Estimated Disease Progression Trajectory of White Matter Disruption in Unilateral Temporal Lobe Epilepsy: A Data-Driven Machine Learning Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. MRI Acquisitions
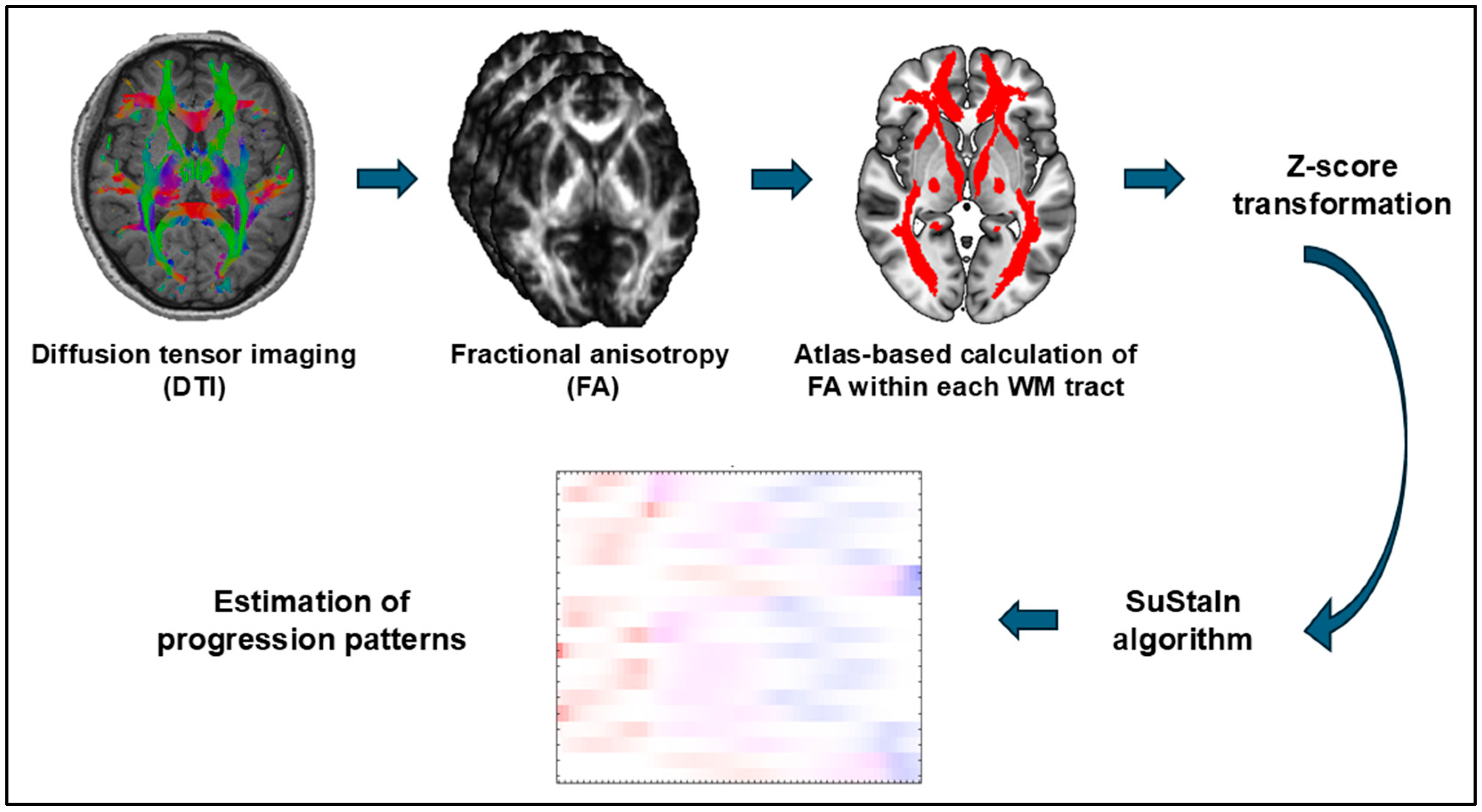
2.3. MRI Processing
2.4. Subtype and Stage Inference (SuStaIn) Analysis
2.5. Separate Analyses for the TLE Patients with and without Hippocampal Sclerosis
2.6. Statistical Analyses
3. Results
3.1. Clinical Demographics
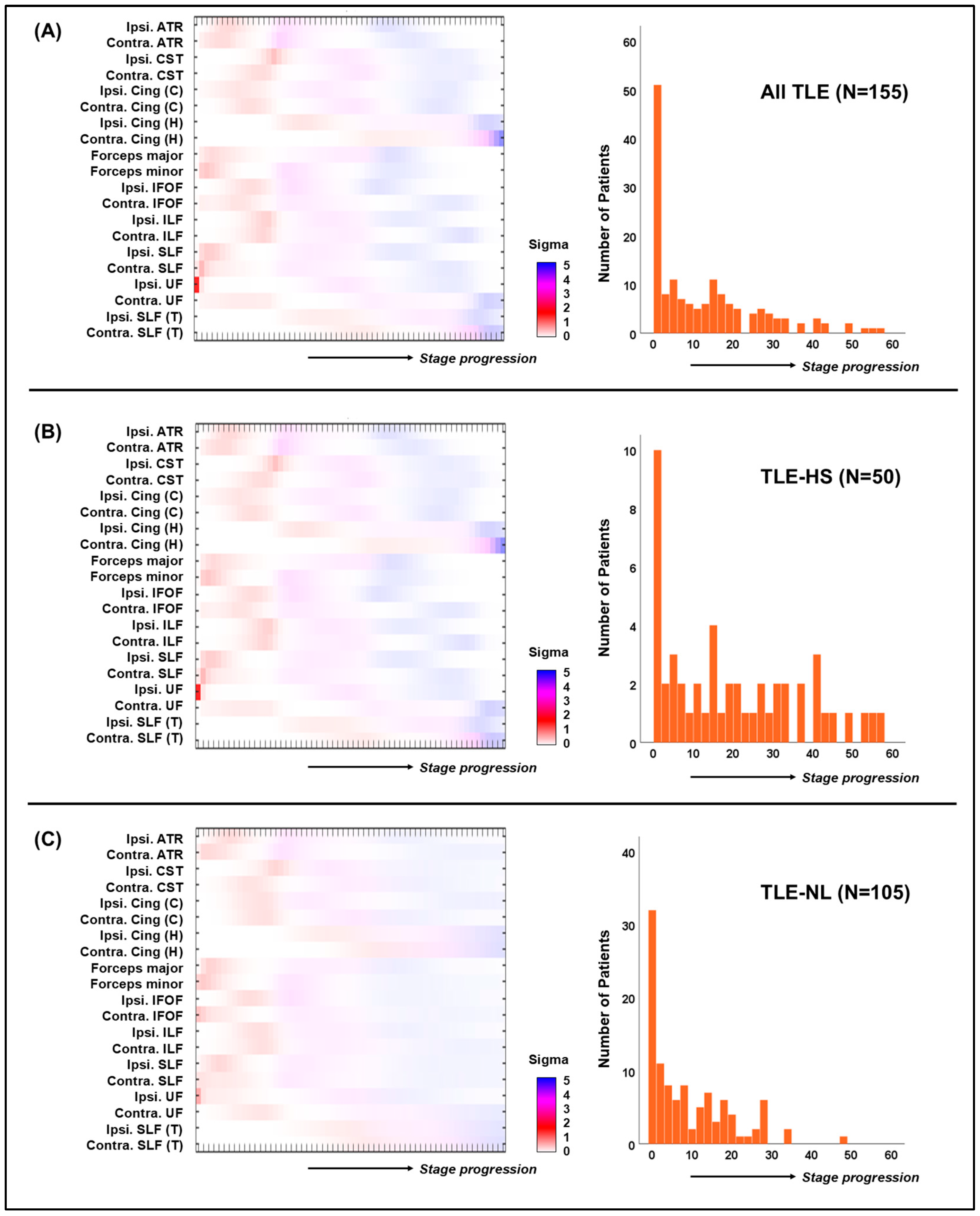
3.2. SuStaIn Algorithm Results
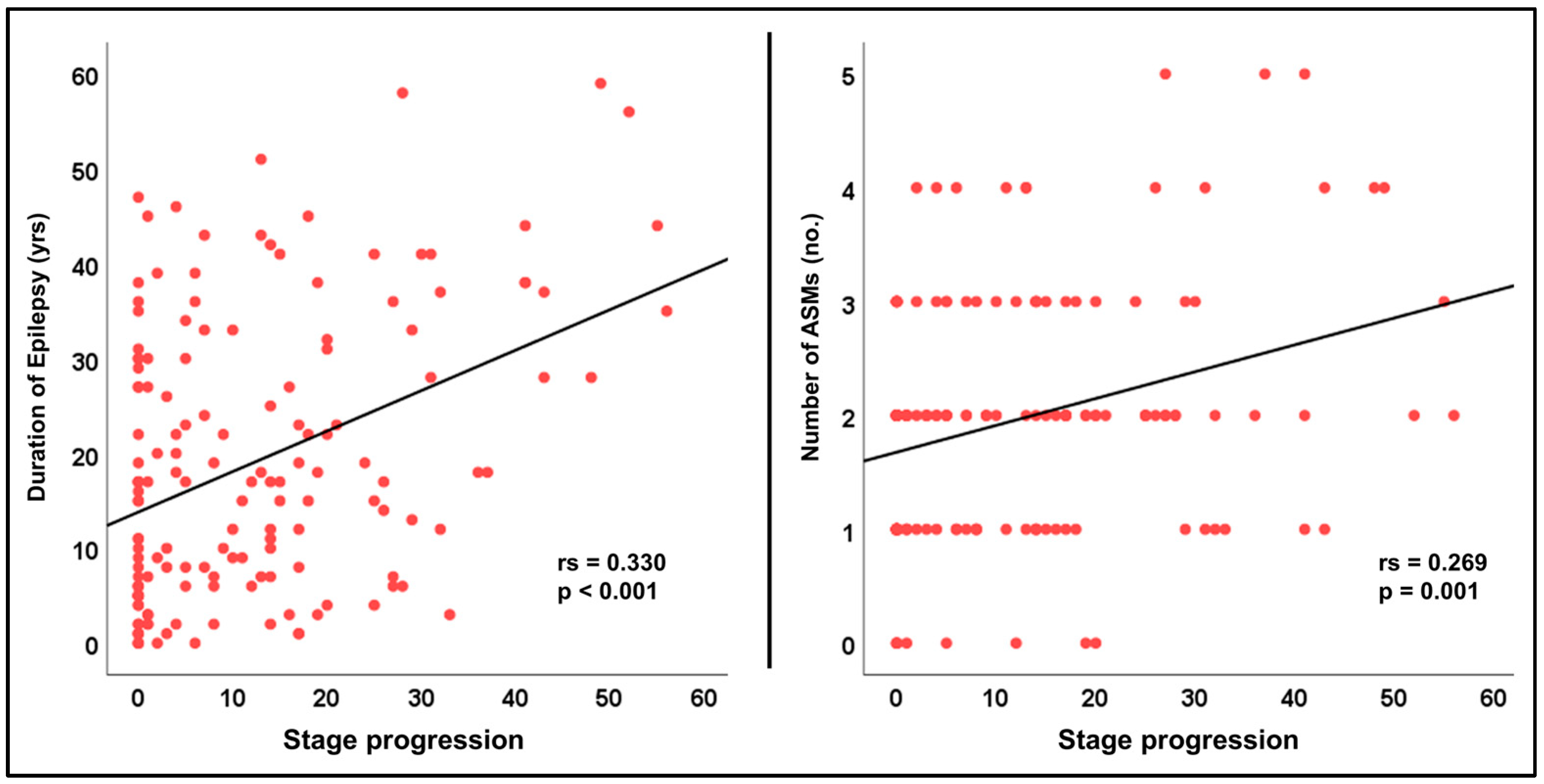
3.3. Associations with Clinical Parameters
3.4. Separate Analyses for the TLE-HS and TLE-NL Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.E. Global, regional, and national burden of epilepsy, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 357–375. [Google Scholar] [CrossRef]
- Spiciarich, M.C.; von Gaudecker, J.R.; Jurasek, L.; Clarke, D.F.; Burneo, J.; Vidaurre, J. Global Health and Epilepsy: Update and Future Directions. Curr. Neurol. Neurosci. Rep. 2019, 19, 30. [Google Scholar] [CrossRef]
- Reynolds, E.H. The origins and early development of the ILAE/IBE/WHO global campaign against epilepsy: Out of the shadows. Epilepsia Open 2024, 9, 77–83. [Google Scholar] [CrossRef]
- Keezer, M.R.; Sisodiya, S.M.; Sander, J.W. Comorbidities of epilepsy: Current concepts and future perspectives. Lancet Neurol. 2016, 15, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Wykes, R.C.; Khoo, H.M.; Caciagli, L.; Blumenfeld, H.; Golshani, P.; Kapur, J.; Stern, J.M.; Bernasconi, A.; Dedeurwaerdere, S.; Bernasconi, N. WONOEP appraisal: Network concept from an imaging perspective. Epilepsia 2019, 60, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Whelan, C.D.; Altmann, A.; Botia, J.A.; Jahanshad, N.; Hibar, D.P.; Absil, J.; Alhusaini, S.; Alvim, M.K.M.; Auvinen, P.; Bartolini, E.; et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain J. Neurol. 2018, 141, 391–408. [Google Scholar] [CrossRef]
