealed by Electrophysiology. Cogn. Affect. Behav. Neurosci. 2018, 18, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-C.; Hsieh, M.H.; Lin, Y.-T.; Chan, P.-Y.S.; Cheng, C.-H. Mismatch Negativity to Different Deviant Changes in Autism Spectrum Disorders: A Meta-Analysis. Clin. Neurophysiol. 2020, 131, 766–777. [Google Scholar] [CrossRef]
- Cléry, H.; Bonnet-Brilhault, F.; Lenoir, P.; Barthelemy, C.; Bruneau, N.; Gomot, M. Atypical Visual Change Processing in Children with Autism: An Electrophysiological Study. Psychophysiology 2013, 50, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Gomot, M.; Giard, M.-H.; Adrien, J.-L.; Barthelemy, C.; Bruneau, N. Hypersensitivity to Acoustic Change in Children with Autism: Electrophysiological Evidence of Left Frontal Cortex Dysfunctioning. Psychophysiology 2002, 39, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Gomot, M.; Blanc, R.; Clery, H.; Roux, S.; Barthelemy, C.; Bruneau, N. Candidate Electrophysiological Endophenotypes of Hyper-Reactivity to Change in Autism. J. Autism Dev. Disord. 2011, 10, 705–714. [Google Scholar] [CrossRef]
- Kovarski, K.; Charpentier, J.; Roux, S.; Batty, M.; Houy-Durand, E.; Gomot, M. Emotional Visual Mismatch Negativity: A Joint Investigation of Social and Non-Social Dimensions in Adults with Autism. Transl. Psychiatry 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Franken, I.H.A.; Nijs, I.; Van Strien, J.W. Impulsivity Affects Mismatch Negativity (MMN) Measures of Preattentive Auditory Processing. Biol. Psychol. 2005, 70, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Rydkjær, J.; Jepsen, J.R.M.; Pagsberg, A.K.; Fagerlund, B.; Glenthøj, B.Y.; Oranje, B. Mismatch Negativity and P3a Amplitude in Young Adolescents with First-Episode Psychosis: A Comparison with ADHD. Psychol. Med. 2017, 47, 377–388. [Google Scholar] [CrossRef]
- Yang, M.-T.; Hsu, C.-H.; Yeh, P.-W.; Lee, W.-T.; Liang, J.-S.; Fu, W.-M.; Lee, C.-Y. Attention Deficits Revealed by Passive Auditory Change Detection for Pure Tones and Lexical Tones in ADHD Children. Front. Hum. Neurosci. 2015, 9, 470. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Sung, J.-Y.; Cheng, Y. Neural Dynamics of Emotional Salience Processing in Response to Voices during the Stages of Sleep. Front. Behav. Neurosci. 2016, 10, 117. [Google Scholar] [CrossRef] [Green Version]
- Baldeweg, T.; Hirsch, S.R. Mismatch Negativity Indexes Illness-Specific Impairments of Cortical Plasticity in Schizophrenia: A Comparison with Bipolar Disorder and Alzheimer’s Disease. Int. J. Psychophysiol. 2015, 95, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Erickson, M.A.; Ruffle, A.; Gold, J.M. A Meta-Analysis of Mismatch Negativity in Schizophrenia: From Clinical Risk to Disease Specificity and Progression. Biol. Psychiatry 2016, 79, 980–987. [Google Scholar] [CrossRef] [Green Version]
- Kirihara, K.; Tada, M.; Koshiyama, D.; Fujioka, M.; Usui, K.; Araki, T.; Kasai, K. A Predictive Coding Perspective on Mismatch Negativity Impairment in Schizophrenia. Front. Psychiatry 2020, 11, 660. [Google Scholar] [CrossRef]
