Predicting Motor Imagery BCI Performance Based on EEG Microstate Analysis
Abstract
:1. Introduction
- A microstate predictor was proposed to predict the performance of MI-BCI by using resting state data of EEG;
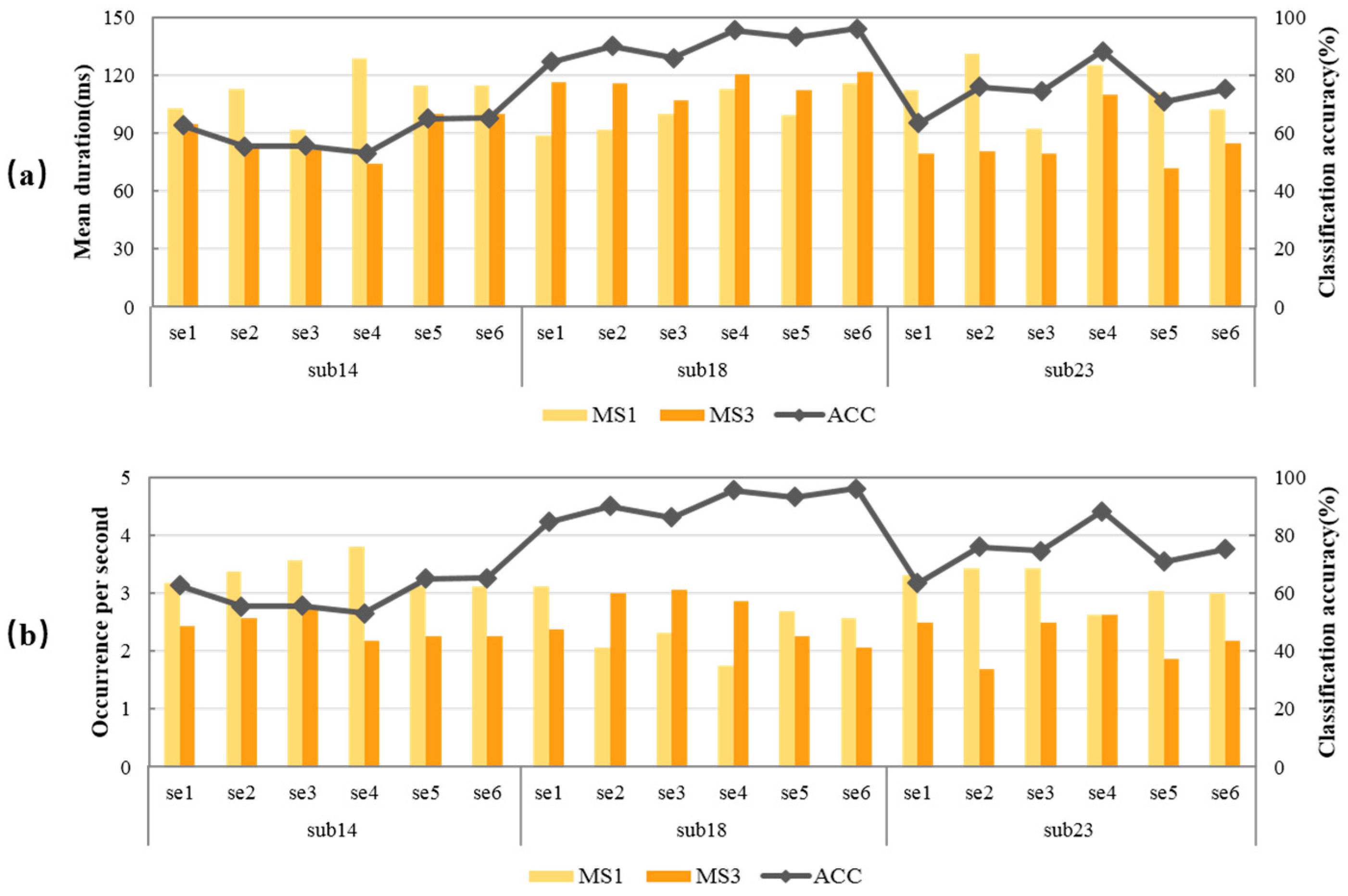
- We found two parameters of microstates that with the occurrence of MS1 and mean