Locked Out: Phoenixin-14 Does Not Cross a Stem-Cell-Derived Blood–Brain Barrier Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation of a Single-Cell Blood–Brain Barrier Model
2.2. Toxicity Studies
2.3. Transport Studies
2.4. ELISA
2.5. Statistical Analysis
3. Results
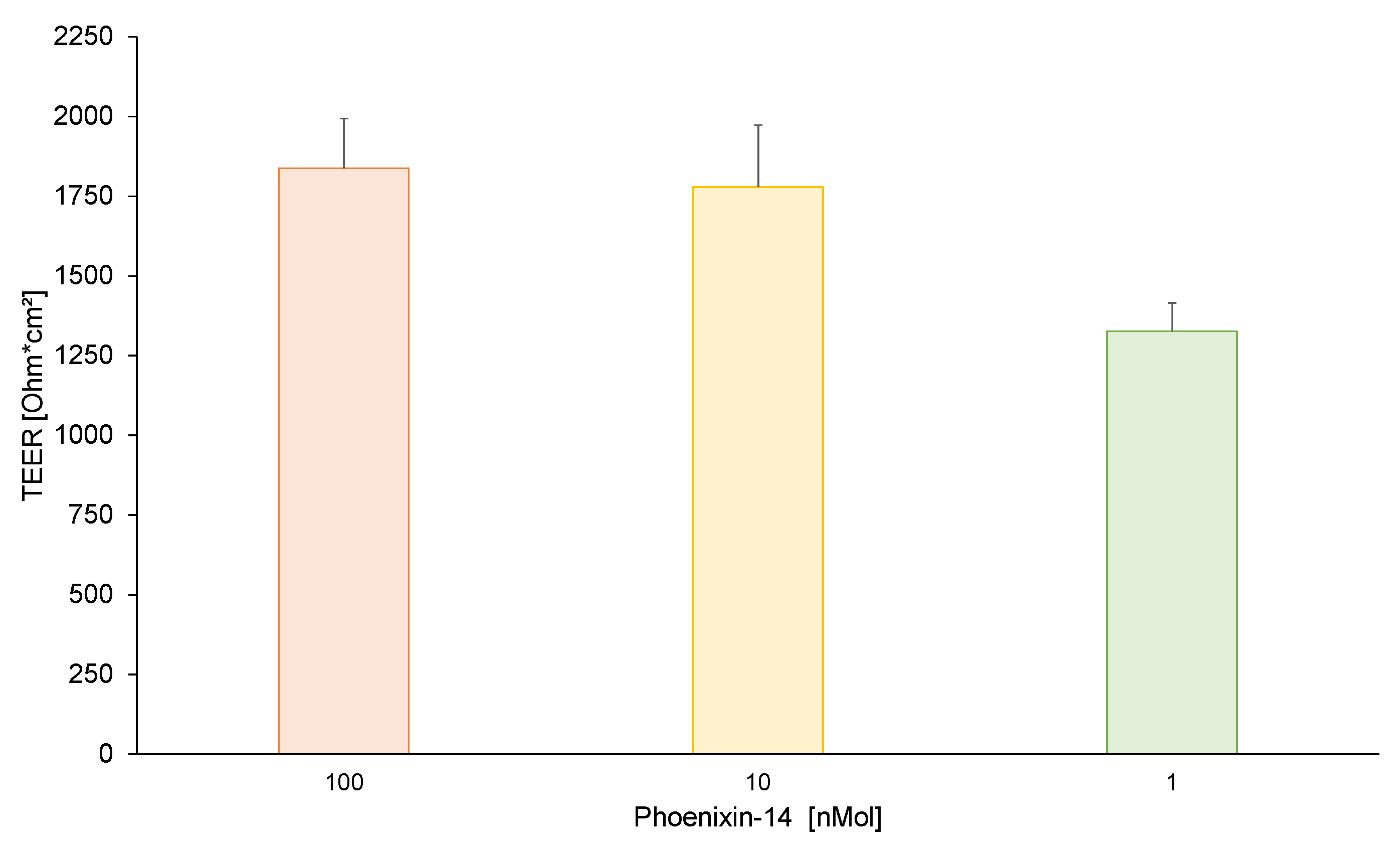
3.1. TEER Values
3.2. Cytotoxicity Studies
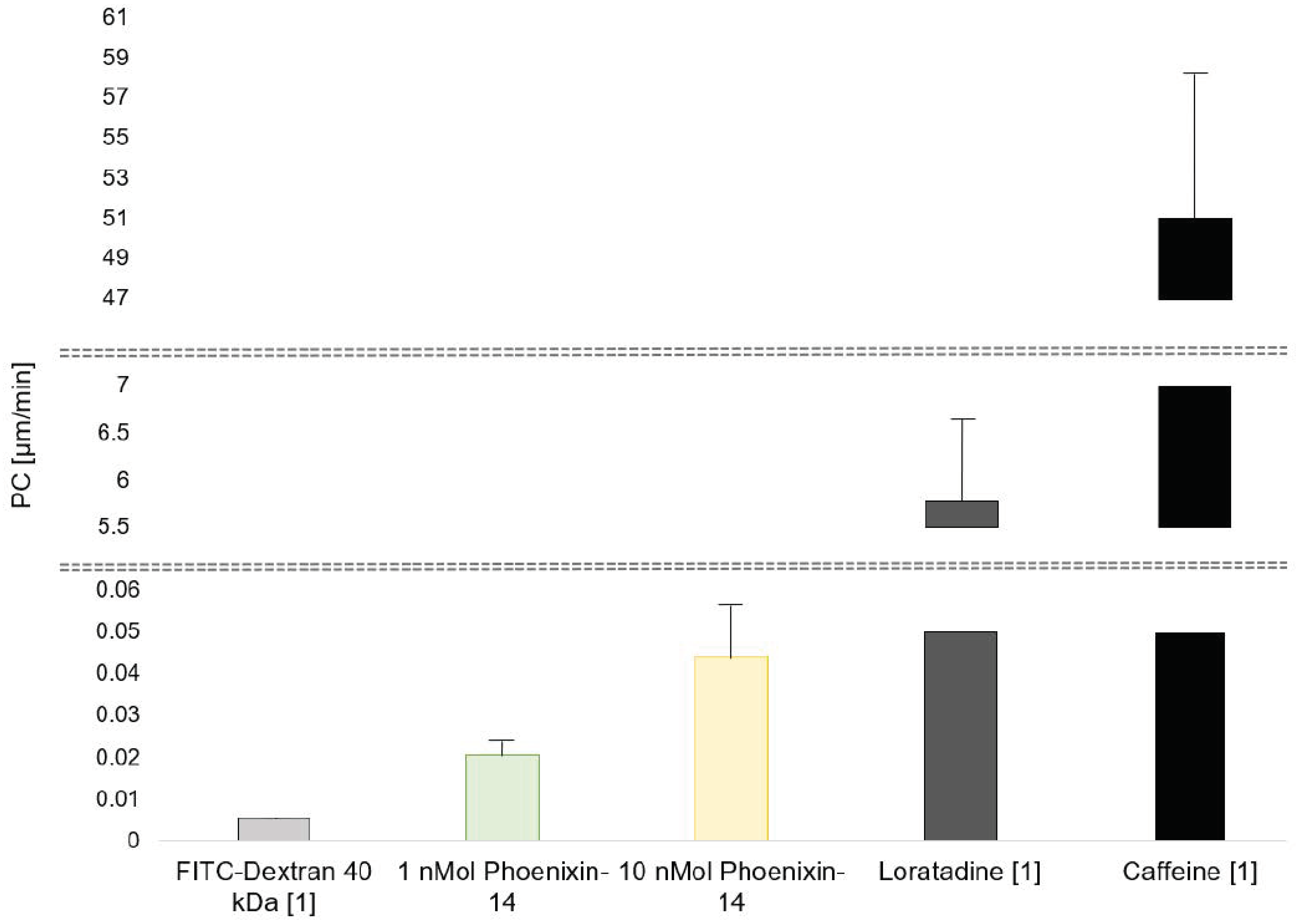
3.3. Transport Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Romijn, J.A.; Corssmit, E.P.; Havekes, L.M.; Pijl, H. Gut-brain axis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 518–521. [Google Scholar] [CrossRef]
- Schalla, M.A.; Taché, Y.; Stengel, A. Neuroendocrine Peptides of the Gut and Their Role in the Regulation of Food Intake. Compr. Physiol. 2021, 11, 1679–1730. [Google Scholar] [CrossRef] [PubMed]
- Yosten, G.L.; Lyu, R.M.; Hsueh, A.J.; Avsian-Kretchmer, O.; Chang, J.K.; Tullock, C.W.; Dun, S.L.; Dun, N.; Samson, W.K. A novel reproductive peptide, phoenixin. J. Neuroendocrinol. 2013, 25, 206–215. [Google Scholar] [CrossRef]
- Prinz, P.; Scharner, S.; Friedrich, T.; Schalla, M.; Goebel-Stengel, M.; Rose, M.; Stengel, A. Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochem. Biophys. Res. Commun. 2017, 493, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Deng, S.P.; Chen, H.P.; Jiang, D.N.; Tian, C.X.; Yang, W.; Wu, T.L.; Zhu, C.H.; Zhang, Y.; Li, G.L. Phoenixin participated in regulation of food intake and growth in spotted scat, Scatophagus argus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 226, 36–44. [Google Scholar] [CrossRef]
- Rajeswari, J.J.; Blanco, A.M.; Unniappan, S. Phoenixin-20 suppresses food intake, modulates glucoregulatory enzymes, and enhances glycolysis in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R917–R928. [Google Scholar] [CrossRef] [PubMed]
- Pałasz, A.; Tyszkiewicz-Nwafor, M.; Suszka-Świtek, A.; Bacopoulou, F.; Dmitrzak-Węglarz, M.; Dutkiewicz, A.; Słopień, A.; Janas-Kozik, M.; Wilczyński, K.M.; Filipczyk, Ł.; et al. Longitudinal study on novel neuropeptides phoenixin, spexin and kisspeptin in adolescent inpatients with anorexia nervosa—Association with psychiatric symptoms. Nutr. Neurosci. 2021, 24, 896–906. [Google Scholar] [CrossRef]
