Strain in the Midbrain: Impact of Traumatic Brain Injury on the Central Serotonin System
Abstract
:1. Introduction
2. Materials and Methods
Search Strategies
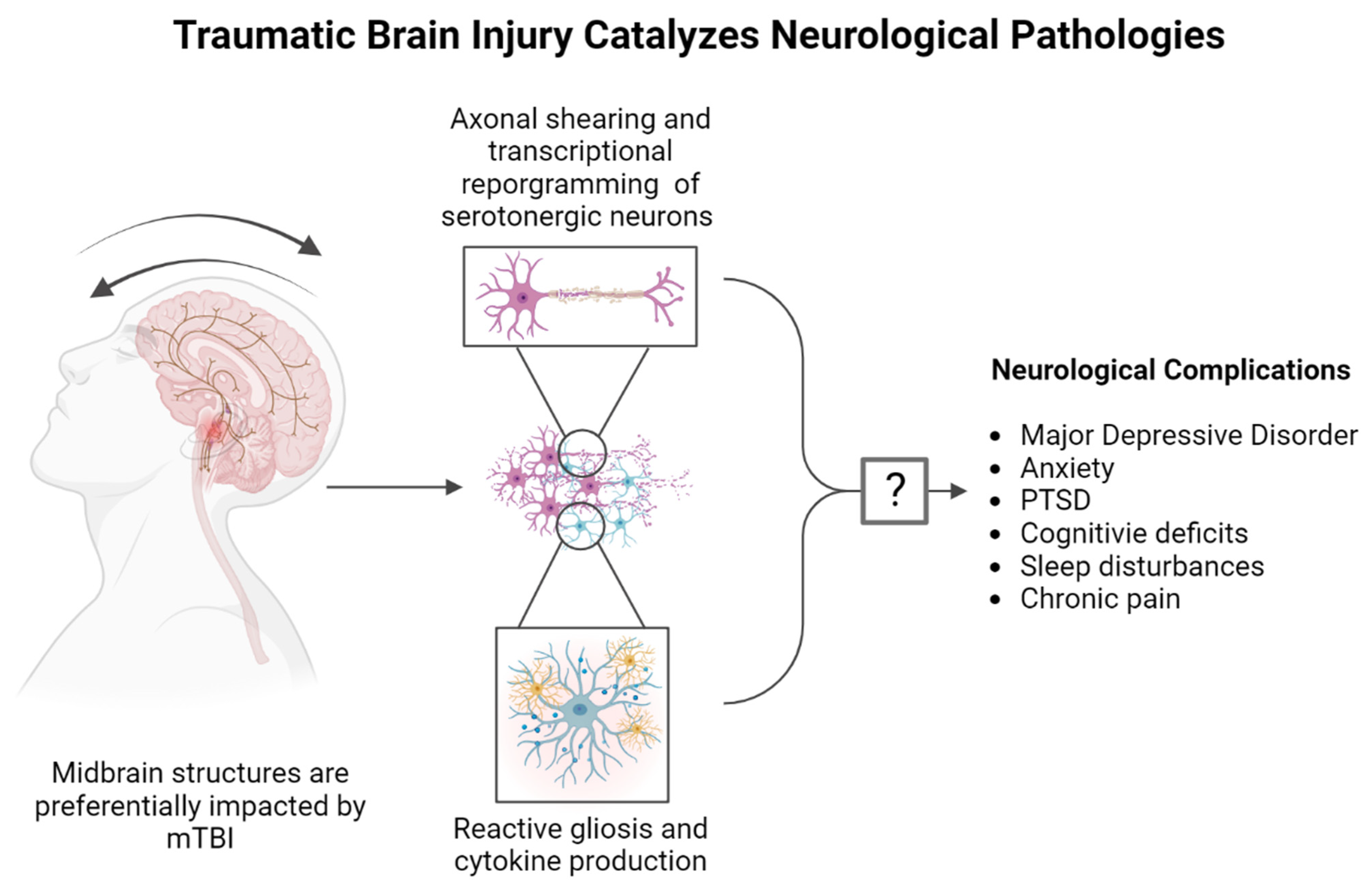
3. Traumatic Brain Injury and Psychiatric Disease
TBI and Mental Health Statistics
4. The 5-HT System and TBI
4.1. History of 5-HT in Traumatic Brain Injuries
4.2. 5-HT Metabolism and TBI
4.3. 5-HT Receptor Pharmacology in TBI-Elicited Behavioral and Nociceptive Pathologies
| Receptor | Species | Study Design | Model|Severity | Pharmacology | Behavioral Outcomes|Measured Effects |
|---|---|---|---|---|---|
| 5-HT1A | Mouse, NMRI | Male (10–11 weeks) N = 10/group | Mild TBI Weight-drop model | Agonism, 8-OH-DPAT Antagonism, WAY-100635 | Subthreshold administration of 8-OH-DPAT improved performance in forced swim, sucrose preference, and tail suspension test, whereas 5-HT1A blockade increased depression-like phenotypes [90]. |
| 5-HT1B | Mouse, C57Bl/6 | Male (8 weeks) N = 6–12/group | Repetitive mild TBI CHIMERA model | Androgenic anabolic steroids | Receptor-specific pharmacology not evaluated in the context of TBI-elicited behavioral changes, though receptor expression was reduced in response to anabolic steroids irrespective of TBI treatment [91]. |
| 5-HT1D | Rat, Sprague-Dawley | Male, N = 128 | Mild TBI Weight-drop model | Agonism, Triptans | Acute sumatriptan treatment attenuated cephalic tactile hypersensitivity at 72 h post-injury and elicited conditioned place preference in injured subjects 7 days post injury [92]. |
| 5-HT2A | Mouse, C57Bl/6 | Male (9–16 weeks) N = 10–12/group | Mild TBI Blast model of mTBI | Agonism, DOI Antagonism, M100907 | Decreases in social preference and social dominance phenotypes measured by 3-chamber test and tube test, respectively, were attenuated by progressive DOI administration post injury [79]. |
| 5-HT2B | Mouse, transgenic | Male (7–9 weeks) N = 3–14/group | - | Agonism, Fluoxetine Antagonism, RS127445 | Not evaluated in the context of TBI-elicited behavioral changes, though receptor activation may be required for antidepressant mechanisms of SSRIs in MDD [81]. |
| 5-HT2C | Rat, Sprague Dawley | Male and Female N = 90 | Surgical craniotomy | Agonism, Agomelatine | Not evaluated in the context of TBI-elicited behavioral changes, though receptor activation acutely reduced locomotor deficits following surgical craniotomy [83]. |
| 5-HT3 | Rat, Sprague Dawley | Male N = 6–8/group | Formalin and carrageenan pain model | Antagonism, SB-269970 | Not evaluated in the context of TBI-elicited behavioral changes, though receptor blockade may attenuate nociception associated with neurotrauma [87]. |
| 5-HT4 | Mouse, C57Bl/6 | Male, (7–8 weeks) N = 7–8/group | - | Agonism, Prucalopride and velusetrag | Not evaluated in the context of TBI-elicited behavioral changes, though receptor activation by agonists prucalopride and velusetrag improved facilitation of contextual fear extinction in a preclinical model of Parkinson’s disease [93]. |
| 5-HT5A | Rat, Wistar | Female (6–7 weeks) N = 6/group | Nerve ligation pain model | Antagonism, Methiothepin and SB-69951 | Not evaluated in the context of TBI-elicited behavioral changes, though receptor blockade reduced neuropathic antiallodynic effect of 5-HT dosing [94]. |
| 5-HT6 | Rat, Sprague Dawley | Male (6–7 weeks) N = 92 | Moderate TBI Controlled cortical impact model | Agonism, WAY-181187 | Impairments in spatial reference memory measured by the Morris water maze were alleviated following WAY-181187 administration [95] |
| 5-HT7 | Rat, Sprague Dawley | Male N = 6–8/group | Formalin and carrageenan pain model | Agonism, AS-19 | Not evaluated in the context of TBI-elicited behavioral changes, though receptor activation may attenuate nociception associated with neurotrauma [87]. |
| Receptor | Study Design | Condition|Disorder | Pharmacology | Behavioral Outcomes|Pharmacological Effects |
|---|---|---|---|---|
| 5-HT1A | Blinded 60 patients enrolled | Severe TBI | Agonism, Repinotan | Proportion of patients having good outcome was slightly greater in repinotan-treated patients (60%) than in controls (50%) measured by the Glasgow coma scale [70]. A greater proportion of severe TBI patients who received repinotan treatment scored more highly on measures of responsivity to commands, including eye opening, orientated verbal responses to questions, and obeying motor movement commands. |
| 5-HT1B | Male and Female 100 individuals | Mild TBI Post-traumatic headache | Agonism, Triptans | Not evaluated in the context of TBI-elicited behavioral changes, though receptor agonism may mitigate post-traumatic headaches [96]. Seventy percent of individuals treated with triptan-lass medications experienced reliable headache relief compared to 42% that were administered alternative headache-abortive medications. |