- Näätänen, R.; Kähkönen, S. Central Auditory Dysfunction in Schizophrenia as Revealed by the Mismatch Negativity (MMN) and Its Magnetic Equivalent MMNm: A Review. Int. J. Neuropsychopharm. 2009, 12, 125–135. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- Baranek, G.T.; Barnett, C.R.; Adams, E.M.; Wolcott, N.A.; Watson, L.R.; Crais, E.R. Object Play in Infants with Autism: Methodological Issues in Retrospective Video Analysis. Am. J. Occup. Ther. 2005, 59, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Liss, M.; Saulnier, C.; Fein, D.; Kinsbourne, M. Sensory and Attention Abnormalities in Autistic Spectrum Disorders. Autism 2006, 10, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Rogers, S.J.; Hepburn, S.; Wehner, E. Parent Reports of Sensory Symptoms in Toddlers with Autism and Those with Other Developmental Disorders. J. Autism Dev. Disord. 2003, 33, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Gomot, M.; Wicker, B. A Challenging, Unpredictable World for People with Autism Spectrum Disorder. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2012, 83, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Brock, J. Alternative Bayesian Accounts of Autistic Perception: Comment on Pellicano and Burr. Trends Cogn. Sci. 2012, 16, 573–574. [Google Scholar] [CrossRef] [PubMed]
- Karaminis, T.; Cicchini, G.M.; Neil, L.; Cappagli, G.; Aagten-Murphy, D.; Burr, D.; Pellicano, E. Central Tendency Effects in Time Interval Reproduction in Autism. Sci. Rep. 2016, 6, 28570. [Google Scholar] [CrossRef] [Green Version]
- Crespo, C.; Santos, S.; Canavarro, M.C.; Kielpikowski, M.; Pryor, J.; Féres-Carneiro, T. Family Routines and Rituals in the Context of Chronic Conditions: A Review. Int. J. Psychol. 2013, 48, 729–746. [Google Scholar] [CrossRef]
- Rogers, S.J.; Ozonoff, S. Annotation: What Do We Know about Sensory Dysfunction in Autism? A Critical Review of the Empirical Evidence. J. Child Psychol. Psychiatry 2005, 46, 1255–1268. [Google Scholar] [CrossRef]
- Cary, E.; Pacheco, D.; Kaplan-Kahn, E.; McKernan, E.; Matsuba, E.; Prieve, B.; Russo, N. Brain Signatures of Early and Late Neural Measures of Auditory Habituation and Discrimination in Autism and Their Relationship to Autistic Traits and Sensory Overresponsivity. J. Autism Dev. Disord. 2023. [Google Scholar] [CrossRef]
- Gonzalez-Gadea, M.L.; Chennu, S.; Bekinschtein, T.A.; Rattazzi, A.; Beraudi, A.; Tripicchio, P.; Moyano, B.; Soffita, Y.; Steinberg, L.; Adolfi, F.; et al. Predictive Coding in Autism Spectrum Disorder and Attention Deficit Hyperactivity Disorder. J. Neurophysiol. 2015, 114, 2625–2636. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, J.A.; Kushnerenko, E.; Tomalski, P.; Davies, K.; Ribeiro, H.; Johnson, M.H. Differential Habituation to Repeated Sounds in Infants at High Risk for Autism. NeuroReport 2011, 22, 845–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Font-Alaminos, M.; Cornella, M.; Costa-Faidella, J.; Hervás, A.; Leung, S.; Rueda, I.; Escera, C. Increased Subcortical Neural Responses to Repeating Auditory Stimulation in Children with Autism Spectrum Disorder. Biol. Psychol. 2020, 149, 107807. [Google Scholar] [CrossRef] [PubMed]
