Target Gene-Based Association Study of High Mobility Group Box Protein 1 in Intracranial Aneurysms in Koreans
Abstract
:1. Introduction
2. Materials and Methods
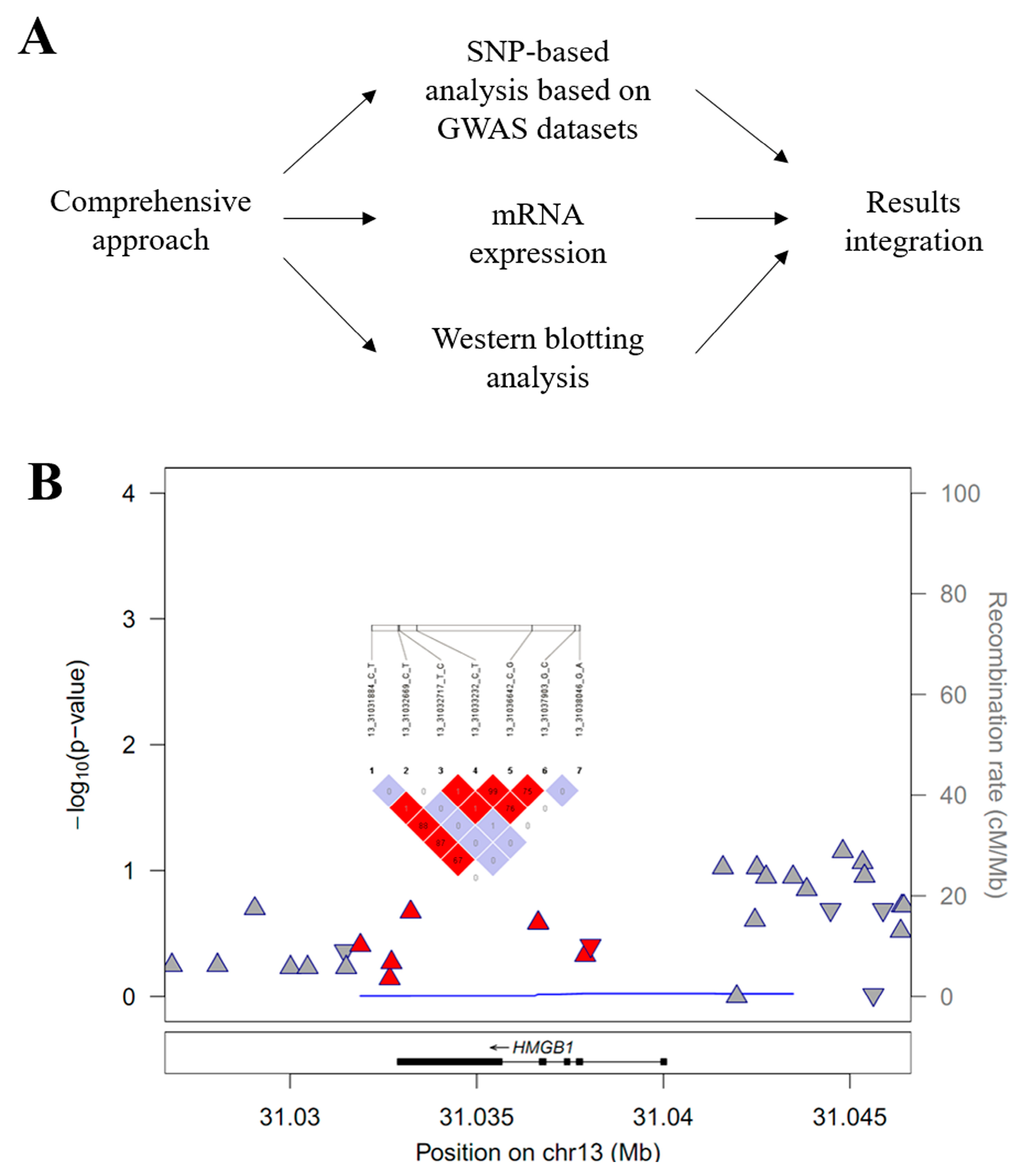
2.1. HMGB1 Target Gene Association Analysis
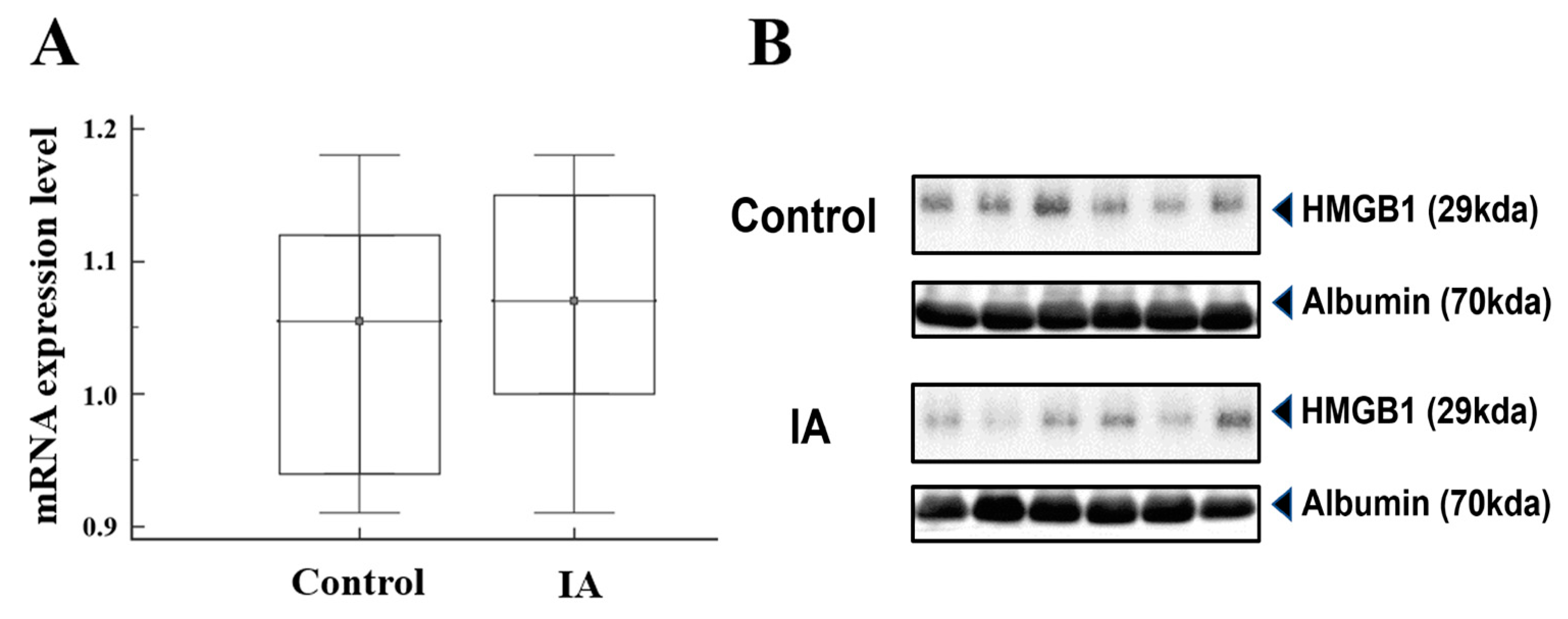
2.2. Real-Time Quantitative PCR and Western Blotting Analysis
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Participants
3.2. High Mobility Group Box 1 (HMGB1) Target Gene Association Analysis
3.3. HMGB1 mRNA and Protein Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Gijn, J.; Kerr, R.S.; Rinkel, G.J. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Ingall, T.; Asplund, K.; Mahonen, M.; Bonita, R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke 2000, 31, 1054–1061. [Google Scholar] [CrossRef]
- Investigators, U.J.; Morita, A.; Kirino, T.; Hashi, K.; Aoki, N.; Fukuhara, S.; Hashimoto, N.; Nakayama, T.; Sakai, M.; Teramoto, A.; et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N. Engl. J. Med. 2012, 366, 2474–2482. [Google Scholar] [CrossRef] [PubMed]
- Hop, J.W.; Rinkel, G.J.; Algra, A.; van Gijn, J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: A systematic review. Stroke 1997, 28, 660–664. [Google Scholar] [CrossRef]
- Brown, R.D., Jr.; Broderick, J.P. Unruptured intracranial aneurysms: Epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014, 13, 393–404. [Google Scholar] [CrossRef]
- Liu, J.; Zou, X.; Zhao, Y.; Jin, Z.; Tu, J.; Ning, X.; Li, J.; Yang, X.; Wang, J. Prevalence and Risk Factors for Unruptured Intracranial Aneurysms in the Population at High Risk for Aneurysm in the Rural Areas of Tianjin. Front. Neurol. 2022, 13, 853054. [Google Scholar] [CrossRef] [PubMed]
- Asaithambi, G.; Adil, M.M.; Chaudhry, S.A.; Qureshi, A.I. Incidences of unruptured intracranial aneurysms and subarachnoid hemorrhage: Results of a statewide study. J. Vasc. Interv. Neurol. 2014, 7, 14–17. [Google Scholar]
- Sliwczynski, A.; Jewczak, M.; Dorobek, M.; Furlepa, K.; Golebiak, I.; Skibinska, E.; Sarzynska-Dlugosz, I. An Analysis of the Incidence and Cost of Intracranial Aneurysm and Subarachnoid Haemorrhage Treatment between 2013 and 2021. Int. J. Environ. Res. Public Health 2023, 20, 3828. [Google Scholar] [CrossRef]