| 5-HT1D | Male and Female 100 individuals | Mild TBI Post-traumatic headache | Agonism, Triptans | Not evaluated in the context of TBI-elicited behavioral changes, though receptor agonism may mitigate post-traumatic headaches [96]. Seventy percent of individuals treated with triptan-class medications experienced reliable headache relief compared to 42% that were administered alternative headache-abortive medications. |
| 5-HT2A | Male Middle aged 35 patients enrolled | Mild to Moderate TBI | - | Not evaluated in the context of TBI-elicited behavioral changes, though 2/3rds of TBI patients harbored 5-HT2A autoantibodies, the concentrations of which were correlated with other neurological comorbidities, including Parkinsons’ disease, other dementias, and painful neuropathies [97]. |
| 5-HT2B | - | Migraine | Antagonism, methysergide, cyproheptadine, pizotifen | Not evaluated in the context of TBI-elicited behavioral changes, though antagonism of the receptor may be prophylactic in the treatment of migraines, whereas other 5-HT2 receptor family antagonists like ketanserin do not exert an anti-headache effect [98]. |
| 5-HT2C | Male and Female 11 individuals | Depression | - | Not evaluated in the context of TBI-elicited behavioral changes, though increased RNA-editing of the transcripts encoding the receptor have been observed in individuals with major depression [99]. Individuals with major depressive disorder exhibited significantly increased expression of the AC’C-edited mRNA and a significantly decreased expression of the A-edited isoform compared to non-depressed individuals. |
| 5-HT3 | Male and Female, 321 patients enrolled | Substance abuse | Antagonist, ondansetron | Not evaluated in the context of TBI-elicited behavioral changes, though antagonism of the receptor in patients with early-onset alcoholism led to less alcohol consumption as compared to placebo by ameliorating underlying serotonergic abnormalities [100]. |
| 5-HT4 | Male and Female 38 individuals | - | Antagonism | Not evaluated in the context of TBI-elicited behavioral changes, though receptor levels were increased in the frontal cortex and caudate nucleus of depressed victims of suicide compared to controls [101]. |
| 5-HT5A | Male and Female 502 individuals | Schizophrenia | - | Not evaluated in the context of TBI-elicited behavioral changes, though the Pro15Ser substitution in the receptor is highly associated with the development or progression of schizophrenia symptoms [102]. |
| 5-HT6 | Male and Female | Alzheimer’s Disease | Antagonism, Latrepirdine, idalopirdine, interpirdine, donepezil | Not evaluated in the context of TBI-elicited behavioral changes, though the effects of pharmacological modulation of the receptor on primary cognitive endpoints associated with Alzheimer’s disease have been evaluated in Phase III clinical trials [103]. All trials have failed to demonstrate clinical efficacy and slow progression of dementia symptoms. |
| 5-HT7 | Male and Female 137 individuals | Schizophrenia and Bipolar affective disorder | - | Not evaluated in the context of TBI-elicited behavioral changes, though two naturally occurring receptor variants were investigated in individuals with schizophrenia and bipolar affective disorder [104], with the data not supporting a role for the receptor in either disorder. |
4.4. SERT, Cessation of 5-HT Signaling, and SSRIs
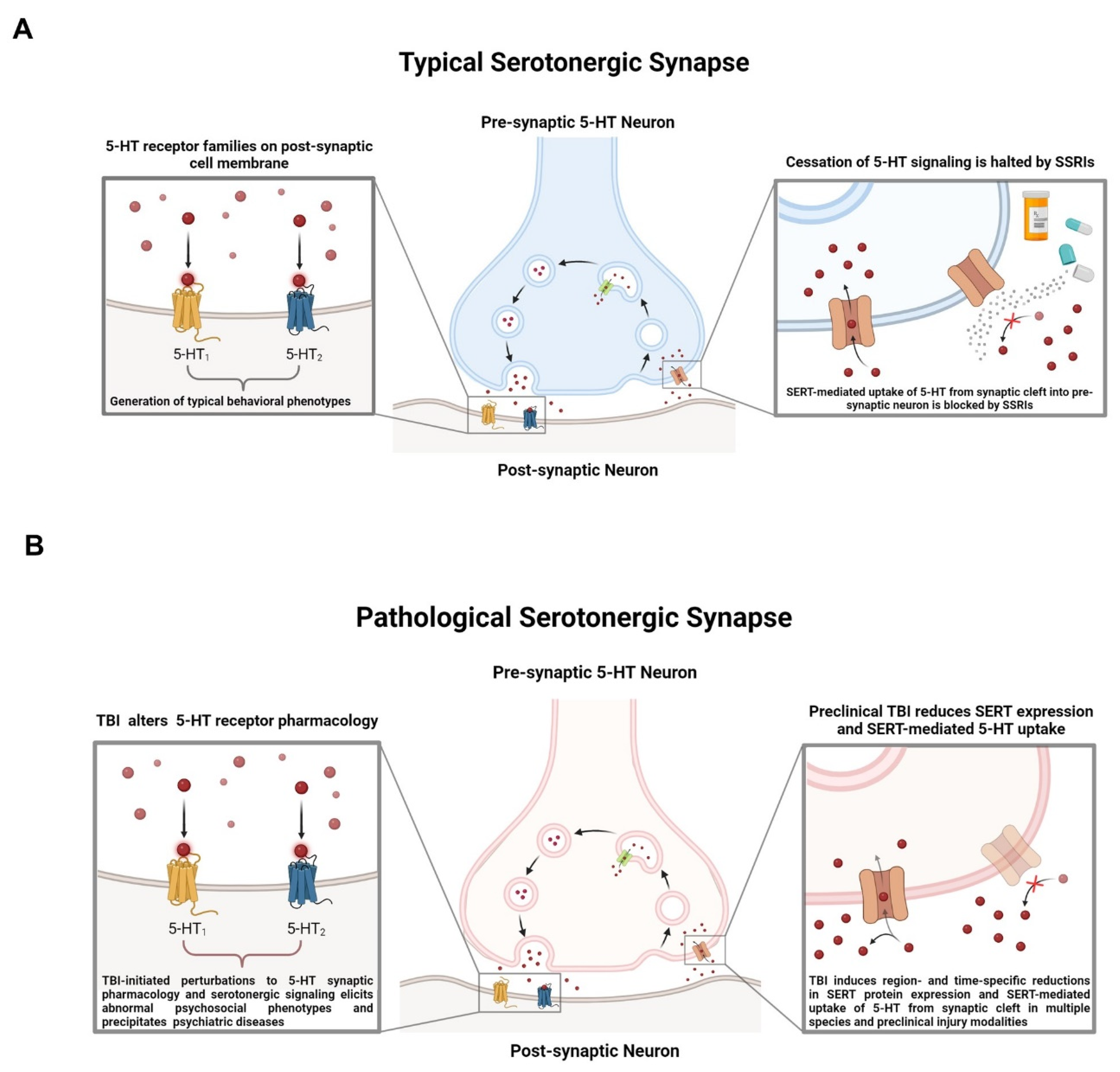
5. Emerging Complexity of the 5-HT System
5.1. Complexity of 5-HT Neuron Architectures and Relevance to Traumatic Brain Injury
5.2. Anatomy, Hodology, and Function of Ascending and Descending 5-HT Neuron Architectures
5.3. 5-HT Neurons Organize into Transcriptionally Distinct Subpopulations
5.4. Therapeutic Perspectives
6. Conclusions and Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. TBI Data and Statistics|Concussion|Traumatic Brain Injury|CDC Injury Center. 2014. Available online: https://cdc.gov/traumaticbraininjury/data/ (accessed on 15 September 2023).
- Kumar, A.; Loane, D.J. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav. Immun. 2012, 26, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Multiple Cause of Death by Single Race 2018–2019; CDC WONDER: Atlanta, GA, USA, 2018–2019.
- Emery, C.A.; Barlow, K.M.; Brooks, B.L.; Max, J.E.; Villavicencio-Requis, A.; Gnanakumar, V.; Robertson, H.L.; Schneider, K.; Yeates, K.O. A Systematic Review of Psychiatric, Psychological, and Behavioural Outcomes following Mild Traumatic Brain Injury in Children and Adolescents. Can. J. Psychiatry 2016, 61, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Ledreux, A.; Pryhoda, M.K.; Gorgens, K.; Shelburne, K.; Gilmore, A.; Linseman, D.A.; Fleming, H.; Koza, L.A.; Campbell, J.; Wolff, A.; et al. Assessment of Long-Term Effects of Sports-Related Concussions: Biological Mechanisms and Exosomal Biomarkers. Front. Neurosci. 2020, 14, 761. [Google Scholar] [CrossRef]
- Meaney, D.F.; Smith, D.H. Biomechanics of concussion. Clin. Sports Med. 2011, 30, 19-vii. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yoganandan, N.; Pintar, F.A.; Gennarelli, T.A. Role of translational and rotational accelerations on brain strain in lateral head impact. Biomed. Sci. Instrum. 2006, 42, 501–506. [Google Scholar] [PubMed]
