Assessment of Interrater Reliability and Accuracy of Cerebral Aneurysm Morphometry Using 3D Virtual Reality, 2D Digital Subtraction Angiography, and 3D Reconstruction: A Randomized Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
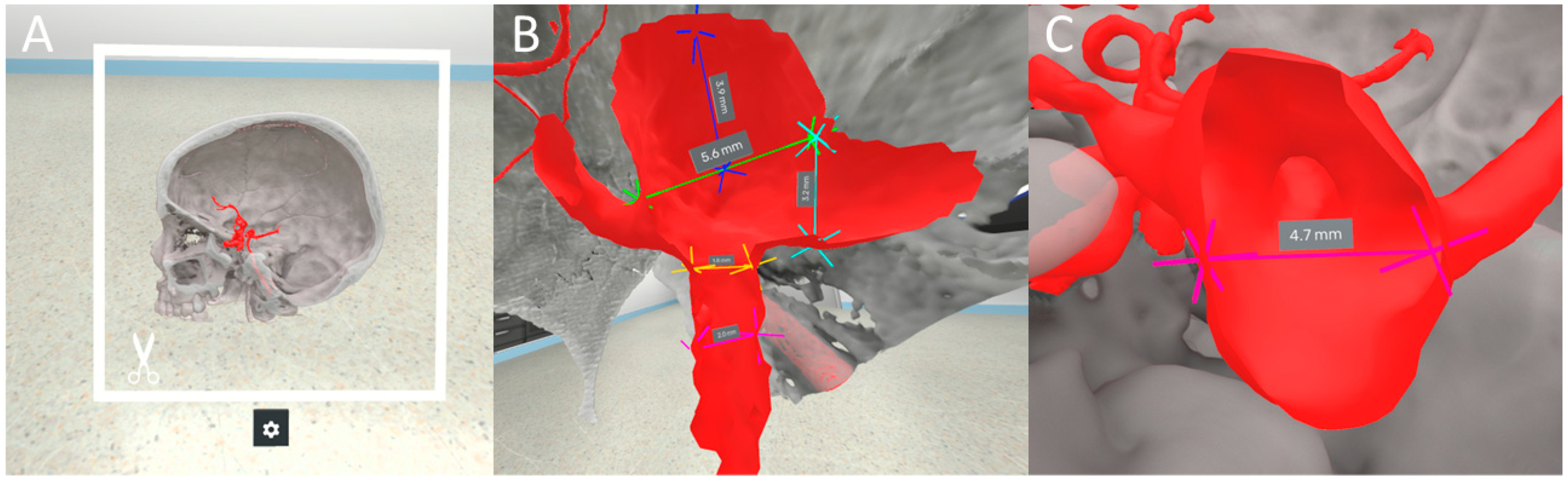
2.2. VR Software
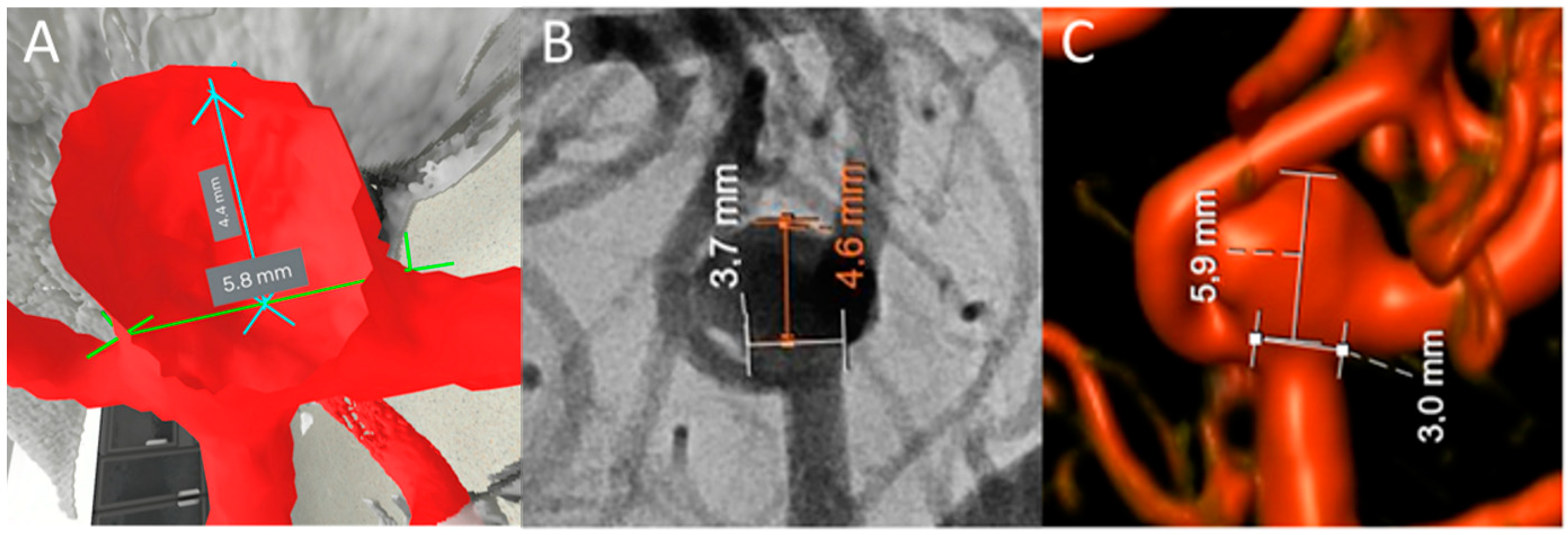
2.3. Measurements
2.4. Outcome Variables
2.5. Statistical Analysis
3. Results
3.1. Patient Population and Rater Cohort
3.2. Primary Outcome
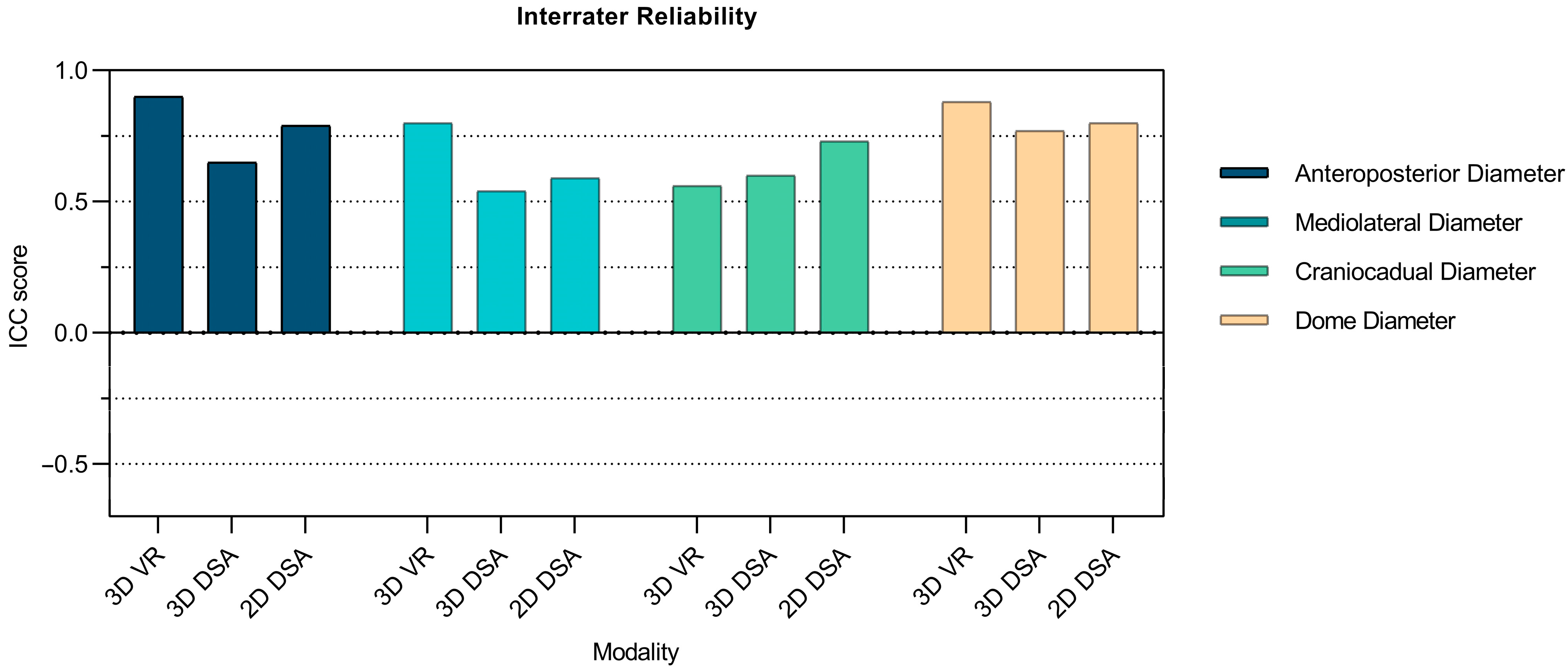
3.2.1. Interrater Reliability of Aneurysm Measurements Comparing 3D VR with 3D DSA and 2D DSA
3.2.2. Interrater Reliability of Morphological Aneurysm Features Comparing 3D VR with 3D DSA and 2D DSA
3.3. Secondary Outcomes
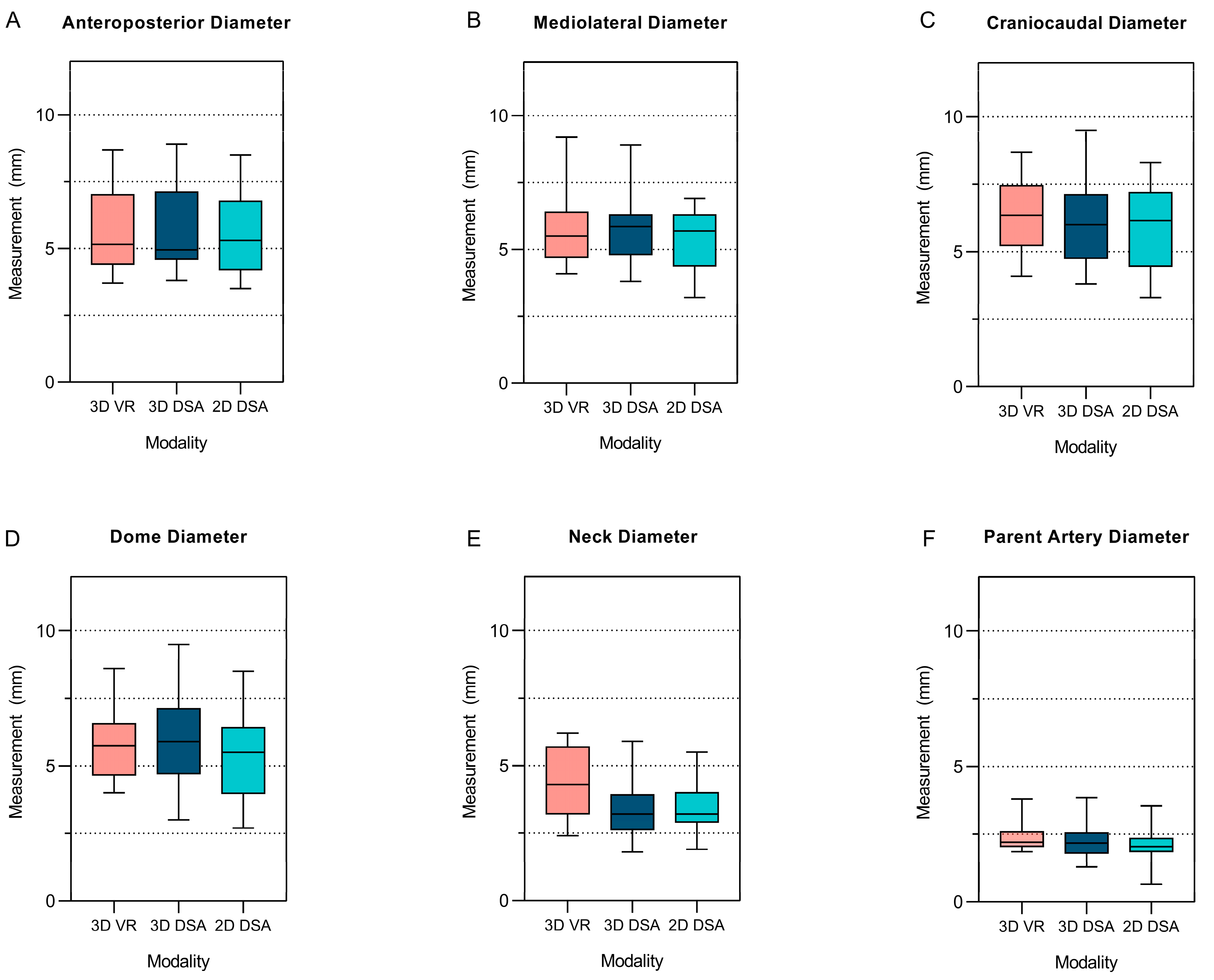
3.3.1. Comparative Differences between the 3D VR Measurements and the 3D and 2D DSA Measurements
3.3.2. Measurement Duration
3.3.3. Subjective Rater Experience
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vlak, M.H.M.; Algra, A.; Brandenburg, R.; Rinkel, G.J.E. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Vernooij, M.W.; Ikram, M.A.; Tanghe, H.L.; Vincent, A.J.; Hofman, A.; Krestin, G.P.; Niessen, W.J.; Breteler, M.M.B.; van der Lugt, A. Incidental Findings on Brain MRI in the General Population. N. Engl. J. Med. 2007, 357, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Nieuwkamp, D.J.; Setz, L.E.; Algra, A.; Linn, F.H.H.; de Rooij, N.K.; Rinkel, G.J.E. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta-analysis. Lancet Neurol. 2009, 8, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.N.; Brown, R.D. Management of unruptured intracranial aneurysms. Neurol. Clin. Pract. 2013, 3, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Korja, M.; Lehto, H.; Juvela, S. Lifelong Rupture Risk of Intracranial Aneurysms Depends on Risk Factors. Stroke 2014, 45, 1958–1963. [Google Scholar] [CrossRef]
- Müller, T.B.; Vik, A.; Romundstad, P.R.; Sandvei, M.S. Risk Factors for Unruptured Intracranial Aneurysms and Subarachnoid Hemorrhage in a Prospective Population-Based Study. Stroke 2019, 50, 2952–2955. [Google Scholar] [CrossRef]