- Hatton, S.N.; Huynh, K.H.; Bonilha, L.; Abela, E.; Alhusaini, S.; Altmann, A.; Alvim, M.K.M.; Balachandra, A.R.; Bartolini, E.; Bender, B.; et al. White matter abnormalities across different epilepsy syndromes in adults: An ENIGMA-Epilepsy study. Brain J. Neurol. 2020, 143, 2454–2473. [Google Scholar] [CrossRef]
- Lariviere, S.; Rodriguez-Cruces, R.; Royer, J.; Caligiuri, M.E.; Gambardella, A.; Concha, L.; Keller, S.S.; Cendes, F.; Yasuda, C.; Bonilha, L.; et al. Network-based atrophy modeling in the common epilepsies: A worldwide ENIGMA study. Sci. Adv. 2020, 6, eabc6457. [Google Scholar] [CrossRef]
- Galovic, M.; van Dooren, V.Q.H.; Postma, T.; Vos, S.B.; Caciagli, L.; Borzi, G.; Rosillo, J.C.; Vuong, K.A.; de Tisi, J.; Nachev, P.; et al. Progressive Cortical Thinning in Patients with Focal Epilepsy. JAMA Neurol. 2019, 76, 1230–1239. [Google Scholar] [CrossRef]
- Young, A.L.; Marinescu, R.V.; Oxtoby, N.P.; Bocchetta, M.; Yong, K.; Firth, N.C.; Cash, D.M.; Thomas, D.L.; Dick, K.M.; Cardoso, J.; et al. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat. Commun. 2018, 9, 4273. [Google Scholar] [CrossRef] [PubMed]
- Young, A.L.; Oxtoby, N.P.; Garbarino, S.; Fox, N.C.; Barkhof, F.; Schott, J.M.; Alexander, D.C. Data-driven modelling of neurodegenerative disease progression: Thinking outside the black box. Nat. Rev. Neurosci. 2024, 25, 111–130. [Google Scholar] [CrossRef]