- Yoganandan, N.; Li, J.; Zhang, J.; Pintar, F.A.; Gennarelli, T.A. Influence of angular acceleration-deceleration pulse shapes on regional brain strains. J. Biomech. 2008, 41, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Ommaya, A.K.; Gennarelli, T.A. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain A J. Neurol. 1974, 97, 633–654. [Google Scholar] [CrossRef]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef]
- Kelly, J.G.; Hawken, M.J. Quantification of neuronal density across cortical depth using automated 3D analysis of confocal image stacks. Brain Struct. Funct. 2017, 222, 3333–3353. [Google Scholar] [CrossRef]
- Smith, D.H.; Nonaka, M.; Miller, R.; Leoni, M.; Chen, X.H.; Alsop, D.; Meaney, D.F. Immediate coma following inertial brain injury dependent on axonal damage in the brainstem. J. Neurosurg. 2000, 93, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.E.; Turner, E.C.; Sawyer, E.K.; Reed, J.L.; Young, N.A.; Flaherty, D.K.; Kaas, J.H. Cortical cell and neuron density estimates in one chimpanzee hemisphere. Proc. Natl. Acad. Sci. USA 2016, 113, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Hirad, A.A.; Bazarian, J.J.; Merchant-Borna, K.; Garcea, F.E.; Heilbronner, S.; Paul, D.; Hintz, E.B.; van Wijngaarden, E.; Schifitto, G.; Wright, D.W.; et al. A common neural signature of brain injury in concussion and subconcussion. Sci. Adv. 2019, 5, eaau3460. [Google Scholar] [CrossRef] [PubMed]
- Barrio, J.R.; Small, G.W.; Wong, K.P.; Huang, S.C.; Liu, J.; Merrill, D.A.; Giza, C.C.; Fitzsimmons, R.P.; Omalu, B.; Bailes, J.; et al. In vivo characterization of chronic traumatic encephalopathy using [F-18]FDDNP PET brain imaging. Proc. Natl. Acad. Sci. USA 2015, 112, E2039–E2047. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, J.; Yano, H.; Nakayama, N. Altered biphasic serotonin discharge hypothesis in mild traumatic brain injury. Concussion 2021, 6, Cnc94. [Google Scholar]
- Khong, E.; Odenwald, N.; Hashim, E.; Cusimano, M.D. Diffusion Tensor Imaging Findings in Post-Concussion Syndrome Patients after Mild Traumatic Brain Injury: A Systematic Review. Front. Neurol. 2016, 7, 156. [Google Scholar] [CrossRef]
- Fann, J.R.; Hart, T.; Schomer, K.G. Treatment for depression after traumatic brain injury: A systematic review. J. Neurotrauma 2009, 26, 2383–2402. [Google Scholar] [CrossRef]
- Komura, A.; Kawasaki, T.; Yamada, Y.; Uzuyama, S.; Asano, Y.; Shinoda, J. Cerebral Glucose Metabolism in Patients with Chronic Mental and Cognitive Sequelae after a Single Blunt Mild Traumatic Brain Injury without Visible Brain Lesions. J. Neurotrauma 2019, 36, 641–649. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Rosenthal, M.E.; Bond, M.R.; Griffith, E.R.; Miller, J. Rehabilitation of the Adult and Child with Traumatic Brain Injury; FA Davis: Philadelphia, PA, USA, 1990. [Google Scholar]
- Hibbard, M.R.; Uysal, S.; Kepler, K.; Bogdany, J.; Silver, J. Axis I psychopathology in individuals with traumatic brain injury. J. Head Trauma Rehabil. 1998, 13, 24–39. [Google Scholar] [CrossRef]
- Kreutzer, J.S.; Marwitz, J.H.; Walker, W.; Sander, A.; Sherer, M.; Bogner, J.; Fraser, R.; Bushnik, T. Moderating factors in return to work and job stability after traumatic brain injury. J. Head Trauma Rehabil. 2003, 18, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Sander, A.M.; Krentzer, J.S.; Rosenthal, M.; Delmonico, R.; Young, M.E. A multicenter longitudinal investigation of return to work and community integration following traumatic brain injury. J. Head Trauma Rehabil. 1996, 11, 70–84. [Google Scholar] [CrossRef]
- Seel, R.T.; Kreutzer, J.S. Depression assessment after traumatic brain injury: An empirically based classification method. Arch. Phys. Med. Rehabil. 2003, 84, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Bombardier, C.H.; Fann, J.R.; Temkin, N.R.; Esselman, P.C.; Barber, J.; Dikmen, S.S. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 2010, 303, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, W.H. Sequelae of concussion caused by minor head injuries. Lancet 1977, 1, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Schoenhuber, R.; Gentilini, M. Anxiety and depression after mild head injury: A case control study. J. Neurol. Neurosurg. Psychiatry 1988, 51, 722–724. [Google Scholar] [CrossRef]
- Fann, J.; Bombardier, C.; Temkin, N.; Esselman, P.; Pelzer, E.; Keough, M.; Romero, H.; Dikmen, S. Incidence, severity, and phenomenology of depression and anxiety in patients with moderate to severe traumatic brain injury. In Psychosomatics; AMER Psychiatric Press: Washington, DC, USA, 2003. [Google Scholar]
- Jorge, R.E.; Robinson, R.G.; Arndt, S.V.; Starkstein, S.E.; Forrester, A.W.; Geisler, F. Depression following traumatic brain injury: A 1 year longitudinal study. J. Affect. Disord. 1993, 27, 233–243. [Google Scholar] [CrossRef]