- Korja, M.; Lehto, H.; Juvela, S. Response to letter regarding article, “Lifelong rupture risk of intracranial aneurysms depends on risk factors: A prospective Finnish cohort study”. Stroke 2014, 45, e211. [Google Scholar] [CrossRef]
- Chalouhi, N.; Ali, M.S.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Koch, W.J.; Dumont, A.S. Biology of intracranial aneurysms: Role of inflammation. J. Cereb. Blood Flow Metab. 2012, 32, 1659–1676. [Google Scholar] [CrossRef]
- Zhang, X.; Ares, W.J.; Taussky, P.; Ducruet, A.F.; Grandhi, R. Role of matrix metalloproteinases in the pathogenesis of intracranial aneurysms. Neurosurg. Focus 2019, 47, E4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, W.; Yan, H.; Jiang, T.; Liu, M.; Yu, Z.; Li, H.; Hang, C. Upregulation of HMGB1 in wall of ruptured and unruptured human cerebral aneurysms: Preliminary results. Neurol. Sci. 2016, 37, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Nin, J.W.; Ferreira, I.; Schalkwijk, C.G.; Jorsal, A.; Prins, M.H.; Parving, H.H.; Tarnow, L.; Rossing, P.; Stehouwer, C.D. Higher plasma high-mobility group box 1 levels are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: A 12 year follow-up study. Diabetologia 2012, 55, 2489–2493. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef]
- Kohno, T.; Anzai, T.; Kaneko, H.; Sugano, Y.; Shimizu, H.; Shimoda, M.; Miyasho, T.; Okamoto, M.; Yokota, H.; Yamada, S.; et al. High-mobility group box 1 protein blockade suppresses development of abdominal aortic aneurysm. J. Cardiol. 2012, 59, 299–306. [Google Scholar] [CrossRef]
- Ousaka, D.; Fujii, Y.; Oozawa, S.; Nishibori, M.; Kuroko, Y.; Masuda, Z.; Sano, S. Decreased Serum Levels of High Mobility Group Box 1 (HMGB-1) after Graft Replacement or Stenting of Abdominal Aortic Aneurysm. Ann. Vasc. Surg. 2017, 41, 265–270. [Google Scholar] [CrossRef]
- Hong, E.P.; Kim, B.J.; Cho, S.S.; Yang, J.S.; Choi, H.J.; Kang, S.H.; Jeon, J.P. Genomic Variations in Susceptibility to Intracranial Aneurysm in the Korean Population. J. Clin. Med. 2019, 8, 275. [Google Scholar] [CrossRef]
- Hong, E.P.; Kim, B.J.; Youn, D.H.; Lee, J.J.; Jeon, H.J.; Choi, H.J.; Cho, Y.J.; Jeon, J.P.; The First Korean Stroke Genetics Association Research (The FirstKSGAR) Study. Updated Genome-Wide Association Study of Intracranial Aneurysms by Genotype Correction and Imputation in Koreans. World Neurosurg. 2022, 166, e109–e117. [Google Scholar] [CrossRef]