- Bijlenga, P.; Gondar, R.; Schilling, S.; Morel, S.; Hirsch, S.; Cuony, J.; Corniola, M.-V.; Perren, F.; Rüfenacht, D.; Schaller, K. PHASES Score for the Management of Intracranial Aneurysm. Stroke 2017, 48, 2105–2112. [Google Scholar] [CrossRef]
- Greving, J.P.; Wermer, M.J.; Brown, R.D.; Morita, A.; Juvela, S.; Yonekura, M.; Ishibashi, T.; Torner, J.C.; Nakayama, T.; Rinkel, G.J.E.; et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: A pooled analysis of six prospective cohort studies. Lancet Neurol. 2014, 13, 59–66. [Google Scholar] [CrossRef]
- Etminan, N.; Brown Jr, R.D.; Beseoglu, K.; Juvela, S.; Raymond, J.; Morita, A.; Torner, J.C.; Derdeyn, C.P.; Raabe, A.; Mocco, J.; et al. The unruptured intracranial aneurysm treatment score: A multidisciplinary consensus. Neurology 2015, 85, 881–889. (In English) [Google Scholar] [CrossRef]
- Ujiie, H.; Tamano, Y.; Sasaki, K.; Hori, T. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery 2001, 48, 495–502; discussion 502–503. (In English) [Google Scholar] [CrossRef]
- Dhar, S.; Tremmel, M.; Mocco, J.; Kim, M.; Yamamoto, J.; Siddiqui, A.H.; Hopkins, L.N.; Meng, H. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery 2008, 63, 185–196; discussion 196–197. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kiyosue, H.; Tanoue, S.; Okahara, M.; Hori, Y.; Nakamura, T.; Nagatomi, H.; Mori, H. Anatomic features predictive of complete aneurysm occlusion can be determined with three-dimensional digital subtraction angiography. AJNR Am. J. Neuroradiol. 2002, 23, 1206–1213. (In English) [Google Scholar] [PubMed]
- Malhotra, A.; Wu, X.; Forman, H.P.; Grossetta Nardini, H.K.; Matouk, C.C.; Gandhi, D.; Moore, C.; Sanelli, P.G. Rupture Risk of Small Unruptured Intracranial Aneurysms: A Systematic Review. Ann. Intern. Med. 2017, 167, 26–33. (In English) [Google Scholar] [CrossRef] [PubMed]
- Ikawa, F.; Morita, A.; Tominari, S.; Nakayama, T.; Shiokawa, Y.; Date, I.; Nozaki, K.; Miyamoto, S.; Kayama, T.; Arai, H.; et al. Rupture risk of small unruptured cerebral aneurysms. J. Neurosurg. 2019, 132, 69–78. (In English) [Google Scholar] [CrossRef]