- Latinus, M.; Cléry, H.; Andersson, F.; Bonnet-Brilhault, F.; Fonlupt, P.; Gomot, M. Inflexibility in Autism Spectrum Disorder: Need for Certainty and Atypical Emotion Processing Share the Blame. Brain Cogn. 2019, 136, 103599. [Google Scholar] [CrossRef]
- Rubenstein, J.L.R.; Merzenich, M.M. Model of Autism: Increased Ratio of Excitation/Inhibition in Key Neural Systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Briend, F.; Barantin, L.; Cléry, H.; Cottier, J.-P.; Bonnet-Brilhault, F.; Houy-Durand, E.; Gomot, M. Glutamate Levels of the Right and Left Anterior Cingulate Cortex in Autistics Adults. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 126, 110801. [Google Scholar] [CrossRef]
- Galineau, L.; Arlicot, N.; Dupont, A.-C.; Briend, F.; Houy-Durand, E.; Tauber, C.; Gomot, M.; Gissot, V.; Barantin, L.; Lefevre, A.; et al. Glutamatergic Synapse in Autism: A Complex Story for a Complex Disorder. Mol. Psychiatry 2023, 28, 801–809. [Google Scholar] [CrossRef]
- Sapey-Triomphe, L.-A.; Temmerman, J.; Puts, N.A.J.; Wagemans, J. Prediction Learning in Adults with Autism and Its Molecular Correlates. Mol. Autism 2021, 12, 64. [Google Scholar] [CrossRef]
- Kolesnik, A.; Begum Ali, J.; Gliga, T.; Guiraud, J.; Charman, T.; Johnson, M.H.; Jones, E.J.H. Increased Cortical Reactivity to Repeated Tones at 8 Months in Infants with Later ASD. Transl. Psychiatry 2019, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Friston, K.J.; Lawson, R.; Frith, C.D. On Hyperpriors and Hypopriors: Comment on Pellicano and Burr. Trends Cogn. Sci. 2013, 17, 1. [Google Scholar] [CrossRef]
- Lawson, R.P.; Rees, G.; Friston, K.J. An Aberrant Precision Account of Autism. Front. Hum. Neurosci. 2014, 8, 302. [Google Scholar] [CrossRef] [Green Version]
- Van de Cruys, S.; Evers, K.; Van der Hallen, R.; Van Eylen, L.; Boets, B.; de-Wit, L.; Wagemans, J. Precise Minds in Uncertain Worlds: Predictive Coding in Autism. Psychol. Rev. 2014, 121, 649. [Google Scholar] [CrossRef] [Green Version]
- Sinha, P.; Kjelgaard, M.M.; Gandhi, T.K.; Tsourides, K.; Cardinaux, A.L.; Pantazis, D.; Diamond, S.P.; Held, R.M. Autism as a Disorder of Prediction. Proc. Natl. Acad. Sci. USA 2014, 111, 15220–15225. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.; O’Brien, A.M.; Bungert, L.; Sinha, P. Prediction in Autism Spectrum Disorder: A Systematic Review of Empirical Evidence. Autism Res. 2021, 14, 604–630. [Google Scholar] [CrossRef] [PubMed]
- Randeniya, R.; Mattingley, J.B.; Garrido, M.I. Increased Context Adjustment Is Associated with Auditory Sensitivities but Not with Autistic Traits. Autism Res. 2022, 15, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Kleinhans, N.M.; Johnson, L.C.; Richards, T.; Mahurin, R.; Greenson, J.; Dawson, G.; Aylward, E. Reduced Neural Habituation in the Amygdala and Social Impairments in Autism Spectrum Disorders. Am. J. Psychiatry 2009, 166, 467–475. [Google Scholar] [CrossRef]