duration of MS3 can fit the performance of MI-BCI well;
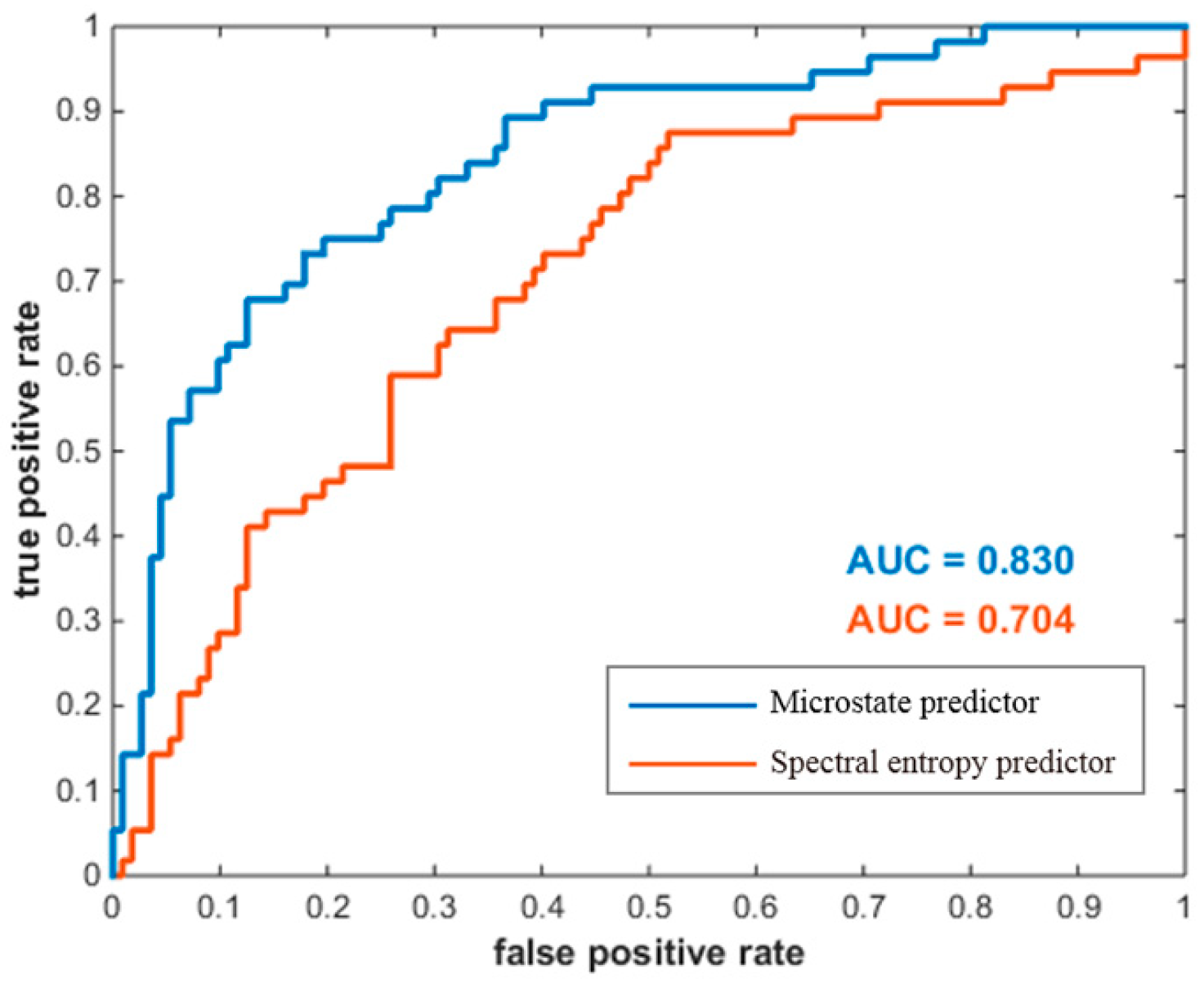
- Our microstate predictor achieved a better prediction performance than the spectral entropy predictor;
- The EEG microstate analysis in this study provides an effective new way to analyze the differences in subjects’ MI-BCI performance.