- Grover, H.M.; Smith, P.M.; Ferguson, A.V. Phoenixin influences the excitability of nucleus of the solitary tract neurones, effects which are modified by environmental and glucocorticoid stress. J. Neuroendocrinol. 2020, 32, e12855. [Google Scholar] [CrossRef]
- Gasparini, S.; Stein, L.M.; Loewen, S.P.; Haddock, C.J.; Soo, J.; Ferguson, A.V.; Kolar, G.R.; Yosten, G.L.C.; Samson, W.K. Novel regulator of vasopressin secretion: Phoenixin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R623–R628. [Google Scholar] [CrossRef]
- Morton, G.J.; Meek, T.H.; Schwartz, M.W. Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 2014, 15, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Woods, S.C.; Porte, D., Jr.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Matafome, P.; Seiça, R. The Role of Brain in Energy Balance. Adv. Neurobiol. 2017, 19, 33–48. [Google Scholar] [CrossRef]
- Rajaei, S.; Zendehdel, M.; Rahnema, M.; Hassanpour, S.; Asle-Rousta, M. Mediatory role of the central NPY, melanocortine and corticotrophin systems on phoenixin-14 induced hyperphagia in neonatal chicken. Gen. Comp. Endocrinol. 2022, 315, 113930. [Google Scholar] [CrossRef]
- Pałasz, A.; Rojczyk, E.; Bogus, K.; Worthington, J.J.; Wiaderkiewicz, R. The novel neuropeptide phoenixin is highly co-expressed with nesfatin-1 in the rat hypothalamus, an immunohistochemical study. Neurosci. Lett. 2015, 592, 17–21. [Google Scholar] [CrossRef]
- Friedrich, T.; Schalla, M.A.; Scharner, S.; Kühne, S.G.; Goebel-Stengel, M.; Kobelt, P.; Rose, M.; Stengel, A. Intracerebroventricular injection of phoenixin alters feeding behavior and activates nesfatin-1 immunoreactive neurons in rats. Brain Res. 2019, 1715, 188–195. [Google Scholar] [CrossRef]
- Goebel-Stengel, M.; Wang, L. Central and peripheral expression and distribution of NUCB2/nesfatin-1. Curr. Pharm. Des. 2013, 19, 6935–6940. [Google Scholar] [CrossRef]
- Schalla, M.A.; Stengel, A. Phoenixin-A Pleiotropic Gut-Brain Peptide. Int. J. Mol. Sci. 2018, 19, 1726. [Google Scholar] [CrossRef] [PubMed]