- D’Mello, A.M.; Frosch, I.R.; Meisler, S.L.; Grotzinger, H.; Perrachione, T.K.; Gabrieli, J.D.E. Diminished Repetition Suppression Reveals Selective and Systems-Level Face Processing Differences in ASD. J. Neurosci. 2023, 43, 1952–1962. [Google Scholar] [CrossRef]
- Millin, R.; Kolodny, T.; Flevaris, A.V.; Kale, A.M.; Schallmo, M.-P.; Gerdts, J.; Bernier, R.A.; Murray, S. Reduced Auditory Cortical Adaptation in Autism Spectrum Disorder. eLife 2018, 7, e36493. [Google Scholar] [CrossRef]
- Lawson, R.P.; Aylward, J.; Roiser, J.P.; Rees, G. Adaptation of Social and Non-Social Cues to Direction in Adults with Autism Spectrum Disorder and Neurotypical Adults with Autistic Traits. Dev. Cogn. Neurosci. 2018, 29, 108–116. [Google Scholar] [CrossRef]
- Hemsley, D.R. The Development of a Cognitive Model of Schizophrenia: Placing It in Context. Neurosci. Biobehav. Rev. 2005, 29, 977–988. [Google Scholar] [CrossRef]
- Sterzer, P.; Adams, R.A.; Fletcher, P.; Frith, C.; Lawrie, S.M.; Muckli, L.; Petrovic, P.; Uhlhaas, P.; Voss, M.; Corlett, P.R. The Predictive Coding Account of Psychosis. Biol. Psychiatry 2018, 84, 634–643. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, K.N.; Silverstein, S.M.; Brascamp, J.W. A Review of Visual Aftereffects in Schizophrenia. Neurosci. Biobehav. Rev. 2019, 101, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Bolino, F.; Di Michele, V.; Di Cicco, L.; Manna, V.; Daneluzzo, E.; Casacchia, M. Sensorimotor Gating and Habituation Evoked by Electro-Cutaneous Stimulation in Schizophrenia. Biol. Psychiatry 1994, 36, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R. Inhibitory Gating of an Evoked Response to Repeated Auditory Stimuli in Schizophrenic and Normal Subjects: Human Recordings, Computer Simulation, and an Animal Model. Arch. Gen. Psychiatry 1996, 53, 1114. [Google Scholar] [CrossRef] [PubMed]
- Avery, S.N.; McHugo, M.; Armstrong, K.; Blackford, J.U.; Vandekar, S.; Woodward, N.D.; Heckers, S. Habituation during Encoding: A New Approach to the Evaluation of Memory Deficits in Schizophrenia. Schizophr. Res. 2020, 223, 179–185. [Google Scholar] [CrossRef]
- Holt, D.J.; Weiss, A.P.; Rauch, S.L.; Wright, C.I.; Zalesak, M.; Goff, D.C.; Ditman, T.; Welsh, R.C.; Heckers, S. Sustained Activation of the Hippocampus in Response to Fearful Faces in Schizophrenia. Biol. Psychiatry 2005, 57, 1011–1019. [Google Scholar] [CrossRef]
- Bodatsch, M.; Brockhaus-Dumke, A.; Klosterkötter, J.; Ruhrmann, S. Forecasting Psychosis by Event-Related Potentials—Systematic Review and Specific Meta-Analysis. Biol. Psychiatry 2015, 77, 951–958. [Google Scholar] [CrossRef]
- Coffman, B.A.; Haigh, S.M.; Murphy, T.K.; Salisbury, D.F. Impairment in Mismatch Negativity but Not Repetition Suppression in Schizophrenia. Brain Topogr. 2017, 30, 521–530. [Google Scholar] [CrossRef]