- Jorge, R.E.; Robinson, R.G.; Moser, D.; Tateno, A.; Crespo-Facorro, B.; Arndt, S. Major depression following traumatic brain injury. Arch. Gen. Psychiatry 2004, 61, 42–50. [Google Scholar] [CrossRef]
- Fann, J.R.; Burington, B.; Leonetti, A.; Jaffe, K.; Katon, W.J.; Thompson, R.S. Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Arch. Gen. Psychiatry 2004, 61, 53–61. [Google Scholar] [CrossRef]
- Hoge, C.W.; McGurk, D.; Thomas, J.L.; Cox, A.L.; Engel, C.C.; Castro, C.A. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 2008, 358, 453–463. [Google Scholar] [CrossRef]
- Vialli, M.V. Richerche sul secreto delle cellule enterocromaffini IX Intorno alla natura chimica della sostanza specifica. Boll. Soc. Med.-Chir. Pavia. 1937, 51, 1111–1116. [Google Scholar]
- Ni, W.; Watts, S.W. 5-hydroxytryptamine in the cardiovascular system: Focus on the serotonin transporter (SERT). Clin. Exp. Pharmacol. Physiol. 2006, 33, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.M.; Cook, E.H.; Murphy, D.L.; Blakely, R.D. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J. Clin. Investig. 2008, 118, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.; Piper, C.; Vogt, J.; Heintze, J.; Butz, T.; Lindner, O.; Burchert, W.; Kersting, C.; Horstkotte, D. Atrial fibrillation in carcinoid heart disease: The role of serotonin. A review of the literature. Clin. Res. Cardiol. 2007, 96, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Trials of new antiarrhythmic drugs for maintenance of sinus rhythm in patients with atrial fibrillation. J. Interv. Card Electrophysiol. 2004, 10 (Suppl. S1), 71–76. [Google Scholar] [CrossRef]
- Kéreveur, A.; Callebert, J.; Humbert, M.; Hervé, P.; Simonneau, G.; Launay, J.M.; Drouet, L. High plasma serotonin levels in primary pulmonary hypertension. Effect of long-term epoprostenol (prostacyclin) therapy. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2233–2239. [Google Scholar] [CrossRef]
- Deraet, M.; Manivet, P.; Janoshazi, A.; Callebert, J.; Guenther, S.; Drouet, L.; Launay, J.M.; Maroteaux, L. The natural mutation encoding a C terminus-truncated 5-hydroxytryptamine 2B receptor is a gain of proliferative functions. Mol. Pharmacol. 2005, 67, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Tecott, L.H.; Abdallah, L. Mouse genetic approaches to feeding regulation: Serotonin 5-HT2C receptor mutant mice. CNS Spectr. 2003, 8, 584–588. [Google Scholar] [CrossRef]
- Lam, D.D.; Heisler, L.K. Serotonin and energy balance: Molecular mechanisms and implications for type 2 diabetes. Expert Rev. Mol. Med. 2007, 9, 1–24. [Google Scholar] [CrossRef]
- Suzuki, A.; Naruse, S.; Kitagawa, M.; Ishiguro, H.; Yoshikawa, T.; Ko, S.B.; Yamamoto, A.; Hamada, H.; Hayakawa, T. 5-hydroxytryptamine strongly inhibits fluid secretion in guinea pig pancreatic duct cells. J. Clin. Investig. 2001, 108, 749–756. [Google Scholar] [CrossRef]
- Dahlström, A.; Fuxe, K. Localization of monoamines in the lower brain stem. Experientia 1964, 20, 398–399. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Isakova, A.; Friedmann, D.; Zeng, J.; Grutzner, S.M.; Pun, A.; Zhao, G.Q.; Kolluru, S.S.; Wang, R.; Lin, R.; et al. Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median raphe nuclei. Elife 2019, 8, e49424. [Google Scholar] [CrossRef] [PubMed]
- Deneris, E.S.; Wyler, S.C. Serotonergic transcriptional networks and potential importance to mental health. Nat. Neurosci. 2012, 15, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Olivier, B. Serotonin: A never-ending story. Eur. J. Pharmacol. 2015, 753, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Mengod, G.; Vilaró, M.; Cortés, R.; López-Giménez, J.; Raurich, A.; Palacios, J. The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics; Springer: Berlin/Heidelberg, Germany, 2006; pp. 319–364. [Google Scholar] [CrossRef]
- Bockaert, J.; Claeysen, S.; Bécamel, C.; Dumuis, A.; Marin, P. Neuronal 5-HT metabotropic receptors: Fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006, 326, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hortnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Coppen, A. The biochemistry of affective disorders. Br. J. Psychiatry J. Ment. Sci. 1967, 113, 1237–1264. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022, 28, 3243–3256. [Google Scholar] [CrossRef]
- Garnett, M.R.; Corkill, R.G.; Blamire, A.M.; Rajagopalan, B.; Manners, D.N.; Young, J.D.; Styles, P.; Cadoux-Hudson, T.A. Altered cellular metabolism following traumatic brain injury: A magnetic resonance spectroscopy study. J. Neurotrauma 2001, 18, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Rasmussen, L.; Saraswati, M.; Koehler, R.C.; Robertson, C.; Kannan, S. Traumatic Injury Leads to Inflammation and Altered Tryptophan Metabolism in the Juvenile Rabbit Brain. J. Neurotrauma 2018, 36, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Hou, J.; Nelson, R.; Tsuda, S.; Jahan, M.; Mohammad, N.S.; Watts, J.V.; Thompson, F.J.; Bose, P. Mild closed head traumatic brain injury-induced changes in monoamine neurotransmitters in the trigeminal subnuclei of a rat model: Mechanisms underlying orofacial allodynias and headache. Neural. Regen. Res. 2017, 12, 981–986. [Google Scholar] [PubMed]