2. Materials and Methods
2.1. Data Description
2.2. Data Preprocessing
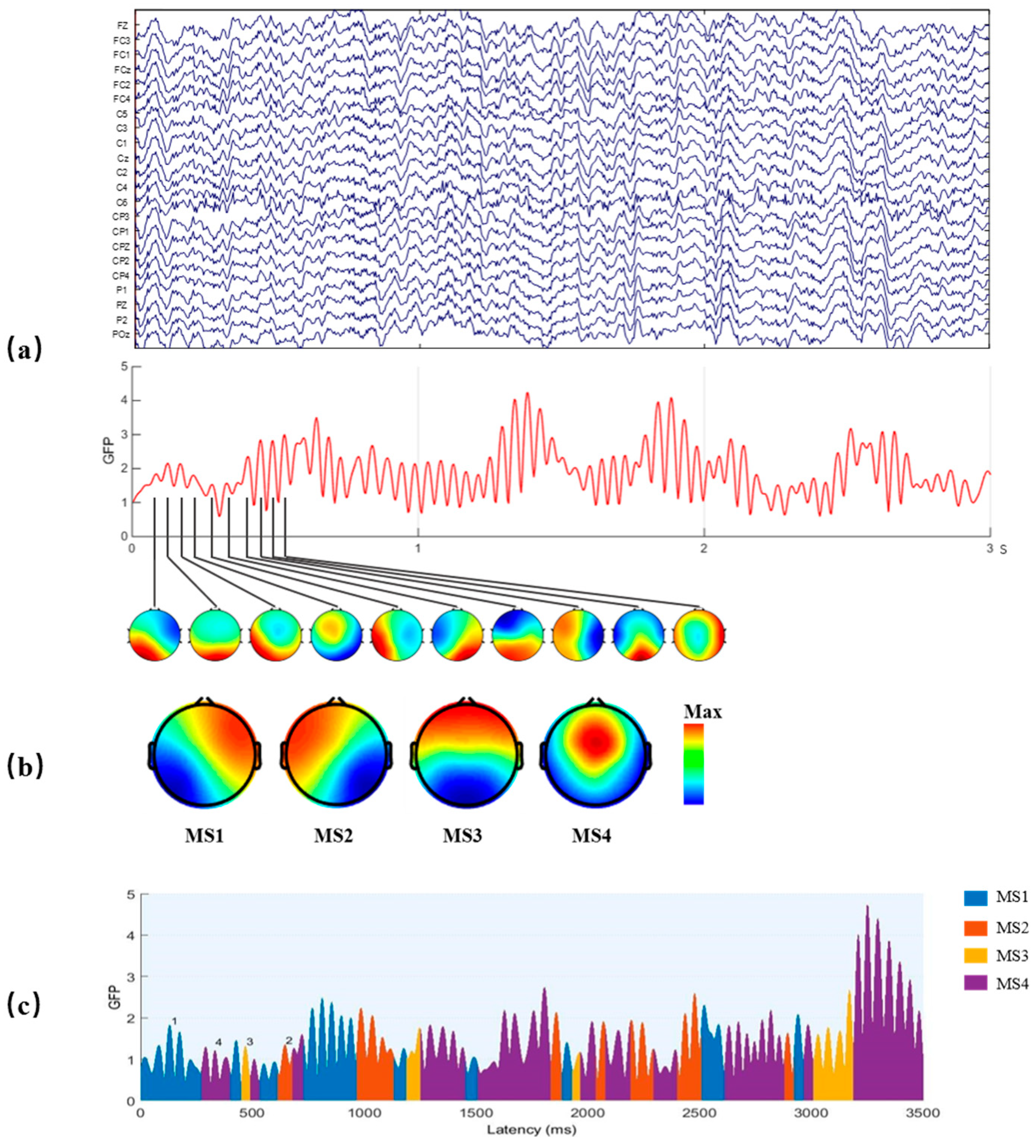
2.3. EEG Microstate Analysis
- Mean duration: the mean length of time that one microstate keeps stable.