- Pettersson, S.D.; Salih, M.; Young, M.; Shutran, M.; Taussky, P.; Ogilvy, C.S. Predictors for Rupture of Small (<7 mm) Intracranial Aneurysms: A Systematic Review and Meta-Analysis. World Neurosurg. 2024, 182, 184–192.e14. (In English) [Google Scholar] [CrossRef]
- Rutledge, C.; Jonzzon, S.; Winkler, E.A.; Raper, D.; Lawton, M.T.; Abla, A.A. Small Aneurysms with Low PHASES Scores Account for Most Subarachnoid Hemorrhage Cases. World Neurosurg. 2020, 139, e580–e584. (In English) [Google Scholar] [CrossRef]
- Ujiie, H.; Tachibana, H.; Hiramatsu, O.; Hazel, A.L.; Matsumoto, T.; Ogasawara, Y.; Nakajima, H.; Hori, T.; Takakura, K.; Kajiya, F. Effects of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: A possible index for surgical treatment of intracranial aneurysms. Neurosurgery 1999, 45, 119–129; discussion 129–130. (In English) [Google Scholar] [CrossRef]
- Beaman, C.; Patel, S.D.; Nael, K.; Colby, G.P.; Liebeskind, D.S. Imaging of Intracranial Saccular Aneurysms. Stroke Vasc. Interv. Neurol. 2023, 3, e000757. [Google Scholar] [CrossRef]
- Backes, D.; Vergouwen, M.D.; Velthuis, B.K.; van der Schaaf, I.C.; Bor AS, E.; Algra, A.; Rinkel, G.J. Difference in Aneurysm Characteristics Between Ruptured and Unruptured Aneurysms in Patients with Multiple Intracranial Aneurysms. Stroke 2014, 45, 1299–1303. [Google Scholar] [CrossRef]
- Kouskouras, C.; Charitanti, A.; Giavroglou, C.; Foroglou, N.; Selviaridis, P.; Kontopoulos, V.; Dimitriadis, A.S. Intracranial aneurysms: Evaluation using CTA and MRA. Correlation with DSA and intraoperative findings. Neuroradiology 2004, 46, 842–850. [Google Scholar] [CrossRef]
- Hochmuth, A.; Spetzger, U.; Schumacher, M. Comparison of three-dimensional rotational angiography with digital subtraction angiography in the assessment of ruptured cerebral aneurysms. AJNR Am. J. Neuroradiol. 2002, 23, 1199–1205. (In English) [Google Scholar] [PubMed]