- Xiao, F.; Caciagli, L.; Wandschneider, B.; Sone, D.; Young, A.L.; Vos, S.B.; Winston, G.P.; Zhang, Y.; Liu, W.; An, D.; et al. Identification of different MRI atrophy progression trajectories in epilepsy by subtype and stage inference. Brain J. Neurol. 2023, 146, 4702–4716. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, W.; Li, J.; Li, X.; Zhang, H.; Sima, X.; Li, L.; Wang, K.; Li, Q.; Fang, J.; et al. Identification of four biotypes in temporal lobe epilepsy via machine learning on brain images. Nat. Commun. 2024, 15, 2221. [Google Scholar] [CrossRef]
- Engel, J., Jr. Introduction to temporal lobe epilepsy. Epilepsy Res. 1996, 26, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Otte, W.M.; van Eijsden, P.; Sander, J.W.; Duncan, J.S.; Dijkhuizen, R.M.; Braun, K.P. A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging. Epilepsia 2012, 53, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Royer, J.; Bernhardt, B.C.; Lariviere, S.; Gleichgerrcht, E.; Vorderwulbecke, B.J.; Vulliemoz, S.; Bonilha, L. Epilepsy and brain network hubs. Epilepsia 2022, 63, 537–550. [Google Scholar] [CrossRef]
- Cui, Z.; Zhong, S.; Xu, P.; He, Y.; Gong, G. PANDA: A pipeline toolbox for analyzing brain diffusion images. Front. Hum. Neurosci. 2013, 7, 42. [Google Scholar] [CrossRef]
- Wakana, S.; Caprihan, A.; Panzenboeck, M.M.; Fallon, J.H.; Perry, M.; Gollub, R.L.; Hua, K.; Zhang, J.; Jiang, H.; Dubey, P.; et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage 2007, 36, 630–644. [Google Scholar] [CrossRef]
- Sone, D.; Young, A.; Shinagawa, S.; Tsugawa, S.; Iwata, Y.; Tarumi, R.; Ogyu, K.; Honda, S.; Ochi, R.; Matsushita, K.; et al. Disease Progression Patterns of Brain Morphology in Schizophrenia: More Progressed Stages in Treatment Resistance. Schizophr. Bull. 2024, 50, 393–402. [Google Scholar] [CrossRef]