- Treen, A.K.; Luo, V.; Belsham, D.D. Phoenixin Activates Immortalized GnRH and Kisspeptin Neurons Through the Novel Receptor GPR173. Mol. Endocrinol. 2016, 30, 872–888. [Google Scholar] [CrossRef]
- Stein, L.M.; Tullock, C.W.; Mathews, S.K.; Garcia-Galiano, D.; Elias, C.F.; Samson, W.K.; Yosten, G.L. Hypothalamic action of phoenixin to control reproductive hormone secretion in females: Importance of the orphan G protein-coupled receptor Gpr173. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R489–R496. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; He, Z.; Peng, Y.L.; Jin, W.D.; Wang, Z.; Mu, L.Y.; Chang, M.; Wang, R. Phoenixin-14 enhances memory and mitigates memory impairment induced by Aβ1-42 and scopolamine in mice. Brain Res. 2015, 1629, 298–308. [Google Scholar] [CrossRef]
- Jiang, J.H.; He, Z.; Peng, Y.L.; Jin, W.D.; Mu, J.; Xue, H.X.; Wang, Z.; Chang, M.; Wang, R. Effects of Phoenixin-14 on anxiolytic-like behavior in mice. Behav. Brain Res. 2015, 286, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Schalla, M.; Prinz, P.; Friedrich, T.; Scharner, S.; Kobelt, P.; Goebel-Stengel, M.; Rose, M.; Stengel, A. Phoenixin-14 injected intracerebroventricularly but not intraperitoneally stimulates food intake in rats. Peptides 2017, 96, 53–60. [Google Scholar] [CrossRef]
- Rocca, C.; Scavello, F.; Granieri, M.C.; Pasqua, T.; Amodio, N.; Imbrogno, S.; Gattuso, A.; Mazza, R.; Cerra, M.C.; Angelone, T. Phoenixin-14: Detection and novel physiological implications in cardiac modulation and cardioprotection. Cell. Mol. Life. Sci. 2018, 75, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Aday, S.; Cecchelli, R.; Hallier-Vanuxeem, D.; Dehouck, M.P.; Ferreira, L. Stem Cell-Based Human Blood-Brain Barrier Models for Drug Discovery and Delivery. Trends Biotechnol. 2016, 34, 382–393. [Google Scholar] [CrossRef]
- Lauschke, K.; Frederiksen, L.; Hall, V.J. Paving the Way Toward Complex Blood-Brain Barrier Models Using Pluripotent Stem Cells. Stem Cells Dev. 2017, 26, 857–874. [Google Scholar] [CrossRef]