- Kawa, L.; Kamnaksh, A.; Long, J.B.; Arborelius, U.P.; Hokfelt, T.; Agoston, D.V.; Risling, M. A Comparative Study of Two Blast-Induced Traumatic Brain Injury Models: Changes in Monoamine and Galanin Systems Following Single and Repeated Exposure. Front. Neurol. 2018, 9, 479. [Google Scholar] [CrossRef]
- Tsuiki, K.; Takada, A.; Nagahiro, S.; Grdisa, M.; Diksic, M.; Pappius, H.M. Synthesis of serotonin in traumatized rat brain. J. Neurochem. 1995, 64, 1319–1325. [Google Scholar] [CrossRef]
- Pithadia, A.B.; Jain, S.M. 5-Hydroxytryptamine Receptor Subtypes and their Modulators with Therapeutic Potentials. J. Clin. Med. Res. 2009, 1, 72–80. [Google Scholar] [CrossRef]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Pike, V.W.; McCarron, J.A.; Lammerstma, A.A.; Hume, S.P.; Poole, K.; Grasby, P.M.; Malizia, A.; Cliffe, I.A.; Fletcher, A.; Bench, C.J. First delineation of 5-HT1A receptors in human brain with PET and [11C]WAY-100635. Eur. J. Pharmacol. 1995, 283, R1–R3. [Google Scholar] [CrossRef]
- Cheng, J.P.; Leary, J.B.; Sembhi, A.; Edwards, C.M.; Bondi, C.O.; Kline, A.E. 5-hydroxytryptamine1A (5-HT1A) receptor agonists: A decade of empirical evidence supports their use as an efficacious therapeutic strategy for brain trauma. Brain Res. 2016, 1640, 5–14. [Google Scholar] [CrossRef]
- De Vry, J.; Dietrich, H.; Glaser, T.; Heine, H.-G.; Horváth, E.; Jork, R.; Maertins, T.; Mauler, F.; Opitz, W.; Scherling, D. BAY x 3702: Neuroprotectant 5-HT1A agonist. Drugs Future 1997, 22, 341–349. [Google Scholar]
- Kline, A.E.; Yu, J.; Massucci, J.L.; Zafonte, R.D.; Dixon, C.E. Protective effects of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin against traumatic brain injury-induced cognitive deficits and neuropathology in adult male rats. Neurosci. Lett. 2002, 333, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Kline, A.E.; Massucci, J.L.; Dixon, C.E.; Zafonte, R.D.; Bolinger, B.D. The therapeutic efficacy conferred by the 5-HT(1A) receptor agonist 8-Hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J. Neurotrauma 2004, 21, 175–185. [Google Scholar] [CrossRef]
- Yelleswarapu, N.K.; Tay, J.K.; Fryer, W.M.; Shah, M.A.; Garcia, A.N.; Cheng, J.P.; Kline, A.E. Elucidating the role of 5-HT(1A) and 5-HT(7) receptors on 8-OH-DPAT-induced behavioral recovery after experimental traumatic brain injury. Neurosci. Lett. 2012, 515, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.M.; Gebhardt, K.M.; Chlebowski, S.M.; Shaw, K.E.; Cheng, J.P.; Henchir, J.J.; Zupa, M.F.; Kline, A.E. A combined therapeutic regimen of buspirone and environmental enrichment is more efficacious than either alone in enhancing spatial learning in brain-injured pediatric rats. J. Neurotrauma 2014, 31, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Ohman, J.; Braakman, R.; Legout, V. Repinotan (BAY × 3702): A 5HT1A agonist in traumatically brain injured patients. J. Neurotrauma 2001, 18, 1313–1321. [Google Scholar] [CrossRef]
- Defrin, R. Chronic post-traumatic headache: Clinical findings and possible mechanisms. J. Man. Manip. Ther. 2014, 22, 36–44. [Google Scholar] [CrossRef]
- Zięba, A.; Stępnicki, P.; Matosiuk, D.; Kaczor, A.A. Overcoming Depression with 5-HT(2A) Receptor Ligands. Int. J. Mol. Sci. 2021, 23, 10. [Google Scholar] [CrossRef]
- Zhang, G.; Stackman, R.W., Jr. The role of serotonin 5-HT2A receptors in memory and cognition. Front. Pharmacol. 2015, 6, 225. [Google Scholar] [CrossRef]
- Zhang, R.; Bi, Y.; Niu, W.; Huang, X.; Chen, S.; Li, X.; Wu, X.; Cao, Y.; Yang, F.; Wang, L.; et al. Association study of 5-HT1A, 5-HT2A polymorphisms with schizophrenia and major depressive disorder in the Han Chinese population. Neurosci. Lett. 2016, 635, 39–43. [Google Scholar] [CrossRef]
- Murnane, K.S. Serotonin 2A receptors are a stress response system: Implications for post-traumatic stress disorder. Behav. Pharmacol. 2019, 30, 151–162. [Google Scholar] [CrossRef]
- Glennon, R.A. Do classical hallucinogens act as 5-HT2 agonists or antagonists? Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 1990, 3, 509–517. [Google Scholar]
- Vamvakopoulou, I.A.; Narine, K.A.D.; Campbell, I.; Dyck, J.R.B.; Nutt, D.J. Mescaline: The forgotten psychedelic. Neuropharmacology 2023, 222, 109294. [Google Scholar] [CrossRef] [PubMed]
- Rahbarnia, A.; Li, Z.; Fletcher, P.J. Effects of psilocybin, the 5-HT2A receptor agonist TCB-2, and the 5-HT2A receptor antagonist M100907 on visual attention in male mice in the continuous performance test. Psychopharmacology 2023. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; O’Connell, C.J.; Reeder, E.L.; Norman, S.V.; Lungani, K.; Gopalan, P.; Gudelsky, G.A.; Robson, M.J. Altered Serotonin 2A (5-HT(2A)) Receptor Signaling Underlies Mild TBI-Elicited Deficits in Social Dominance. Front. Pharmacol. 2022, 13, 930346. [Google Scholar] [CrossRef] [PubMed]