- Zhou, Y.; Yang, P.F.; Fang, Y.B.; Xu, Y.; Hong, B.; Zhao, W.Y.; Li, Q.; Zhao, R.; Huang, Q.-H.; Liu, J.-M. Parent Artery Reconstruction for Large or Giant Cerebral Aneurysms Using the Tubridge Flow Diverter: A Multicenter, Randomized, Controlled Clinical Trial (PARAT). Am. J. Neuroradiol. 2018, 39, 807. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Nawawi, O.; Ramli, N.; Kadir, K.A.A. Benefits of 3D Rotational DSA Compared with 2D DSA in the Evaluation of Intracranial Aneurysm. Acad. Radiol. 2012, 19, 701–707. [Google Scholar] [CrossRef] [PubMed]
- van der Kruk, S.R.; Zielinski, R.; MacDougall, H.; Hughes-Barton, D.; Gunn, K.M. Virtual reality as a patient education tool in healthcare: A scoping review. Patient Educ. Couns. 2022, 105, 1928–1942. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.; Cheng, C.; Pan, Z.; Liu, L.; Du, J.; Shen, X.; Shen, Z.; Zhu, H.; Liu, J.; et al. Can virtual reality improve traditional anatomy education programmes? A mixed-methods study on the use of a 3D skull model. BMC Med. Educ. 2020, 20, 395. [Google Scholar] [CrossRef]
- Asadzadeh, A.; Samad-Soltani, T.; Salahzadeh, Z.; Rezaei-Hachesu, P. Effectiveness of virtual reality-based exercise therapy in rehabilitation: A scoping review. Inform. Med. Unlocked 2021, 24, 100562. [Google Scholar] [CrossRef]
- Mishra, R.; Narayanan, M.D.K.; Umana, G.E.; Montemurro, N.; Chaurasia, B.; Deora, H. Virtual Reality in Neurosurgery: Beyond Neurosurgical Planning. Int. J. Environ. Res. Public Health 2022, 19, 1719. [Google Scholar] [CrossRef]
- Saemann, A.; Schmid, S.; Licci, M.; Zelechowski, M.; Faludi, B.; Cattin, P.C.; Soleman, J.; Guzman, R. Enhancing educational experience through establishing a VR database in craniosynostosis: Report from a single institute and systematic literature review. Front. Surg. 2024, 11, 1440042. [Google Scholar] [CrossRef]
- Maloca, P.M.; Faludi, B.; Zelechowski, M.; Jud, C.; Vollmar, T.; Hug, S. Validation of virtual reality orbitometry bridges digital and physical worlds. Sci. Rep. 2020, 10, 11815. (In English) [Google Scholar] [CrossRef]
- Maloca, P.M.; de Carvalho JE, R.; Heeren, T.; Hasler, P.W.; Mushtaq, F.; Mon-Williams, M.; Scholl, H.P.N.; Balaskas, K.; Egan, C.; Tufail, A.; et al. High-Performance Virtual Reality Volume Rendering of Original Optical Coherence Tomography Point-Cloud Data Enhanced with Real-Time Ray Casting. Transl. Vis. Sci. Technol. 2018, 7, 2. [Google Scholar] [CrossRef]