- Young, A.L.; Bocchetta, M.; Russell, L.L.; Convery, R.S.; Peakman, G.; Todd, E.; Cash, D.M.; Greaves, C.V.; van Swieten, J.; Jiskoot, L.; et al. Characterizing the Clinical Features and Atrophy Patterns of MAPT-Related Frontotemporal Dementia with Disease Progression Modeling. Neurology 2021, 97, e941–e952. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.W.; Young, A.L.; Oxtoby, N.P.; Smith, R.; Ossenkoppele, R.; Strandberg, O.T.; La Joie, R.; Aksman, L.M.; Grothe, M.J.; Iturria-Medina, Y.; et al. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat. Med. 2021, 27, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Von Der Heide, R.J.; Skipper, L.M.; Klobusicky, E.; Olson, I.R. Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain J. Neurol. 2013, 136, 1692–1707. [Google Scholar] [CrossRef] [PubMed]
- Sone, D.; Beheshti, I. Clinical Application of Machine Learning Models for Brain Imaging in Epilepsy: A Review. Front. Neurosci. 2021, 15, 684825. [Google Scholar] [CrossRef]
- Liu, L.; Sun, S.; Kang, W.; Wu, S.; Lin, L. A review of neuroimaging-based data-driven approach for Alzheimer’s disease heterogeneity analysis. Rev. Neurosci. 2024, 35, 121–139. [Google Scholar] [CrossRef]
| TLE | HC | p-Value | TLE-HS | TLE-NL | p-Value | |
|---|---|---|---|---|---|---|
| N | 155 | 270 | NA | 50 | 105 | NA |
| Age (yrs) median (IQR) | 42 (26) | 45 (16) | 0.354 | 44 (21) | 40 (27) | 0.997 |
| Gender (M:F) | 68:87 | 119:151 | 0.968 | 18:32 | 50:55 | 0.173 |
| Onset age (yrs) median (IQR) | 20 (22) | NA | NA | 10 (15) | 24 (30) | <0.001 |
| Duration (yrs) median (IQR) | 17 (24) | NA | NA | 28 (20) | 9 (19) | <0.001 |
| Laterality | L = 107, R = 48 | NA | NA | L = 32, R = 18 | L = 75, R = 30 | 0.35 |
| Etiology | HS = 50, NL = 105 | NA | NA | NA | NA | NA |
| Number of ASMs median (IQR) * | 2 (2) | NA | NA | 2 (1) | 2 (1) | 0.002 |
| Seizure freedom | SF = 14, not SF = 141 | NA | NA | SF = 2, not SF = 48 | SF = 12, not SF = 93 | 0.131 |