- GenomeAsia, K.C. The GenomeAsia 100K Project enables genetic discoveries across Asia. Nature 2019, 576, 106–111. [Google Scholar] [CrossRef]
- Youn, D.H.; Kim, Y.; Kim, B.J.; Jeong, M.S.; Lee, J.; Rhim, J.K.; Kim, H.C.; Jeon, J.P. Mitochondrial dysfunction associated with autophagy and mitophagy in cerebrospinal fluid cells of patients with delayed cerebral ischemia following subarachnoid hemorrhage. Sci. Rep. 2021, 11, 16512. [Google Scholar] [CrossRef]
- Youn, D.H.; Kim, B.J.; Hong, E.P.; Jeon, J.P.; first Korean Stroke Genetics Association Research. Bioinformatics Analysis of Autophagy and Mitophagy Markers Associated with Delayed Cerebral Ischemia Following Subarachnoid Hemorrhage. J. Korean Neurosurg. Soc. 2022, 65, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.H.; Lan, J.; Esposito, E.; Ning, M.; Balaj, L.; Ji, X.; Lo, E.H.; Hayakawa, K. Extracellular Mitochondria in Cerebrospinal Fluid and Neurological Recovery After Subarachnoid Hemorrhage. Stroke 2017, 48, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Sathyan, S.; Koshy, L.V.; Srinivas, L.; Easwer, H.V.; Premkumar, S.; Nair, S.; Bhattacharya, R.N.; Alapatt, J.P.; Banerjee, M. Pathogenesis of intracranial aneurysm is mediated by proinflammatory cytokine TNFA and IFNG and through stochastic regulation of IL10 and TGFB1 by comorbid factors. J. J. Neuroinflamm. 2015, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Bown, M.J.; Burton, P.R.; Horsburgh, T.; Nicholson, M.L.; Bell, P.R.; Sayers, R.D. The role of cytokine gene polymorphisms in the pathogenesis of abdominal aortic aneurysms: A case-control study. J. Vasc. Surg. 2003, 37, 999–1005. [Google Scholar] [CrossRef]
- Bown, M.J.; Lloyd, G.M.; Sandford, R.M.; Thompson, J.R.; London, N.J.; Samani, N.J.; Sayers, R.D. The interleukin-10-1082 ‘A’ allele and abdominal aortic aneurysms. J. Vasc. Surg. 2007, 46, 687–693. [Google Scholar] [CrossRef]
- Wang, F.; Quan, Q.Q.; Zhang, C.L.; Li, Y.B.; Jiang, T.B. Association between polymorphisms in the interleukin-10 gene and risk of abdominal aortic aneurysm. Genet. Mol. Res. 2015, 14, 17599–17604. [Google Scholar] [CrossRef]