- Van Rooij, W.J.; Sprengers, M.E.; De Gast, A.N.; Peluso, J.P.P.; Sluzewski, M. 3D Rotational Angiography: The New Gold Standard in the Detection of Additional Intracranial Aneurysms. Am. J. Neuroradiol. 2008, 29, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Timmins, K.M.; Kuijf, H.J.; Vergouwen MD, I.; Otten, M.J.; Ruigrok, Y.M.; Velthuis, B.K.; van der Schaaf, I.C. Reliability and Agreement of 2D and 3D Measurements on MRAs for Growth Assessment of Unruptured Intracranial Aneurysms. Am. J. Neuroradiol. 2021, 42, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Kiura, Y.; Shibukawa, M.; Ohba, S.; Arita, K.; Kurisu, K. Subtracted 3D CT Angiography for Evaluation of Internal Carotid Artery Aneurysms: Comparison with Conventional Digital Subtraction Angiography. Am. J. Neuroradiol. 2006, 27, 1332. [Google Scholar] [PubMed]
- Forbes, G.; Fox, A.J.; Huston, J., 3rd; Wiebers, D.O.; Torner, J. Interobserver variability in angiographic measurement and morphologic characterization of intracranial aneurysms: A report from the International Study of Unruptured Intracranial Aneurysms. AJNR. Am. J. Neuroradiol. 1996, 17, 1407–1415. (In English) [Google Scholar] [PubMed]
- Zawy Alsofy, S.; Sakellaropoulou, I.; Nakamura, M.; Ewelt, C.; Salma, A.; Lewitz, M.; Welzel Saravia, H.; Sarkis, H.M.; Fortmann, T.; Stroop, R.; et al. Impact of Virtual Reality in Arterial Anatomy Detection and Surgical Planning in Patients with Unruptured Anterior Communicating Artery Aneurysms. Brain Sci. 2020, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Steineke, T.C.; Barbery, D. Microsurgical clipping of middle cerebral artery aneurysms: Preoperative planning using virtual reality to reduce procedure time. Neurosurg. Focus 2021, 51, E12. [Google Scholar] [CrossRef]
- Greuter, L.; De Rosa, A.; Cattin, P.; Croci, D.M.; Soleman, J.; Guzman, R. Randomized study comparing 3D virtual reality and conventional 2D on-screen teaching of cerebrovascular anatomy. Neurosurg. Focus 2021, 51, E18. [Google Scholar] [CrossRef]