- Braccagni, G.; Scheggi, S.; Bortolato, M. Elevated levels of serotonin 5-HT(2A) receptors in the orbitofrontal cortex of antisocial individuals. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.L.; Doly, S.; Narboux-Nême, N.; Fernández, S.; Mazot, P.; Banas, S.M.; Boutourlinsky, K.; Moutkine, I.; Belmer, A.; Roumier, A.; et al. 5-HT(2B) receptors are required for serotonin-selective antidepressant actions. Mol. Psychiatry 2012, 17, 154–163. [Google Scholar] [CrossRef]
- Pogorelov, V.M.; Rodriguiz, R.M.; Cheng, J.; Huang, M.; Schmerberg, C.M.; Meltzer, H.Y.; Roth, B.L.; Kozikowski, A.P.; Wetsel, W.C. 5-HT(2C) Agonists Modulate Schizophrenia-Like Behaviors in Mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 2163–2177. [Google Scholar] [CrossRef]
- Lad, K.A.; Maheshwari, A.; Saxena, B. Repositioning of an anti-depressant drug, agomelatine as therapy for brain injury induced by craniotomy. Drug Discov. Ther. 2019, 13, 189–197. [Google Scholar] [CrossRef]
- Martin, J.R.; Bös, M.; Jenck, F.; Moreau, J.; Mutel, V.; Sleight, A.J.; Wichmann, J.; Andrews, J.S.; Berendsen, H.H.; Broekkamp, C.L.; et al. 5-HT2C receptor agonists: Pharmacological characteristics and therapeutic potential. J. Pharmacol. Exp. Ther. 1998, 286, 913–924. [Google Scholar]
- Hao, S.; Shi, W.; Liu, W.; Chen, Q.Y.; Zhuo, M. Multiple modulatory roles of serotonin in chronic pain and injury-related anxiety. Front. Synaptic. Neurosci. 2023, 15, 1122381. [Google Scholar] [CrossRef]
- Paredes, S.; Cantillo, S.; Candido, K.D.; Knezevic, N.N. An Association of Serotonin with Pain Disorders and Its Modulation by Estrogens. Int. J. Mol. Sci. 2019, 20, 5729. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bae, H.B.; Ki, H.G.; Oh, J.M.; Kim, W.M.; Lee, H.G.; Yoon, M.H.; Choi, J.I. Different role of spinal 5-HT(hydroxytryptamine)7 receptors and descending serotonergic modulation in inflammatory pain induced in formalin and carrageenan rat models. Br. J. Anaesth. 2014, 113, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, M. Cortical excitation and chronic pain. Trends Neurosci. 2008, 31, 199–207. [Google Scholar] [CrossRef]
- Sahbaie, P.; Irvine, K.A.; Liang, D.Y.; Shi, X.; Clark, J.D. Mild Traumatic Brain Injury Causes Nociceptive Sensitization through Spinal Chemokine Upregulation. Sci. Rep. 2019, 9, 19500. [Google Scholar] [CrossRef] [PubMed]
- Kosari-Nasab, M.; Shokouhi, G.; Azarfarin, M.; Bannazadeh Amirkhiz, M.; Mesgari Abbasi, M.; Salari, A.A. Serotonin 5-HT1A receptors modulate depression-related symptoms following mild traumatic brain injury in male adult mice. Metab. Brain Dis. 2019, 34, 575–582. [Google Scholar] [CrossRef]
- Namjoshi, D.R.; Cheng, W.H.; Carr, M.; Martens, K.M.; Zareyan, S.; Wilkinson, A.; McInnes, K.A.; Cripton, P.A.; Wellington, C.L. Chronic Exposure to Androgenic-Anabolic Steroids Exacerbates Axonal Injury and Microgliosis in the CHIMERA Mouse Model of Repetitive Concussion. PLoS ONE 2016, 11, e0146540. [Google Scholar] [CrossRef]
- Bree, D.; Levy, D. Development of CGRP-dependent pain and headache related behaviours in a rat model of concussion: Implications for mechanisms of post-traumatic headache. Cephalalgia 2018, 38, 246–258. [Google Scholar] [CrossRef]
- Ishii, T.; Kinoshita, K.I.; Muroi, Y. Serotonin 5-HT(4) Receptor Agonists Improve Facilitation of Contextual Fear Extinction in an MPTP-Induced Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 5340. [Google Scholar] [CrossRef]
- Avila-Rojas, S.H.; Velázquez-Lagunas, I.; Salinas-Abarca, A.B.; Barragán-Iglesias, P.; Pineda-Farias, J.B.; Granados-Soto, V. Role of spinal 5-HT5A, and 5-HT1A/1B/1D, receptors in neuropathic pain induced by spinal nerve ligation in rats. Brain Res. 2015, 1622, 377–385. [Google Scholar] [CrossRef]
- Ou, F.Y.; Ning, Y.L.; Yang, N.; Chen, X.; Peng, Y.; Zhao, Y.; Li, P.; Zhou, Y.G.; Liu, Y. A 5-HT(6)R agonist alleviates cognitive dysfunction after traumatic brain injury in rats by increasing BDNF expression. Behav. Brain Res. 2022, 433, 113997. [Google Scholar] [CrossRef]
- Erickson, J.C. Treatment outcomes of chronic post-traumatic headaches after mild head trauma in US soldiers: An observational study. Headache 2011, 51, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Zimering, M.B.; Pulikeyil, A.T.; Myers, C.E.; Pang, K.C. Serotonin 2A Receptor Autoantibodies Increase in Adult Traumatic Brain Injury In Association with Neurodegeneration. J. Endocrinol. Diabetes 2020, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Segelcke, D.; Messlinger, K. Putative role of 5-HT(2B) receptors in migraine pathophysiology. Cephalalgia 2017, 37, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, I.; Tamir, H.; Arango, V.; Dwork, A.J.; Mann, J.J.; Schmauss, C. Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron 2002, 34, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.A.; Roache, J.D.; Javors, M.A.; DiClemente, C.C.; Cloninger, C.R.; Prihoda, T.J.; Bordnick, P.S.; Ait-Daoud, N.; Hensler, J. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: A randomized controlled trial. JAMA 2000, 284, 963–971. [Google Scholar] [CrossRef]