- Koshiyama, D.; Kirihara, K.; Tada, M.; Nagai, T.; Fujioka, M.; Usui, K.; Araki, T.; Kasai, K. Reduced Auditory Mismatch Negativity Reflects Impaired Deviance Detection in Schizophrenia. Schizophr. Bull. 2020, 46, 937–946. [Google Scholar] [CrossRef] [Green Version]
- Rentzsch, J.; Shen, C.; Jockers-Scherübl, M.C.; Gallinat, J.; Neuhaus, A.H. Auditory Mismatch Negativity and Repetition Suppression Deficits in Schizophrenia Explained by Irregular Computation of Prediction Error. PLoS ONE 2015, 10, e0126775. [Google Scholar] [CrossRef]
- Mazer, P.; Macedo, I.; Paiva, T.O.; Ferreira-Santos, F.; Pasion, R.; Barbosa, F.; Almeida, P.; Silveira, C.; Cunha-Reis, C.; Marques-Teixeira, J. Abnormal Habituation of the Auditory Event-Related Potential P2 Component in Patients With Schizophrenia. Front. Psychiatry 2021, 12, 630406. [Google Scholar] [CrossRef]
- McCleery, A.; Wynn, J.K.; Green, M.F. Hallucinations, Neuroplasticity, and Prediction Errors in Schizophrenia. Scand. J. Psychol. 2018, 59, 41–48. [Google Scholar] [CrossRef]
- Kovács, G.; Grotheer, M.; Münke, L.; Kéri, S.; Nenadić, I. Significant Repetition Probability Effects in Schizophrenia. Psychiatry Res. Neuroimaging 2019, 290, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E.; Blackford, J.U.; Luksik, A.; Gauthier, I.; Heckers, S. Reduced Habituation in Patients with Schizophrenia. Schizophr. Res. 2013, 151, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Reavis, E.A.; Engel, S.A.; Altshuler, L.L.; Cohen, M.S.; Glahn, D.C.; Nuechterlein, K.H.; Wynn, J.K.; Green, M.F. FMRI Evidence of Aberrant Neural Adaptation for Objects in Schizophrenia and Bipolar Disorder. Hum. Brain Mapp. 2019, 40, 1608–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansiewicz, E.M.; Newschaffer, C.J.; Denckla, M.B.; Mostofsky, S.H. Impaired Habituation in Children with Attention Deficit Hyperactivity Disorder. Cogn. Behav. Neurol. 2004, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Massa, J.; O’Desky, I.H. Impaired Visual Habituation in Adults With ADHD. J. Atten. Disord. 2011, 16, 553–561. [Google Scholar] [CrossRef]
- Conzelmann, A.; Pauli, P.; Mucha, R.F.; Jacob, C.P.; Gerdes, A.B.M.; Romanos, J.; Bähne, C.G.; Heine, M.; Boreatti-Hümmer, A.; Alpers, G.W.; et al. Early Attentional Deficits in an Attention-to-Prepulse Paradigm in ADHD Adults. J. Abnorm. Psychol. 2010, 119, 594. [Google Scholar] [CrossRef] [Green Version]
- Herpertz, S.C.; Mueller, B.; Wenning, B.; Qunaibi, M.; Lichterfeld, C.; Herpertz-Dahlmann, B. Autonomic Responses in Boys with Externalizing Disorders. J. Neural Transm. 2003, 110, 1181–1195. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Medina, D.J.; Hawk, L.W.; Fosco, W.D.; Richards, J.B. Habituation of Reinforcer Effectiveness. Front. Integr. Neurosci. 2014, 7, 107. [Google Scholar] [CrossRef] [Green Version]
- Friston, K. The Free-Energy Principle: A Rough Guide to the Brain? Trends Cogn. Sci. 2009, 13, 293–301. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Chan, P.-Y.S.; Hsieh, Y.-W.; Chen, K.-F. A Meta-Analysis of Mismatch Negativity in Children with Attention Deficit-Hyperactivity Disorders. Neurosci. Lett. 2016, 612, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Kanner, L. Autistic Disturbances of Affective Contact. Nerv. Child 1943, 2, 217–250. [Google Scholar]