- Occurrence per second: the number of times one microstate occurs per second.
- Time coverage ratio: the ratio of the sum of the duration of one microstate to the duration of the EEG signal.
- Transition probability (TP): Percent of transition from one microstate to another.
3. Results
3.1. MI-BCI Performance
3.2. Relationship between Microstate Feature Parameters and Subjects’ MI-BCI Performance
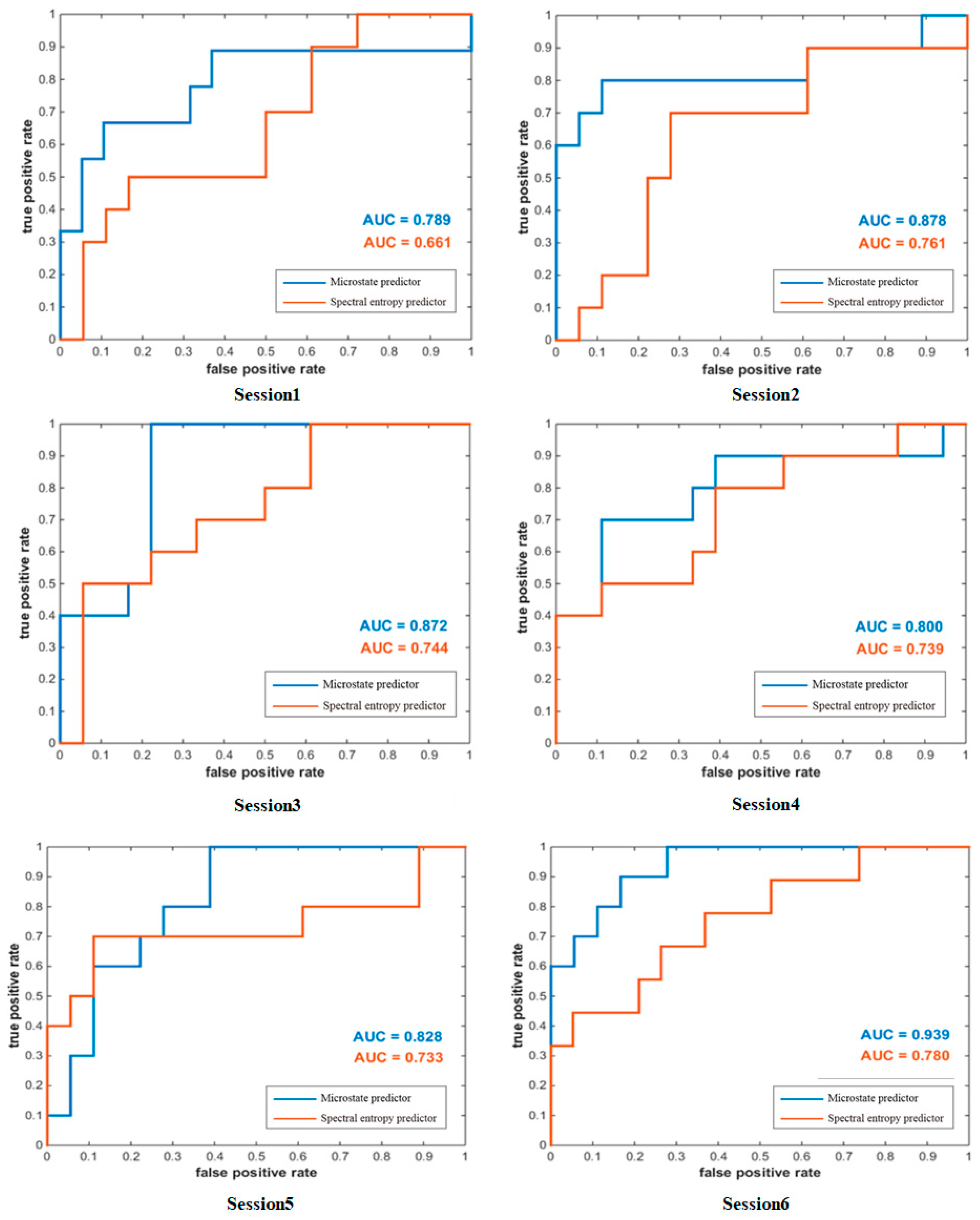
3.3. Microstate Prediction Performance
4. Discussion
4.1. Subjects’ MI-BCI Performance
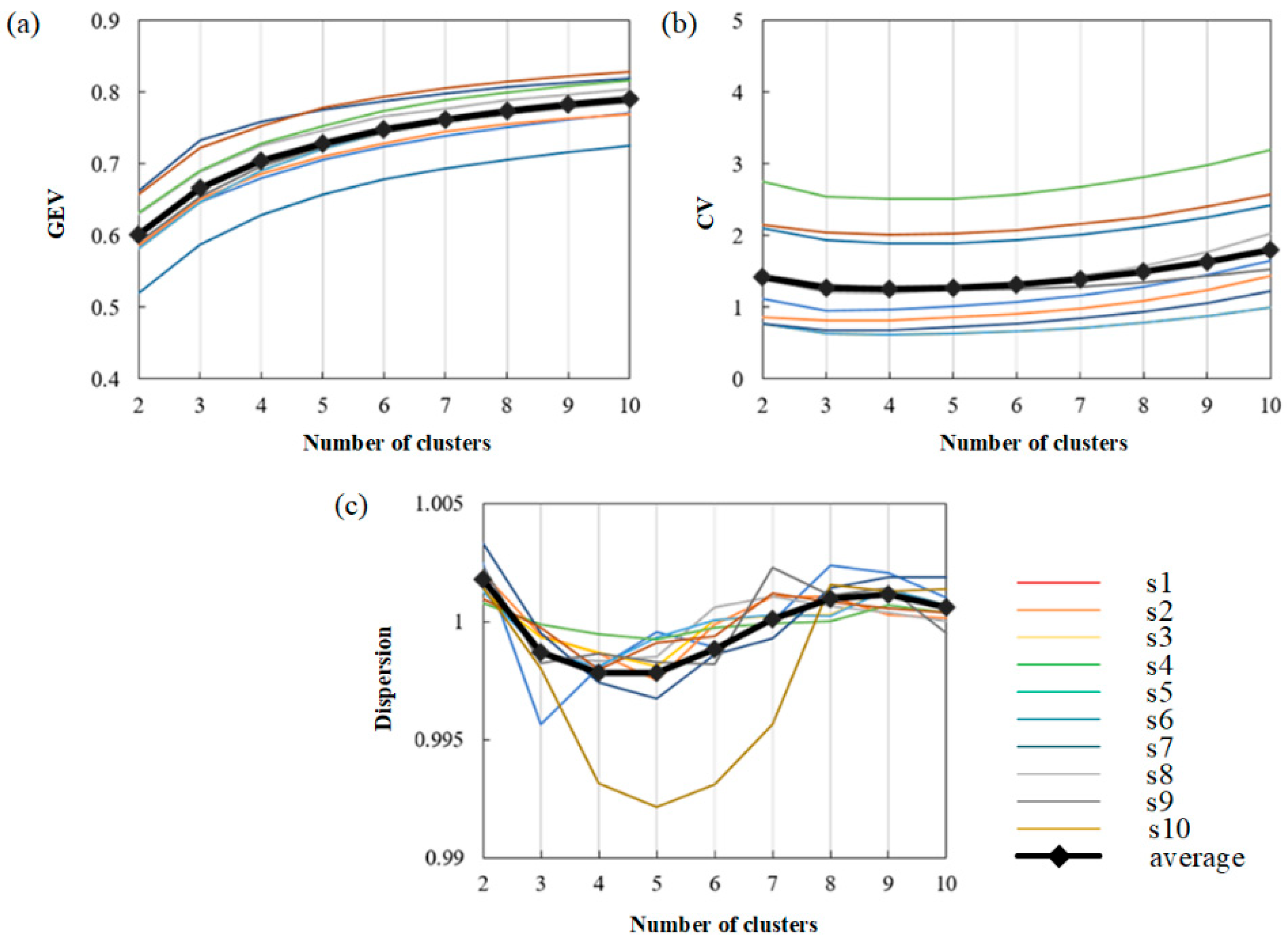
4.2. Number of Microstate Maps
4.3. Microstate Predictor and Spectral Entropy Predictor
4.4. Cross-Session Analysis of the Subject’s MI-BCI Performance Prediction
4.5. Future Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haas, L.F. Hans Berger (1873–1941), Richard Caton (1842–1926), and electroencephalography. J. Neurol. Neurosurg. Psychiatry 2003, 74, 9. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.K.M.; Joadder, M.A.M. A Review on the Components of EEG-based Motor Imagery Classification with Quantitative Comparison. Appl. Theory Comput. Technol. 2017, 2, 1. [Google Scholar] [CrossRef]
- Vogt, S.; Di Rienzo, F.; Collet, C.; Collins, A.; Guillot, A. Multiple roles of motor imagery during action observation. Front. Hum. Neurosci. 2013, 7, 807. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Xie, S.; Xie, X.; Obermayer, K.; Cui, Y.; Wang, Z. An Online Data Visualization Feedback Protocol for Motor Imagery-Based BCI Training. Front. Hum. Neurosci. 2021, 15, 625983. [Google Scholar] [CrossRef] [PubMed]
- Idowu, O.P.; Adelopo, O.; Ilesanmi, A.E.; Li, X.; Samuel, O.W.; Fang, P.; Li, G. Neuro-evolutionary approach for optimal selection of EEG channels in motor imagery based BCI application. Biomed. Signal Proces. 2021, 68, 102621. [Google Scholar] [CrossRef]