- Farrington, G.K.; Caram-Salas, N.; Haqqani, A.S.; Brunette, E.; Eldredge, J.; Pepinsky, B.; Antognetti, G.; Baumann, E.; Ding, W.; Garber, E.; et al. A novel platform for engineering blood-brain barrier-crossing bispecific biologics. FASEB J. 2014, 28, 4764–4778. [Google Scholar] [CrossRef]
- Lippmann, E.S.; Azarin, S.M.; Kay, J.E.; Nessler, R.A.; Wilson, H.K.; Al-Ahmad, A.; Palecek, S.P.; Shusta, E.V. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 2012, 30, 783–791. [Google Scholar] [CrossRef]
- Lippmann, E.S.; Al-Ahmad, A.; Azarin, S.M.; Palecek, S.P.; Shusta, E.V. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 2014, 4, 4160. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.K.; Canfield, S.G.; Hjortness, M.K.; Palecek, S.P.; Shusta, E.V. Exploring the effects of cell seeding density on the differentiation of human pluripotent stem cells to brain microvascular endothelial cells. Fluids Barriers CNS 2015, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Schalla, M.A.; Goebel-Stengel, M.; Friedrich, T.; Kühne, S.G.; Kobelt, P.; Rose, M.; Stengel, A. Restraint stress affects circulating NUCB2/nesfatin-1 and phoenixin levels in male rats. Psychoneuroendocrinology 2020, 122, 104906. [Google Scholar] [CrossRef] [PubMed]
- Appelt-Menzel, A.; Cubukova, A.; Günther, K.; Edenhofer, F.; Piontek, J.; Krause, G.; Stüber, T.; Walles, H.; Neuhaus, W.; Metzger, M. Establishment of a Human Blood-Brain Barrier Co-culture Model Mimicking the Neurovascular Unit Using Induced Pluri- and Multipotent Stem Cells. Stem Cell Rep. 2017, 8, 894–906. [Google Scholar] [CrossRef]
- Hawkins, B.T.; Davis, T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005, 57, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Crone, C.; Olesen, S.P. Electrical resistance of brain microvascular endothelium. Brain Res. 1982, 241, 49–55. [Google Scholar] [CrossRef]
- Butt, A.M.; Jones, H.C.; Abbott, N.J. Electrical resistance across the blood-brain barrier in anaesthetized rats: A developmental study. J. Physiol. 1990, 429, 47–62. [Google Scholar] [CrossRef]
- Ma, H.; Su, D.; Wang, Q.; Chong, Z.; Zhu, Q.; He, W.; Wang, W. Phoenixin 14 inhibits ischemia/reperfusion-induced cytotoxicity in microglia. Arch. Biochem. Biophys. 2020, 689, 108411. [Google Scholar] [CrossRef]