- Andronikof, A.; Fontan, P. Grounia Efimovna Soukhareva: La Première Description Du Syndrome Dit d’Asperger. Neuropsychiatr. L’enfance L’adolescence 2016, 64, 58–70. [Google Scholar] [CrossRef]
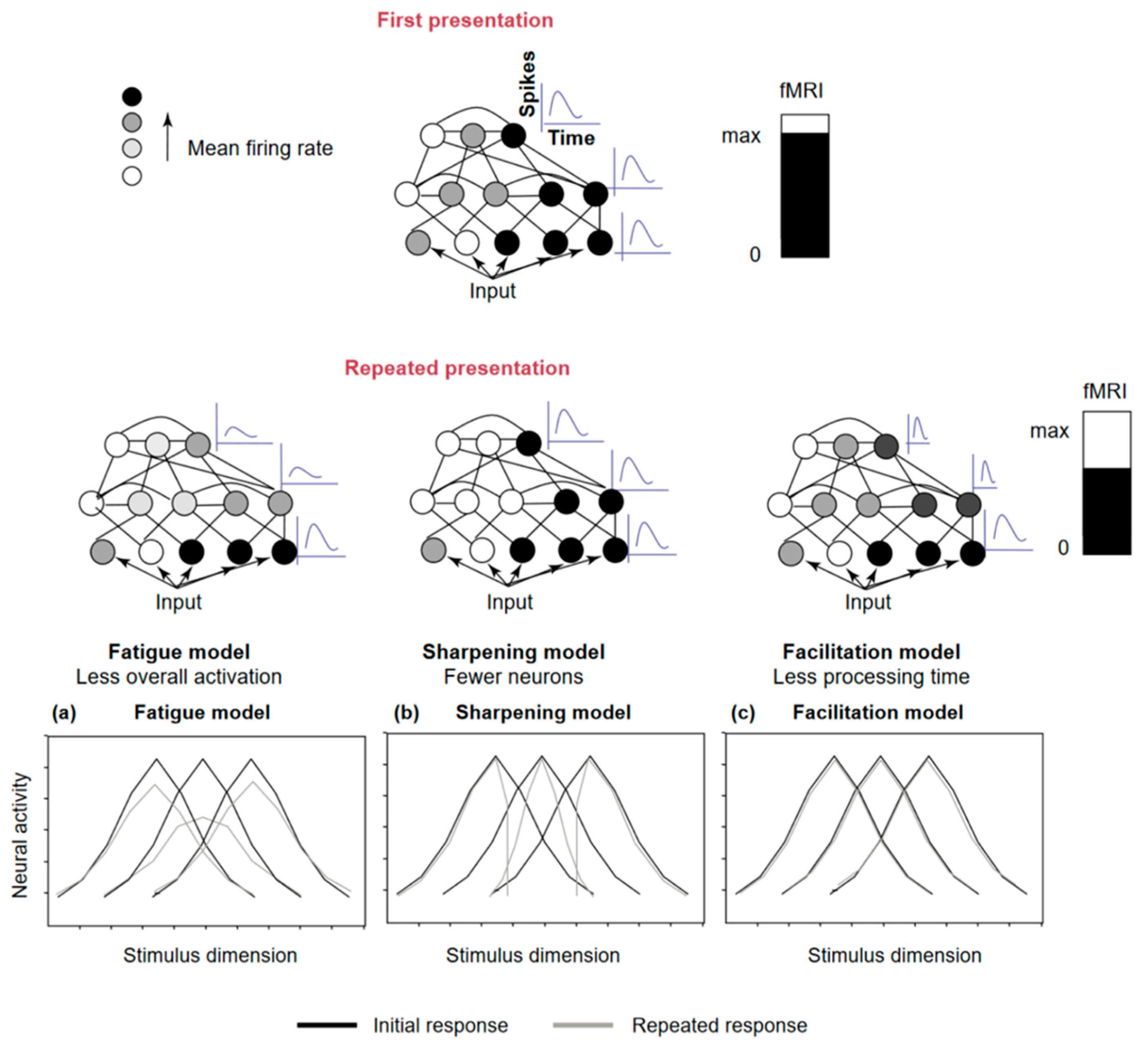
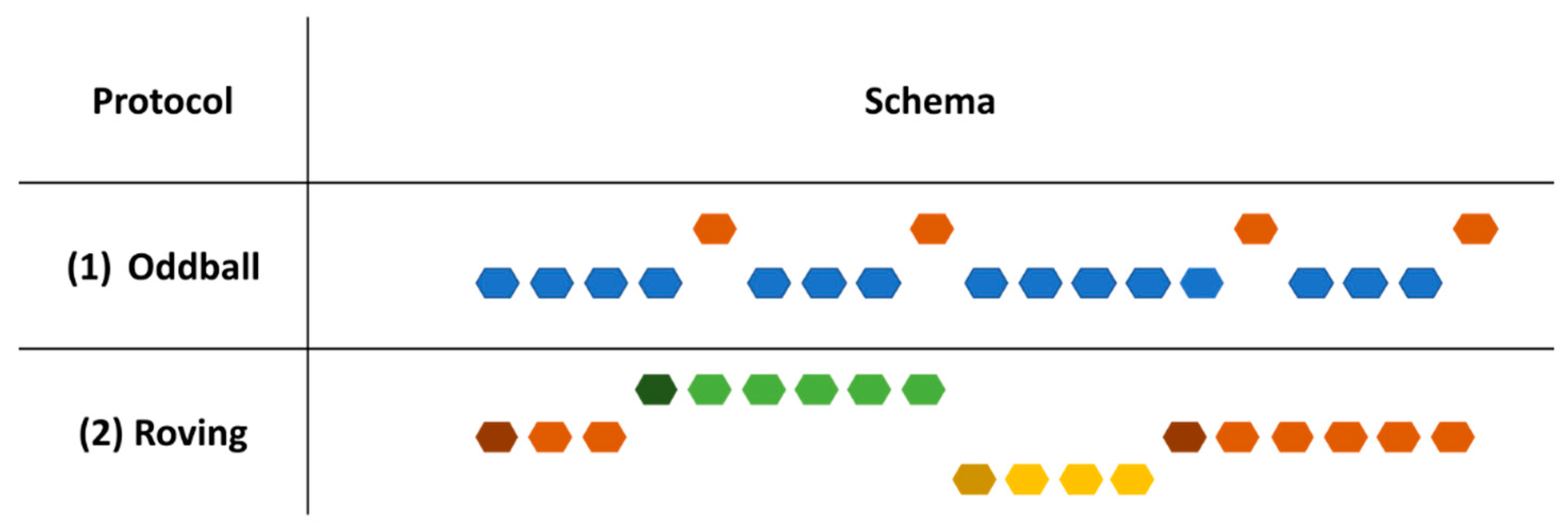
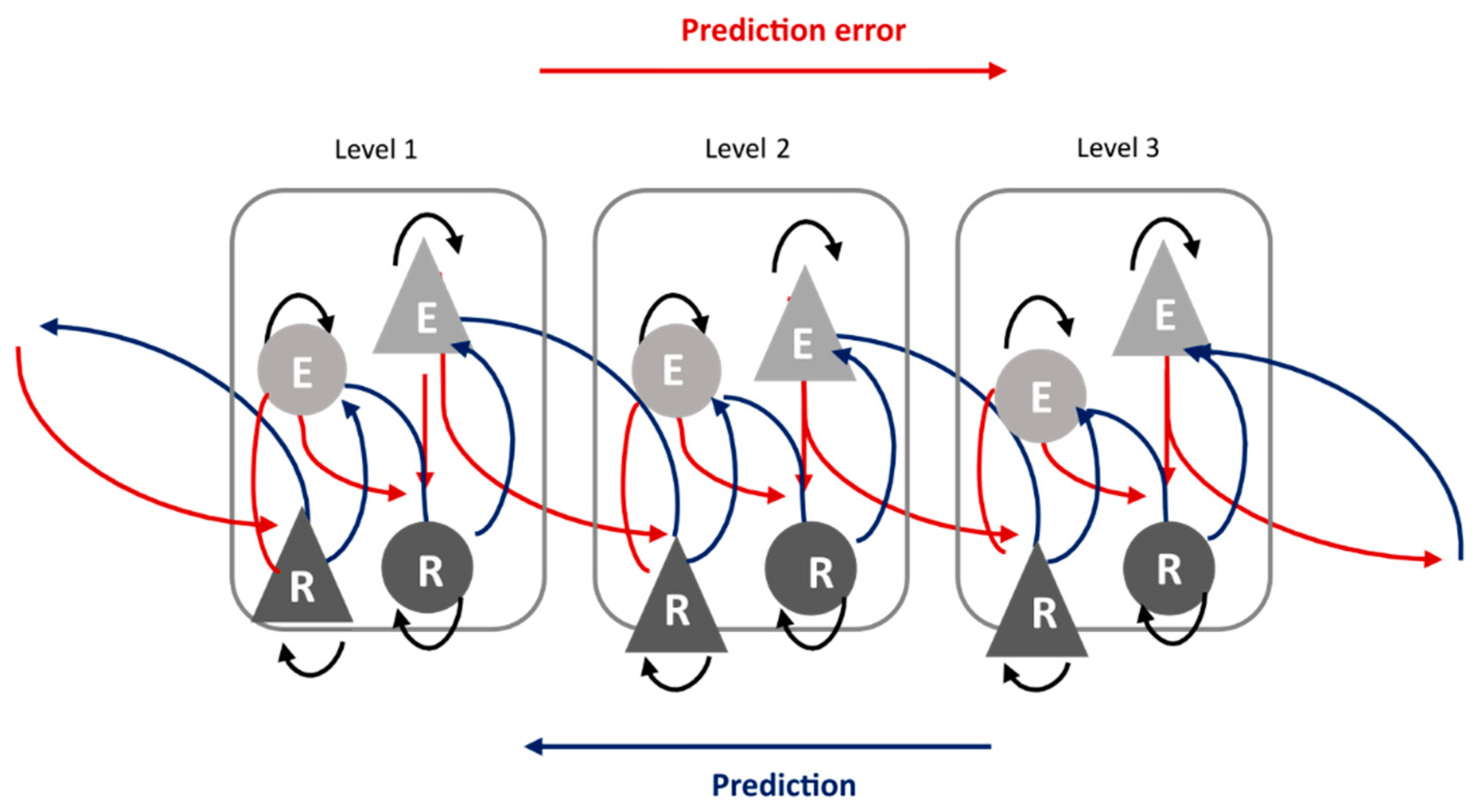
| Repetition Suppression | Repetition Enhancement | |
|---|---|---|
| Decrease in neural activity to repeated presentation of the same stimulus → Reflects novelty preference | Increase in the response as the number of repetitions of a stimulus increases. More marked for degraded stimuli, reflecting missing access to memory representation and construction of this representation. → Reflects familiarity preference | |
| Grill-Spector et al.’s Models Grill-Spector et al., 2006 [23] | The fatigue model: decrease in the amplitude of firing of neurons responding to the stimulus, proportional to the initial response | The novel network formation: presentation of a novel stimulus that is learned and its representation is established with the creation of a new neural network coded for this stimulus. |
| The sharpening model: decrease in the number of neurons that respond to the stimulus with repeated presentation | ||
| The facilitation (or accumulation) model James and Gauthier, 2006 [27] | Faster processing of the stimulus by the neurons involved, resulting in shorter latency or reaction time | Repeated exposure to stimulus with low visibility generally leads to increased perceptual performance. |
| Predictive coding Friston, 2005 [93] | Regularity encoding, displaying a decrease in the demand that occurs when expected and observed sensory information coincide. Reflects the increasing precision of prediction, the correct prediction of the upcoming stimulus | Increase in the prediction weight when expected and observed sensory information are the same. |
| Study | Cortical/Subcortical | Modality | Population | Paradigm | Protocol | Stimuli Duration and SOA (ms) | Results |
|---|---|---|---|---|---|---|---|
| Baldeweg et al., 2004 [4] | Cortical | Auditory (pure tones) | 20 adults | Roving (2, 6, 18 and 38 repetitions) | 1 sequence | Stimuli: 25 for standard and 50 for deviant |
|
| Haenschel et al., 2005 [66] | Cortical | Auditory (pure tones) | 40 adults | Roving (trains of 2, 6 and 36 repetitions) | 2 blocks with passive listening 2 blocks with active discrimination | Stimuli: 200 SOA: 500 |
|
| Ylinen and Huotilainen, 2007 [73] | Cortical | Auditory (synthetized vowels and vowels-like equivalents) | 9 adults | Roving (3–4 repetitions 5–6 repetitions) | 1 sequence of familiar stimuli (vowels) 1 sequence of unfamiliar stimuli (vowels-like) | Stimuli: 400 SOA: 700 |
|
| Garrido et al., 2009 [118] | Cortical | Auditory (pure tones) | 10 adults | Roving | 1 sequence | Stimuli: 70 SOA: 570 |
|