- Rosel, P.; Arranz, B.; Urretavizcaya, M.; Oros, M.; San, L.; Navarro, M.A. Altered 5-HT2A and 5-HT4 postsynaptic receptors and their intracellular signalling systems IP3 and cAMP in brains from depressed violent suicide victims. Neuropsychobiology 2004, 49, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Ozaki, N.; Inada, T.; Goldman, D. Association of a 5-HT(5A) receptor polymorphism, Pro15Ser, to schizophrenia. Mol. Psychiatry 2001, 6, 217–219. [Google Scholar] [CrossRef]
- Andrews, M.; Tousi, B.; Sabbagh, M.N. 5HT6 Antagonists in the Treatment of Alzheimer’s Dementia: Current Progress. Neurol. Ther. 2018, 7, 51–58. [Google Scholar] [CrossRef]
- Erdmann, J.; Nöthen, M.M.; Shimron-Abarbanell, D.; Rietschel, M.; Albus, M.; Borrmann, M.; Maier, W.; Franzek, E.; Körner, J.; Weigelt, B.; et al. The human serotonin 7 (5-HT7) receptor gene: Genomic organization and systematic mutation screening in schizophrenia and bipolar affective disorder. Mol. Psychiatry 1996, 1, 392–397. [Google Scholar]
- Adams, S.M.; Miller, K.E.; Zylstra, R.G. Pharmacologic management of adult depression. Am. Fam. Physician 2008, 77, 785–792. [Google Scholar]
- Albert, P.R.; Benkelfat, C.; Descarries, L. The neurobiology of depression--revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2012, 367, 2378–2381. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Nutt, D.J. Serotonin and brain function: A tale of two receptors. J. Psychopharmacol. 2017, 31, 1091–1120. [Google Scholar] [CrossRef]
- Celada, P.; Puig, M.; Amargós-Bosch, M.; Adell, A.; Artigas, F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J. Psychiatry Neurosci. 2004, 29, 252–265. [Google Scholar] [PubMed]
- Lanzenberger, R.R.; Mitterhauser, M.; Spindelegger, C.; Wadsak, W.; Klein, N.; Mien, L.K.; Holik, A.; Attarbaschi, T.; Mossaheb, N.; Sacher, J.; et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol. Psychiatry 2007, 61, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef]
- Olivier, B.; Mos, J. Rodent models of aggressive behavior and serotonergic drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry 1992, 16, 847–870. [Google Scholar] [CrossRef] [PubMed]
- Borgogna, N.C.; Aita, S.L. Is the serotonin hypothesis dead? If so, how will clinical psychology respond? Front. Psychol. 2022, 13, 1027375. [Google Scholar] [CrossRef] [PubMed]
- Feighner, J.P. Mechanism of action of antidepressant medications. J. Clin. Psychiatry 1999, 60 (Suppl. S4), 4–11; discussion 12–13. [Google Scholar]
- Yang, D.; Gouaux, E. Illumination of serotonin transporter mechanism and role of the allosteric site. Sci. Adv. 2021, 7, eabl3857. [Google Scholar] [CrossRef]
- Yue, J.K.; Burke, J.F.; Upadhyayula, P.S.; Winkler, E.A.; Deng, H.; Robinson, C.K.; Pirracchio, R.; Suen, C.G.; Sharma, S.; Ferguson, A.R.; et al. Selective Serotonin Reuptake Inhibitors for Treating Neurocognitive and Neuropsychiatric Disorders Following Traumatic Brain Injury: An Evaluation of Current Evidence. Brain Sci. 2017, 7, 93. [Google Scholar] [CrossRef]
- Fann, J.R.; Bombardier, C.H.; Temkin, N.; Esselman, P.; Warms, C.; Barber, J.; Dikmen, S. Sertraline for Major Depression during the Year Following Traumatic Brain Injury: A Randomized Controlled Trial. J. Head Trauma Rehabil. 2017, 32, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, N.D.; Panenka, W.J. Antidepressants for depression after concussion and traumatic brain injury are still best practice. BMC Psychiatry 2019, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Shimada, R.; Okada, Y.; Kibayashi, K. Traumatic brain injury decreases serotonin transporter expression in the rat cerebrum. Neurol. Res. 2016, 38, 358–363. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, C.J.; Reeder, E.L.; Collins, S.M.; Lungani, K.; Norman, S.V.; Robson, M.J. Mild traumatic brain injury elicits time- and region-specific reductions in serotonin transporter protein expression and uptake capacity. Neuroreport 2022, 33, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Langlieb, J.; Sachdev, N.; Balderrama, K.; Nadaf, N.; Raj, M.; Murray, E.; Webber, J.; Vanderburg, C.; Gazestani, V.; Tward, D.; et al. The cell type composition of the adult mouse brain revealed by single cell and spatial genomics. bioRxiv 2023, 27. [Google Scholar] [CrossRef]
- Alonso, A.; Merchán, P.; Sandoval, J.E.; Sánchez-Arrones, L.; Garcia-Cazorla, A.; Artuch, R.; Ferrán, J.L.; Martínez-de-la-Torre, M.; Puelles, L. Development of the serotonergic cells in murine raphe nuclei and their relations with rhombomeric domains. Brain Struct. Funct. 2013, 218, 1229–1277. [Google Scholar] [CrossRef]
- Cordes, S.P. Molecular genetics of the early development of hindbrain serotonergic neurons. Clin. Genet. 2005, 68, 487–494. [Google Scholar] [CrossRef]
- Steinbusch, H.W. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 1981, 6, 557–618. [Google Scholar] [CrossRef]