- Zandeh-Rahimi, Y.; Panahi, N.; Hesaraki, S.; Shirazi-Beheshtiha, S.H. Protective Effects of Phoenixin-14 Peptide in the Indomethacin-Induced Duodenal Ulcer: An Experimental Study. Int. J. Pept. Res. Ther. 2022, 28, 43. [Google Scholar] [CrossRef]
- Watanabe, D.; Nakagawa, S.; Morofuji, Y.; Tóth, A.E.; Vastag, M.; Aruga, J.; Niwa, M.; Deli, M.A. Characterization of a Primate Blood-Brain Barrier Co-Culture Model Prepared from Primary Brain Endothelial Cells, Pericytes and Astrocytes. Pharmaceutics 2021, 13, 1484. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Bredno, J.; Wendland, M.; Derugin, N.; Ohara, P.; Wintermark, M. High and Low Molecular Weight Fluorescein Isothiocyanate (FITC)-Dextrans to Assess Blood-Brain Barrier Disruption: Technical Considerations. Transl. Stroke Res. 2011, 2, 106–111. [Google Scholar] [CrossRef]
- Guvenc, G.; Altinbas, B.; Kasikci, E.; Ozyurt, E.; Bas, A.; Udum, D.; Niaz, N.; Yalcin, M. Contingent role of phoenixin and nesfatin-1 on secretions of the male reproductive hormones. Andrologia 2019, 51, e13410. [Google Scholar] [CrossRef] [PubMed]
- Ganong, W.F. Circumventricular organs: Definition and role in the regulation of endocrine and autonomic function. Clin. Exp. Pharmacol. Physiol. 2000, 27, 422–427. [Google Scholar] [CrossRef]
- Battaglia, S.; Di Fazio, C.; Vicario, C.M.; Avenanti, A. Neuropharmacological Modulation of N-methyl-D-aspartate, Noradrenaline and Endocannabinoid Receptors in Fear Extinction Learning: Synaptic Transmission and Plasticity. Int. J. Mol. Sci. 2023, 24, 5926. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, H.; Wang, Y. Phoenixin-14 Promotes the Recovery of Neurological Dysfunction After Spinal Cord Injury by Regulating Microglial Polarization via PTEN/Akt Signaling Pathway. Hum. Exp. Toxicol. 2022, 41, 9603271221111345. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, X.; Liu, F.; Zhu, W. Phoenixin-14 Ameliorates Cellular Senescence Against Morphine in M17 Neuronal Cells. Neurotox. Res. 2022, 40, 498–507. [Google Scholar] [CrossRef]