- Padfield, V.R.J. EEG-Based Brain-Computer Interfaces Using Motor-Imagery: Techniques and Challenges. Nat. Rev. Cancer 2019, 19, 1423. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Jun, S.C. Performance variation in motor imagery brain-computer interface: A brief review. J. Neurosci. Meth. 2015, 243, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Neuper, C.; Schlogl, A.; Pfurtscheller, G. Enhancement of left-right sensorimotor EEG differences during feedback-regulated motor imagery. J. Clin. Neurophysiol. 1999, 16, 373–382. [Google Scholar] [CrossRef]
- Hamedi, M.; Salleh, S.; Noor, A.M. Electroencephalographic Motor Imagery Brain Connectivity Analysis for BCI: A Review. Neural Comput. 2016, 28, 999–1041. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.C. Critiquing the Concept of BCI Illiteracy. Sci. Eng. Ethics 2019, 25, 1217–1233. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Sheng, X.; Zhang, D.; Jiang, N.; Mrachacz-Kersting, N.; Zhu, X.; Farina, D. A Stimulus-Independent Hybrid BCI Based on Motor Imagery and Somatosensory Attentional Orientation. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1674–1682. [Google Scholar] [CrossRef]
- Volosyak, I.; Rezeika, A.; Benda, M.; Gembler, F.; Stawicki, P. Towards solving of the Illiteracy phenomenon for VEP-based brain-computer interfaces. Biomed. Phys. Eng. Express 2020, 6, 035034. [Google Scholar] [CrossRef]
- Yuan, H.; He, B. Brain–Computer Interfaces Using Sensorimotor Rhythms: Current State and Future Perspectives. IEEE Trans. Biomed. Eng. 2014, 61, 1425–1435. [Google Scholar] [CrossRef]
- Blankertz, B.; Sannelli, C.; Halder, S.; Hammer, E.M.; Kübler, A.; Müller, K.; Curio, G.; Dickhaus, T. Neurophysiological predictor of SMR-based BCI performance. Neuroimage 2010, 51, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Minkyu, A.; Hohyun, C.; Sangtae, A.; Chan, J.S.; Dewen, H. High Theta and Low Alpha Powers May Be Indicative of BCI-Illiteracy in Motor Imagery. PLoS ONE 2013, 8, e80886. [Google Scholar]