- Hendrix, P.; Foreman, P.M.; Harrigan, M.R.; Fisher, W.S.R.; Vyas, N.A.; Lipsky, R.H.; Lin, M.; Walters, B.C.; Tubbs, R.S.; Shoja, M.M.; et al. Impact of High-Mobility Group Box 1 Polymorphism on Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2017, 101, 325–330. [Google Scholar] [CrossRef]
- Javaherian, K.; Liu, J.F.; Wang, J.C. Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science 1978, 199, 1345–1346. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Chen, Q.; Jiao, F.; Shi, C.; Pei, M.; Lv, J.; Zhang, H.; Wang, L.; Gong, Z. TNF-alpha/HMGB1 inflammation signalling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif. 2020, 53, e12829. [Google Scholar] [CrossRef]
- Pedrazzi, M.; Patrone, M.; Passalacqua, M.; Ranzato, E.; Colamassaro, D.; Sparatore, B.; Pontremoli, S.; Melloni, E. Selective proinflammatory activation of astrocytes by high-mobility group box 1 protein signaling. J. Immunol. 2007, 179, 8525–8532. [Google Scholar] [CrossRef]
- Cai, J.; Yuan, H.; Wang, Q.; Yang, H.; Al-Abed, Y.; Hua, Z.; Wang, J.; Chen, D.; Wu, J.; Lu, B.; et al. HMGB1-Driven Inflammation and Intimal Hyperplasia After Arterial Injury Involves Cell-Specific Actions Mediated by TLR4. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2579–2593. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.P.; Cho, S.M.; Rhim, J.K.; Park, J.J.; Ahn, J.H.; Youn, D.H.; Kim, J.T.; Park, C.H.; Lee, Y.; Jeon, J.P. Updated Trans-Ethnic Meta-Analysis of Associations between Inflammation-Related Genes and Intracranial Aneurysm. J. Korean Neurosurg. Soc. 2023, 66, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Medina-Suarez, J.; Rodriguez-Esparragon, F.; Sosa-Perez, C.; Cazorla-Rivero, S.; Torres-Mata, L.B.; Jimenez-O’Shanahan, A.; Clavo, B.; Morera-Molina, J. A Review of Genetic Polymorphisms and Susceptibilities to Complications after Aneurysmal Subarachnoid Hemorrhage. Int. J. Mol. Sci. 2022, 23, 15427. [Google Scholar] [CrossRef] [PubMed]
- Haruma, J.; Teshigawara, K.; Hishikawa, T.; Wang, D.; Liu, K.; Wake, H.; Mori, S.; Takahashi, H.K.; Sugiu, K.; Date, I.; et al. Anti-high mobility group box-1 (HMGB1) antibody attenuates delayed cerebral vasospasm and brain injury after subarachnoid hemorrhage in rats. Sci. Rep. 2016, 6, 37755. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Chen, L.; Hu, W.; Wang, M.; Li, S.; Gu, X.; Tao, H.; Zhao, B.; Ma, G.; et al. Genetic predisposition to ischaemic stroke by RAGE and HMGB1 gene variants in Chinese Han population. Oncotarget 2017, 8, 100150–100164. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, D.M.; Pace, L.A.; Pascale, C.L.; Kutchin, A.C.; O’Neill, B.E.; Starke, R.M.; Dumont, A.S. Lymphocytes influence intracranial aneurysm formation and rupture: Role of extracellular matrix remodeling and phenotypic modulation of vascular smooth muscle cells. J. Neuroinflamm. 2016, 13, 185. [Google Scholar] [CrossRef]
- Sun, Q.; Wu, W.; Hu, Y.C.; Li, H.; Zhang, D.; Li, S.; Li, W.; Li, W.D.; Ma, B.; Zhu, J.H.; et al. Early release of high-mobility group box 1 (HMGB1) from neurons in experimental subarachnoid hemorrhage in vivo and in vitro. J. Neuroinflamm. 2014, 11, 106. [Google Scholar] [CrossRef]
- Chyatte, D.; Bruno, G.; Desai, S.; Todor, D.R. Inflammation and intracranial aneurysms. Neurosurgery 1999, 45, 1137–1146; discussion in 1146–1147. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Zhang, S.; Yu, Q.; Xiong, F.; Huang, K.; Wang, C.Y.; Yang, P. HMGB1, an innate alarmin, plays a critical role in chronic inflammation of adipose tissue in obesity. Mol. Cell. Endocrinol. 2017, 454, 103–111. [Google Scholar] [CrossRef]
- Chalouhi, N.; Points, L.; Pierce, G.L.; Ballas, Z.; Jabbour, P.; Hasan, D. Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke 2013, 44, 2594–2597. [Google Scholar] [CrossRef]
- Gholampour, S.; Mehrjoo, S. Effect of bifurcation in the hemodynamic changes and rupture risk of small intracranial aneurysm. Neurosurg. Rev. 2021, 44, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.K.; Kanning, J.P.; Abraham, G.; Martinsen, A.E.; Winsvold, B.S.; Zwart, J.A.; Bourcier, R.; Sawada, T.; Koido, M.; Kamatani, Y.; et al. Genetic Risk Score for Intracranial Aneurysms: Prediction of Subarachnoid Hemorrhage and Role in Clinical Heterogeneity. Stroke 2023, 54, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Kong, X.; Liu, B.; Lou, J. Bioinformatics analysis of gene expression profiling for identification of potential key genes among ischemic stroke. Medicine 2017, 96, e7564. [Google Scholar] [CrossRef] [PubMed]
| GWAS Datasets | mRNA and Western Blotting | |||||
|---|---|---|---|---|---|---|
| Variables | IA (n = 250) | Control (n = 296) | p-Value | IA (n = 12) | Control (n = 12) | p-Value |