- Olson, L.; Seiger, A. Early prenatal ontogeny of central monoamine neurons in the rat: Fluorescence histochemical observations. Z. Anat. Entwicklungsgesch. 1972, 137, 301–316. [Google Scholar] [CrossRef]
- Deneris, E.; Gaspar, P. Serotonin neuron development: Shaping molecular and structural identities. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e301. [Google Scholar] [CrossRef]
- Meneses, A.; Liy-Salmeron, G. Serotonin and emotion, learning and memory. Rev. Neurosci. 2012, 23, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Okaty, B.W.; Commons, K.G.; Dymecki, S.M. Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci. 2019, 20, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Pollak Dorocic, I.; Fürth, D.; Xuan, Y.; Johansson, Y.; Pozzi, L.; Silberberg, G.; Carlén, M.; Meletis, K. A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 2014, 83, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Weissbourd, B.; Ren, J.; DeLoach, K.E.; Guenthner, C.J.; Miyamichi, K.; Luo, L. Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron 2014, 83, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.K.; Cohen, J.Y.; Hwang, D.; Uchida, N.; Watabe-Uchida, M. Organization of monosynaptic inputs to the serotonin and dopamine neuromodulatory systems. Cell Rep. 2014, 8, 1105–1118. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef]
- Muzerelle, A.; Scotto-Lomassese, S.; Bernard, J.F.; Soiza-Reilly, M.; Gaspar, P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5-B9) to the forebrain and brainstem. Brain Struct. Funct. 2016, 221, 535–561. [Google Scholar] [CrossRef]
- Vertes, R.P. A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J. Comp. Neurol. 1991, 313, 643–668. [Google Scholar] [CrossRef]
- Vertes, R.P.; Fortin, W.J.; Crane, A.M. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 1999, 407, 555–582. [Google Scholar] [CrossRef]
- Ren, J.; Friedmann, D.; Xiong, J.; Liu, C.D.; Ferguson, B.R.; Weerakkody, T.; DeLoach, K.E.; Ran, C.; Pun, A.; Sun, Y.; et al. Anatomically Defined and Functionally Distinct Dorsal Raphe Serotonin Sub-systems. Cell 2018, 175, 472–487.e420. [Google Scholar] [CrossRef]
- Senft, R.A.; Freret, M.E.; Sturrock, N.; Dymecki, S.M. Neurochemically and Hodologically Distinct Ascending VGLUT3 versus Serotonin Subsystems Comprise the r2-Pet1 Median Raphe. J. Neurosci. Off. J. Soc. Neurosci. 2021, 41, 2581–2600. [Google Scholar] [CrossRef]
- Okaty, B.W.; Sturrock, N.; Escobedo Lozoya, Y.; Chang, Y.; Senft, R.A.; Lyon, K.A.; Alekseyenko, O.V.; Dymecki, S.M. A single-cell transcriptomic and anatomic atlas of mouse dorsal raphe Pet1 neurons. Elife 2020, 9, e55523. [Google Scholar] [CrossRef]
- Kawa, L.; Arborelius, U.P.; Yoshitake, T.; Kehr, J.; Hokfelt, T.; Risling, M.; Agoston, D. Neurotransmitter Systems in a Mild Blast Traumatic Brain Injury Model: Catecholamines and Serotonin. J. Neurotrauma 2015, 32, 1190–1199. [Google Scholar] [CrossRef]
- Jin, Y.; Dougherty, S.E.; Wood, K.; Sun, L.; Cudmore, R.H.; Abdalla, A.; Kannan, G.; Pletnikov, M.; Hashemi, P.; Linden, D.J. Regrowth of Serotonin Axons in the Adult Mouse Brain Following Injury. Neuron 2016, 91, 748–762. [Google Scholar] [CrossRef]
- Kajstura, T.J.; Dougherty, S.E.; Linden, D.J. Serotonin axons in the neocortex of the adult female mouse regrow after traumatic brain injury. J. Neurosci. Res. 2018, 96, 512–526. [Google Scholar] [CrossRef]
- Xing, J.; Ren, L.; Xu, H.; Zhao, L.; Wang, Z.H.; Hu, G.D.; Wei, Z.L. Single-Cell RNA Sequencing Reveals Cellular and Transcriptional Changes Associated With Traumatic Brain Injury. Front. Genet. 2022, 13, 861428. [Google Scholar] [CrossRef]
- Crick, F. Central dogma of molecular biology. Nature 1970, 227, 561–563. [Google Scholar] [CrossRef]
- Santiago, C.; Bashaw, G.J. Transcription factors and effectors that regulate neuronal morphology. Development 2014, 141, 4667–4680. [Google Scholar] [CrossRef]
- Kerman, I.A.; Bernard, R.; Bunney, W.E.; Jones, E.G.; Schatzberg, A.F.; Myers, R.M.; Barchas, J.D.; Akil, H.; Watson, S.J.; Thompson, R.C. Evidence for transcriptional factor dysregulation in the dorsal raphe nucleus of patients with major depressive disorder. Front. Neurosci. 2012, 6, 135. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connell, C.J.; Brown, R.S.; Peach, T.M.; Traubert, O.D.; Schwierling, H.C.; Notorgiacomo, G.A.; Robson, M.J. Strain in the Midbrain: Impact of Traumatic Brain Injury on the Central Serotonin System. Brain Sci. 2024, 14, 51. https://doi.org/10.3390/brainsci14010051
O’Connell CJ, Brown RS, Peach TM, Traubert OD, Schwierling HC, Notorgiacomo GA, Robson MJ. Strain in the Midbrain: Impact of Traumatic Brain Injury on the Central Serotonin System. Brain Sciences. 2024; 14(1):51. https://doi.org/10.3390/brainsci14010051
Chicago/Turabian StyleO’Connell, Christopher J., Ryan S. Brown, Taylor M. Peach, Owen D. Traubert, Hana C. Schwierling, Gabrielle A. Notorgiacomo, and Matthew J. Robson. 2024. "Strain in the Midbrain: Impact of Traumatic Brain Injury on the Central Serotonin System" Brain Sciences 14, no. 1: 51. https://doi.org/10.3390/brainsci14010051