- Zhang, R.; Xu, P.; Chen, R.; Li, F.; Guo, L.; Li, P.; Zhang, T.; Yao, D. Predicting Inter-session Performance of SMR-Based Brain-Computer Interface Using the Spectral Entropy of Resting-State EEG. Brain Topogr. 2015, 28, 680–690. [Google Scholar] [CrossRef]
- Lee, M.; Yoon, J.; Lee, S. Predicting Motor Imagery Performance from Resting-State EEG Using Dynamic Causal Modeling. Front. Hum. Neurosci. 2020, 14, 321. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, K.; Shah, R.; Daulat, S.R.; Moodley, V.; Yao, Y.; Sengupta, P.; Ramu, V.; Madathil, D. Evaluation of EEG Oscillatory Patterns and Classification of Compound Limb Tactile Imagery. Brain Sci. 2023, 13, 656. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Mora, D.A.; Aldridge, A.; Jorge, C.; Vourvopoulos, A.; Figueiredo, P.; Badia, S.B.I. Impact of age, VR, immersion, and spatial resolution on classifier performance for a MI-based BCI. Brain Comput. Interfaces 2022, 9, 169–178. [Google Scholar] [CrossRef]
- Tarailis, P.; Koenig, T.; Michel, C.M.; Griškova-Bulanova, I. The Functional Aspects of Resting EEG Microstates: A Systematic Review. Brain Topogr. 2023. [Google Scholar] [CrossRef]
- Milz, P.; Pascual-Marqui, R.D.; Achermann, P.; Kochi, K.; Faber, P.L. The EEG microstate topography is predominantly determined by intracortical sources in the alpha band. Neuroimage 2017, 162, 353–361. [Google Scholar] [CrossRef]
- Khanna, A.; Pascual-Leone, A.; Michel, C.M.; Farzan, F. Microstates in resting-state EEG: Current status and future directions. Neurosci. Biobehav. R. 2015, 49, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Englitz, B.; Cohen, M.X. EEG microstates as a continuous phenomenon. Neuroimage 2020, 208, 116454. [Google Scholar] [CrossRef]
- Xu, J.; Pan, Y.; Zhou, S.Q.; Zou, G.Y.; Liu, J.Y.; Su, Z.H.; Zou, Q.H.; Gao, J.H. EEG microstates are correlated with brain functional networks during slow-wave sleep. Neuroimage 2020, 215, 116786. [Google Scholar] [CrossRef] [PubMed]