| Male | 104 (41.6%) | 142 (48.0%) | 0.74 | 6 (50.0%) | 5 (41.7%) | 0.68 |
| Age, years | 59.3 ± 0.8 | 52.1 ± 1.0 | <0.01 | 59.6 ± 11.6 | 56.3 ± 12.8 | 0.51 |
| Hypertension | 93 (37.2%) | 88 (29.7%) | 0.87 | 5 (41.7%) | 4 (33.3%) | 0.67 |
| Diabetes mellitus | 17 (6.8%) | 37 (12.5%) | <0.01 | 2 (16.7%) | 2 (16.7%) | 1.00 |
| Hyperlipidemia | 29 (11.6%) | 27 (9.1%) | 0.48 | 3 (25.0%) | 2 (16.7%) | 0.62 |
| Smoking | 26 (10.4%) | 37 (12.5%) | 0.62 | 3 (25.0%) | 2 (16.7%) | 0.62 |
| Gene | Chr | SNP | Position | Function | M/m a | MAF a | HWEp a | OR b | L95 b | U95 b | P b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HMGB1 | 13q12.3 | rs1360485 | 31,031,884 | Near UTR-3 | T/C | 0.144/0.171 | 0.838 | 0.86 | 0.60 | 1.22 | 0.394 |
| HMGB1 | 13q12.3 | rs185382445 | 31,032,669 | Near UTR-3 | C/T | 0.018/0.017 | 1.000 | 0.84 | 0.32 | 2.18 | 0.7172 |
| HMGB1 | 13q12.3 | rs2039338 | 31,032,717 | Near UTR-3 | T/C | 0.07/0.078 | 0.690 | 0.86 | 0.53 | 1.40 | 0.5355 |
| HMGB1 | 13q12.3 | rs1045411 | 31,033,232 | UTR-3 | C/T | 0.124/0.159 | 0.384 | 0.79 | 0.54 | 1.15 | 0.2138 |
| HMGB1 | 13q12.3 | rs3742305 | 31,036,642 | Intronic | C/G | 0.124/0.157 | 0.386 | 0.81 | 0.56 | 1.17 | 0.2615 |
| HMGB1 | 13q12.3 | rs2249825 | 31,037,903 | Intronic | G/C | 0.102/0.122 | 0.593 | 0.86 | 0.57 | 1.29 | 0.4686 |
| HMGB1 | 13q12.3 | rs189034241 | 31,038,046 | Intronic | G/A | 0.024/0.025 | 1.000 | 1.44 | 0.62 | 3.34 | 0.3951 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, E.P.; Han, S.W.; Kim, B.J.; Youn, D.H.; Rhim, J.K.; Jeon, J.P.; Park, J.J. Target Gene-Based Association Study of High Mobility Group Box Protein 1 in Intracranial Aneurysms in Koreans. Brain Sci. 2024, 14, 969. https://doi.org/10.3390/brainsci14100969
Hong EP, Han SW, Kim BJ, Youn DH, Rhim JK, Jeon JP, Park JJ. Target Gene-Based Association Study of High Mobility Group Box Protein 1 in Intracranial Aneurysms in Koreans. Brain Sciences. 2024; 14(10):969. https://doi.org/10.3390/brainsci14100969
Chicago/Turabian StyleHong, Eun Pyo, Sung Woo Han, Bong Jun Kim, Dong Hyuk Youn, Jong Kook Rhim, Jin Pyeong Jeon, and Jeong Jin Park. 2024. "Target Gene-Based Association Study of High Mobility Group Box Protein 1 in Intracranial Aneurysms in Koreans" Brain Sciences 14, no. 10: 969. https://doi.org/10.3390/brainsci14100969