- Rychen, J.; Saemann, A.; Gehweiler, J.E.; Roethlisberger, M.; Soleman, J.; Hutter, G.; Müller-Gerbl, M.; Mariani, L.; Guzman, R. The sylvian keyhole approach for surgical clipping of middle cerebral artery aneurysms: Technical nuance to the minipterional craniotomy. Front. Surg. 2022, 9, 1078735. [Google Scholar] [CrossRef]
- Alaraj, A.; Luciano, C.J.; Bailey, D.P.; Elsenousi, A.; Roitberg, B.Z.; Bernardo, A.; Banerjee, P.P.; Charbel, F.T. Virtual Reality Cerebral Aneurysm Clipping Simulation with Real-Time Haptic Feedback. Oper. Neurosurg. 2015, 11, 52–58. [Google Scholar] [CrossRef]
- Kockro, R.A.; Killeen, T.; Ayyad, A.; Glaser, M.; Stadie, A.; Reisch, R.; Giese, A.; Schwandt, E. Aneurysm Surgery with Preoperative Three-Dimensional Planning in a Virtual Reality Environment: Technique and Outcome Analysis. World Neurosurg. 2016, 96, 489–499. [Google Scholar] [CrossRef]
- Briganti, F.; Tortora, M.; Loiudice, G.; Tarantino, M.; Guida, A.; Buono, G.; Marseglia, M.; Caranci, F.; Tortora, F. Utility of virtual stenting in treatment of cerebral aneurysms by flow diverter devices. Radiol. Med. 2023, 128, 480–491. [Google Scholar] [CrossRef] [PubMed]



| Patient ID | Parent Vessel | Side |
|---|---|---|
| 1 | MCA | Left |
| 2 | ACOM | Left |
| 3 | MCA | Right |
| 4 | MCA | Right |
| 5 | ACOM | Right |
| 6 | PCOM | Right |
| 7 | PCOM | Right |
| 8 | MCA | Right |
| 9 | ACOM | Left |
| 10 | MCA | Right |
| 3D VR (n = 30) | 3D DSA (n = 30) | 2D DSA (n = 30) | ||||
|---|---|---|---|---|---|---|
| Aneurysm Dimension | ICC (95% CI) | p Value | ICC (95% CI) | p Value | ICC (95% CI) | p Value |
| Anteroposterior Diameter | 0.9 (0.74–0.97) | 0.000000003 | 0.65 (0.30–0.88) | 0.00025 | 0.79 (0.51–0.94) | 0.000007 |
| Mediolateral Diameter | 0.8 (0.55–0.94) | 0.0000014 | 0.54 (0.16–0.84) | 0.0026 | 0.59 (0.22–0.86) | 0.00097 |
| Cephalocaudal Diameter | 0.56 (0.19–0.85) | 0.0016 | 0.6 (0.24–0.87) | 0.00075 | 0.73 (0.43–0.92) | 0.00002 |
| Smallest Axial Diameter | 0.88 (0.71–0.97) | 0.00000001 | 0.77 (0.49–0.93) | 0.000005 | 0.8 (0.55–0.94) | 0.0000013 |