- Tajti, J.; Szok, D.; Csáti, A.; Szabó, Á.; Tanaka, M.; Vécsei, L. Exploring Novel Therapeutic Targets in the Common Pathogenic Factors in Migraine and Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 4114. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, T.; Schalla, M.A.; Lommel, R.; Goebel-Stengel, M.; Kobelt, P.; Rose, M.; Stengel, A. Restraint stress increases the expression of phoenixin immunoreactivity in rat brain nuclei. Brain Res. 2020, 1743, 146904. [Google Scholar] [CrossRef]
- Lin, C.C.; Cheng, P.Y.; Hsiao, M.; Liu, Y.P. Effects of RU486 in Treatment of Traumatic Stress-Induced Glucocorticoid Dysregulation and Fear-Related Abnormalities: Early versus Late Intervention. Int. J. Mol. Sci. 2022, 23, 5494. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Halmi, K.A. Psychological Comorbidity of Eating Disorders. The Oxford Handbook of Eating Disorders; Oxford University Press: Oxford, UK, 2010; Volume 292, p. 303. [Google Scholar]
- Schalla, M.A.; Stengel, A. Pharmacological Modulation of Ghrelin to Induce Weight Loss: Successes and Challenges. Curr. Diabetes Rep. 2019, 19, 102. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schalla, M.A.; Oerter, S.; Cubukova, A.; Metzger, M.; Appelt-Menzel, A.; Stengel, A. Locked Out: Phoenixin-14 Does Not Cross a Stem-Cell-Derived Blood–Brain Barrier Model. Brain Sci. 2023, 13, 980. https://doi.org/10.3390/brainsci13070980
Schalla MA, Oerter S, Cubukova A, Metzger M, Appelt-Menzel A, Stengel A. Locked Out: Phoenixin-14 Does Not Cross a Stem-Cell-Derived Blood–Brain Barrier Model. Brain Sciences. 2023; 13(7):980. https://doi.org/10.3390/brainsci13070980
Chicago/Turabian StyleSchalla, Martha A., Sabrina Oerter, Alevtina Cubukova, Marco Metzger, Antje Appelt-Menzel, and Andreas Stengel. 2023. "Locked Out: Phoenixin-14 Does Not Cross a Stem-Cell-Derived Blood–Brain Barrier Model" Brain Sciences 13, no. 7: 980. https://doi.org/10.3390/brainsci13070980
APA StyleSchalla, M. A., Oerter, S., Cubukova, A., Metzger, M., Appelt-Menzel, A., & Stengel, A. (2023). Locked Out: Phoenixin-14 Does Not Cross a Stem-Cell-Derived Blood–Brain Barrier Model. Brain Sciences, 13(7), 980. https://doi.org/10.3390/brainsci13070980