- Britz, J.; Van De Ville, D.; Michel, C.M. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage 2010, 52, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Jatupornpoonsub, T.; Thimachai, P.; Supasyndh, O.; Wongsawat, Y. EEG Delta/Theta Ratio and Microstate Analysis Originating Novel Biomarkers for Malnutrition-Inflammation Complex Syndrome in ESRD Patients. Front. Hum. Neurosci. 2022, 15, 795237. [Google Scholar] [CrossRef]
- May, E.S.; Avila, C.G.; Dinh, S.T.; Heitmann, H.; Hohn, V.D.; Nickel, M.M.; Tiemann, L.; Tolle, T.R.; Ploner, M. Dynamics of brain function in patients with chronic pain assessed by microstate analysis of resting-state electroencephalography. Pain 2021, 162, 2894–2908. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Wang, X.; Cai, L.; Zhang, L.; Wang, J.; Liu, C.; Zhu, X. Spatiotemporal EEG microstate analysis in drug-free patients with Parkinson’s disease. Neuroimage Clin. 2020, 25, 102132. [Google Scholar] [CrossRef]
- Kikuchi, M.; Koenig, T.; Munesue, T.; Hanaoka, A.; Strik, W.; Dierks, T.; Koshino, Y.; Minabe, Y. EEG Microstate Analysis in Drug-Naive Patients with Panic Disorder. PLoS ONE 2011, 6, e22912. [Google Scholar] [CrossRef]
- Bell, A.J. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1994, 7, 1129. [Google Scholar] [CrossRef]
- Michalopoulos, K.; Zervakis, M.; Deiber, M.; Bourbakis, N. Classification of EEG Single Trial Microstates Using Local Global Graphs and Discrete Hidden Markov Models. Int. J. Neural Syst. 2016, 26, 1650036. [Google Scholar] [CrossRef] [PubMed]
- Pascualmarqui, R.D.; Michel, C.M.; Lehmann, D. Segmentation of brain electrical-activity into microstates—Model estimation and validation. IEEE Trans. Biomed. Eng. 1995, 42, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Ramoser, H.; Muller-Gerking, J.; Pfurtscheller, G. Optimal spatial filtering of single trial EEG during imagined hand movement. IEEE Trans. Rehabil. Eng. 2000, 8, 441–446. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Sawan, M. Exploring the Role of Visual Guidance in Motor Imagery-Based Brain-Computer Interface: An EEG Microstate-Specific Functional Connectivity Study. Bioengineering 2023, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, K.; Xu, M.P.; Yi, W.B.; Xu, F.Z.; Ming, D. Transformed common spatial pattern for motor imagery-based brain-computer interfaces. Front. Neurosci. 2023, 17, 1116721. [Google Scholar] [CrossRef] [PubMed]



| Parameters | MS1 | MS2 | MS3 | MS4 | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Duration | −0.388 | 0.025 * | −0.036 | 0.482 | 0.593 | <0.001 ** | −0.057 | 0.464 |
| Occurrence | −0.544 | <0.001 ** | −0.022 | 0.575 | 0.263 | 0.032 * | −0.035 | 0.483 |
| Coverage | −0.141 | 0.067 | −0.093 | 0.240 | 0.176 | 0.053 | −0.071 | 0.355 |
| TP1 | / | / | −0.204 | 0.041 * | −0.055 | 0.474 | −0.090 | 0.245 |
| TP2 | 0.004 | 0.961 | / | / | −0.004 | 0.962 | −0.218 | 0.039 * |
| TP3 | 0.146 | 0.059 | 0.495 | <0.001 ** | / | / | 0.463 | <0.001 ** |
| TP4 | −0.143 | 0.064 | −0.077 | 0.324 | 0.057 | 0.464 | / | / |
| High Group | Low Group | |
|---|---|---|
| Session 1 | 7 | 21 |
| Session 2 | 10 | 18 |
| Session 3 | 9 | 19 |
| Session 4 | 10 | 18 |
| Session 5 | 11 | 17 |
| Session 6 | 11 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Xie, S.; Fu, Y.; Xie, X. Predicting Motor Imagery BCI Performance Based on EEG Microstate Analysis. Brain Sci. 2023, 13, 1288. https://doi.org/10.3390/brainsci13091288
Cui Y, Xie S, Fu Y, Xie X. Predicting Motor Imagery BCI Performance Based on EEG Microstate Analysis. Brain Sciences. 2023; 13(9):1288. https://doi.org/10.3390/brainsci13091288
Chicago/Turabian StyleCui, Yujie, Songyun Xie, Yingxin Fu, and Xinzhou Xie. 2023. "Predicting Motor Imagery BCI Performance Based on EEG Microstate Analysis" Brain Sciences 13, no. 9: 1288. https://doi.org/10.3390/brainsci13091288