| Neck Diameter | 0.8 (0.50–0.95) | 0.000016 | −0.49 (−0.50–−0.08) | 0.98 | 0.26 (−0.26–0.91) | 0.18 |
| Parent Vessel Diameter | 0.93 (0.82–0.98) | 0.00000000004 | 0.5 (0.12–0.82) | 0.0047 | 0.03 (−0.26–0.50) | 0.41 |
| Maximum Aneurysm Height | 0.35 (−0.03–0.74) | 0.037 | 0.57 (0.20–0.85) | 0.0014 | 0.27 (−0.09–0.69) | 0.079 |
| Maximum Perpendicular Height | 0.78 (0.50–0.93) | 0.000004 | 0.52 (0.14–0.83) | 0.0033 | 0.25 (−0.11–0.68) | 0.099 |
| Morphological Parameter | ||||||
| Aspect Ratio | 0.33 (−0.09–0.77) | 0.066 | 0.23 (−0.33–0.96) | 0.23 | 0.34 (−0.21–0.92) | 0.13 |
| Dome-to-Neck Ratio | 0.55 (0.13–0.87) | 0.0051 | 0.14 (−0.36–0.95) | 0.30 | 0.72 (0.17–0.98) | 0.0067 |
| Size Ratio | 0.76 (0.47–0.93) | 0.0000086 | −0.04 (−0.3–0.43) | 0.55 | −0.04 (0.3–0.43) | 0.55 |
| 3D VR (n = 30) | 3D DSA (n = 30) | 2D DSA (n = 30) | 3D VR vs. 3D DSA | 3D VR vs. 2D DSA | 3D DSA vs. 2D DSA | |
|---|---|---|---|---|---|---|
| Aneurysm Dimension | Mean ± Range (mm) | Mean ± Range (mm) | Mean ± Range (mm) | p Value | p Value | p Value |
| Anteroposterior Diameter | 5.7 ± 5 | 5.8 ± 5.1 | 5.7 ± 5 | 0.84 | 0.84 | 0.68 |
| Mediolateral Diameter | 5.7 ± 5.1 | 5.8 ± 5.1 | 5.4 ± 3.7 | 0.62 | 0.69 | 0.46 |
| Craniocaudal Diameter | 6.3 ± 4.6 | 6.1 ± 5.7 | 5.8 ± 5 | 0.42 | 0.23 | 0.63 |
| Dome Diameter | 5.8 ± 4.6 | 6.1 ± 6.5 | 5.3 ± 5.8 | 0.004 | 0.14 | 0.85 |
| Neck Diameter | 4.4 ± 3.8 | 3.3 ± 4.1 | 3.4 ± 3.6 | 0.68 | 0.005 | 0.10 |
| Parent Vessel Diameter | 2.3 ± 1.8 | 2.3 ± 2.55 | 1.9 ± 2.8 | 0.48 | 0.017 | 0.047 |
| Maximum Height | 6.5 ± 8.5 | 5.8 ± 4.4 | 5.7 ± 3.9 | 0.016 | 0.009 | 0.80 |
| Maximum Perpendicular Height | 6.3 ± 4.3 | 5.3 ± 4.5 | 5.3 ± 5.3 | 0.016 | 0.004 | 0.95 |
| Morphological Parameter | ||||||
| Aspect Ratio | 1.6 ± 2.1 | 1.6 ± 1.8 | 1.7 ± 3.3 | 0.63 | 0.38 | 0.93 |
| Dome-to-Neck Ratio | 1.4 ± 2.4 | 1.9 ± 3.2 | 1.7 ± 2.6 | 0.0044 | 0.14 | 0.60 |
| Size Ratio | 3 ± 5.2 | 2.5 ± 2.4 | 3.3 ± 10.3 | 0.057 | 0.75 | 0.088 |
| Rater Assessment | |
|---|---|
| Which modality do you find easier to detect and describe aneurysms? | 5/5 (100%) virtual reality model |