| Costa-Faidella et al., 2011 [56] | Cortical | Auditory (pure tones) | 17 adults | Roving (trains of 3, 6 and 12 repetitions) | Predictable condition and unpredictable condition | Stimuli: 50 SOA: 708 (predictable) or 364–1062 (unpredictable) |
|
| Costa-Faidella et al., 2011 [5] | Cortical | Auditory (pure tones) | 20 adults | Oddball (2, 6 and 12 repetitions) | 1 sequence composed of 2 runs. Run 1: S1 repeated 2, 6 or 12 times, followed by deviant S2. Run 2: S2 repeated 2, 6 or 12 times, followed by deviant S1 | Stimuli: 40 |
|
| Cooper et al., 2013 [68] | Cortical | Auditory (pure tones) | 24 adults | Roving and oddball (4, 8 or 16 standards) | 1 roving sequence 1 oddball sequence | Stimuli: 50 for standard and 100 for deviant |
|
| Recasens et al., 2015 [67] | Cortical | Auditory (pure tones) | 13 adults | Roving (3, 12 or 24 repetitions) | 2 runs: 198 trains of each length | Stimuli: 50 SOA: 500 |
|
| Gorina-Careta et al., 2016 [119] | Subcortical | Auditory (consonant-vowel) | 30 adults | Sequence of repeated stimuli | Predictable timing condition and unpredictable timing condition (8 blocks of 1001 repetitions per condition) | Stimuli: 170 SOA: 366 (predictable) or 183– 549 (unpredictable) |
|
| Pinheiro et al., 2017 [72] | Cortical | Auditory (human vocalizations) | 23 adults | Oddball | 4 blocks of 1050 standards and 150 deviants. Three different stimuli, standard or deviant, depending on the block: neutral, angry and happy | Stimuli 700 SOA: 1200 |
|
| McCleery et al., 2019 [70] | Cortical | Auditory (pure tones) | 29 adults | Roving (3, 8 or 33 repetitions) | 1 sequence composed of 5 blocks at two different times (2 weeks apart) Stimuli vary in pitch + duration | Stimuli: 50 or 100 |
|
| Fryer et al., 2020 [120] | Cortical | Auditory (pure tones) | 241 adults | Oddball | 3 blocks consisting of a standard (85%), a deviant in duration (5%), a deviant in frequency (5%), and a deviant in duration and in frequency (5%) | SOA: 500 Stimuli standard in duration: 50 Deviant in duration: 100 |
|
| Jamal et al., 2020 [121] | Cortical | Auditory (pure tones) and visual (radial checkerboard) | 22 children 7.1–12.8 years | Sequences of repeated stimulus | 2 sequences (1 visual and 1 auditive) with 300 repetitions of the same stimulus | SOA: 1116 Stimuli duration: 116 |
|
| Ruiz-Martínez et al., 2020 [57] | Cortical | Auditory (Electronic and human sounds) | 15 children 5–11 years | Oddball | 8 blocks (4/sound type) (deviant separated by at least 2 standards; each block begins with 10 standards) | Stimuli: 85 SOA: 685–885 |
|
| Feuerriegel et al., 2021 [122] | Cortical | Visual (faces) | 43 adults | Oddball | 34 sequences: 6 faces presented, then oddball face identity (different identity than the base face). Blocks of 6 consecutive sequences were used with two oddball face identities possible, the proportion of both is known | Images were presented at a rate of 6 Hz |
|
| Heurteloup et al., 2022 [71] | Cortical | Auditory (complex tones and human voice) | 20 adults (18 to 30 years) | Roving (4, 8 or 16 repetitions) | 2 roving sequences: one for vocal sounds and one for complex non-vocal sounds | SOA: 646 Stimuli duration: 300 |