| Categorical Comparison | ||
|---|---|---|
| Categories and median (IQR) Stages | p-value | |
| Male | Female | |
| 5.5 (18) | 9 (21) | 0.382 |
| HS | NL | |
| 16 (28) | 5 (15) | <0.001 |
| Left TLE | Right TLE | |
| 7 (17) | 11.5 (24) | 0.205 |
| SF | not SF | |
| 6.5 (13) | 8 (19) | 0.427 |
| Correlation analysis | ||
| Parameters | Spearman’s rs | p-value |
| Age | 0.102 | 0.207 |
| Onset age | −0.191 | 0.017 |
| Duration | 0.330 | <0.001 |
| Number of ASMs | 0.269 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sone, D.; Sato, N.; Shigemoto, Y.; Beheshti, I.; Kimura, Y.; Matsuda, H. Estimated Disease Progression Trajectory of White Matter Disruption in Unilateral Temporal Lobe Epilepsy: A Data-Driven Machine Learning Approach. Brain Sci. 2024, 14, 992. https://doi.org/10.3390/brainsci14100992
Sone D, Sato N, Shigemoto Y, Beheshti I, Kimura Y, Matsuda H. Estimated Disease Progression Trajectory of White Matter Disruption in Unilateral Temporal Lobe Epilepsy: A Data-Driven Machine Learning Approach. Brain Sciences. 2024; 14(10):992. https://doi.org/10.3390/brainsci14100992
Chicago/Turabian StyleSone, Daichi, Noriko Sato, Yoko Shigemoto, Iman Beheshti, Yukio Kimura, and Hiroshi Matsuda. 2024. "Estimated Disease Progression Trajectory of White Matter Disruption in Unilateral Temporal Lobe Epilepsy: A Data-Driven Machine Learning Approach" Brain Sciences 14, no. 10: 992. https://doi.org/10.3390/brainsci14100992
APA StyleSone, D., Sato, N., Shigemoto, Y., Beheshti, I., Kimura, Y., & Matsuda, H. (2024). Estimated Disease Progression Trajectory of White Matter Disruption in Unilateral Temporal Lobe Epilepsy: A Data-Driven Machine Learning Approach. Brain Sciences, 14(10), 992. https://doi.org/10.3390/brainsci14100992