| Overall agreement (1 = strongly agree, 2 = agree, 3 = neutral, 4 = disagree, 5 = strongly disagree) | |
| Mean (SD) | |
| Everyday thoughts and concerns faded out during the measurement. | 2.1 (0.75) |
| I experienced fatigue, eyestrain, difficulty focusing, headache, blurred vision, dizziness (eyes closed), or vertigo. | 4.5 (0.54) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saemann, A.; de Wilde, D.; Rychen, J.; Roethlisberger, M.; Żelechowski, M.; Faludi, B.; Cattin, P.C.; Psychogios, M.-N.; Soleman, J.; Guzman, R. Assessment of Interrater Reliability and Accuracy of Cerebral Aneurysm Morphometry Using 3D Virtual Reality, 2D Digital Subtraction Angiography, and 3D Reconstruction: A Randomized Comparative Study. Brain Sci. 2024, 14, 968. https://doi.org/10.3390/brainsci14100968
Saemann A, de Wilde D, Rychen J, Roethlisberger M, Żelechowski M, Faludi B, Cattin PC, Psychogios M-N, Soleman J, Guzman R. Assessment of Interrater Reliability and Accuracy of Cerebral Aneurysm Morphometry Using 3D Virtual Reality, 2D Digital Subtraction Angiography, and 3D Reconstruction: A Randomized Comparative Study. Brain Sciences. 2024; 14(10):968. https://doi.org/10.3390/brainsci14100968
Chicago/Turabian StyleSaemann, Attill, Daniel de Wilde, Jonathan Rychen, Michel Roethlisberger, Marek Żelechowski, Balázs Faludi, Philippe Claude Cattin, Marios-Nikos Psychogios, Jehuda Soleman, and Raphael Guzman. 2024. "Assessment of Interrater Reliability and Accuracy of Cerebral Aneurysm Morphometry Using 3D Virtual Reality, 2D Digital Subtraction Angiography, and 3D Reconstruction: A Randomized Comparative Study" Brain Sciences 14, no. 10: 968. https://doi.org/10.3390/brainsci14100968
APA StyleSaemann, A., de Wilde, D., Rychen, J., Roethlisberger, M., Żelechowski, M., Faludi, B., Cattin, P. C., Psychogios, M.-N., Soleman, J., & Guzman, R. (2024). Assessment of Interrater Reliability and Accuracy of Cerebral Aneurysm Morphometry Using 3D Virtual Reality, 2D Digital Subtraction Angiography, and 3D Reconstruction: A Randomized Comparative Study. Brain Sciences, 14(10), 968. https://doi.org/10.3390/brainsci14100968