|
| Study | Cortical/Subcortical | Modality | Population | Paradigm | Protocol | Stimuli Duration and SOA (ms) | Results |
|---|---|---|---|---|---|---|---|
| Baldeweg et al., 2004 [4] | Cortical | Auditory (pure tones) | 28 adults | Roving (2, 6, 18 and 38 repetitions) | 1 sequence | Stimuli: 25 for standard and 50 for deviant |
|
| Rentzsch et al., 2015 [183] | Cortical | Auditory (pure tones and click sound) | 25 adults | Oddball and paired click paradigm | Oddball: standard and deviant stimuli differed in frequency Click: pair of identical stimuli repeated | Oddball: Pure tone duration: 80 Inter-stimulus interval: between 350 and 650 ms Click sound: SOA: 500 and inter-trial interval of 3400 |
|
| Coffman et al., 2017 [181] | Cortical | Auditory (pure tones) | 26 adults | Oddball | 2 tasks:
| Stimuli: 50 SOA: 330 Inter-group: 750 Stimuli duration mismatch: 100 |
|
| McCleery et al., 2019 [70] | Cortical | Auditory (pure tones) | 43 adults | Roving (3, 8 or 33 repetitions) | 1 sequence composed of 5 blocks at two different times (2 weeks apart). Pitch + duration stimuli variation | Stimuli: 50 or 100 |
|
| Fryer et al., 2020 [120] | Cortical | Auditory (pure tones) | 54 adults with Psychosis Risk syndrome—Conversion (PRS-C) | Oddball | 3 blocks consisting of a standard (85%), a deviant in duration (5%), a deviant in frequency (5%), and a deviant in duration and in frequency (5%) | SOA: 500 Stimuli standard in duration: 50 Deviant in duration: 100 |
|
| Koshiyama et al., 2020 [182] | Cortical | Auditory (pure tones) | 25 adults | Oddball and many-standards paradigm | Oddball: 2 sequences
| Stimulus standard: 50 Oddball duration sequence → deviants 100 Many-standards paradigm: between 10 and 225 |
|
| Mazer et al., 2021 [184] | Cortical | Auditory (bird song, voice and pure tone) | 26 adults | Roving | 7 repetitions for trains for bird song and voice—target tone appears between train | Complex sounds (bird songs and voice): 200 Inter-stimulus interval: 1000 Pure tone: 70 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merchie, A.; Gomot, M. Habituation, Adaptation and Prediction Processes in Neurodevelopmental Disorders: A Comprehensive Review. Brain Sci. 2023, 13, 1110. https://doi.org/10.3390/brainsci13071110
Merchie A, Gomot M. Habituation, Adaptation and Prediction Processes in Neurodevelopmental Disorders: A Comprehensive Review. Brain Sciences. 2023; 13(7):1110. https://doi.org/10.3390/brainsci13071110
Chicago/Turabian StyleMerchie, Annabelle, and Marie Gomot. 2023. "Habituation, Adaptation and Prediction Processes in Neurodevelopmental Disorders: A Comprehensive Review" Brain Sciences 13, no. 7: 1110. https://doi.org/10.3390/brainsci13071110
APA StyleMerchie, A., & Gomot, M. (2023). Habituation, Adaptation and Prediction Processes in Neurodevelopmental Disorders: A Comprehensive Review. Brain Sciences, 13(7), 1110. https://doi.org/10.3390/brainsci13071110





