Contrast Sensitivity Is Impaired in Suspected Primary Open-Angle Glaucoma Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
2.1.1. Participants
2.1.2. Apparatus
2.1.3. Measurement Conditions
2.1.4. Data Analysis
2.2. Specific Methods
2.2.1. Experiment 1: Contrast Sensitivity in Suspected POAG Patients
2.2.2. Experiment 2: Comparison with Early POAG Patients
3. Results
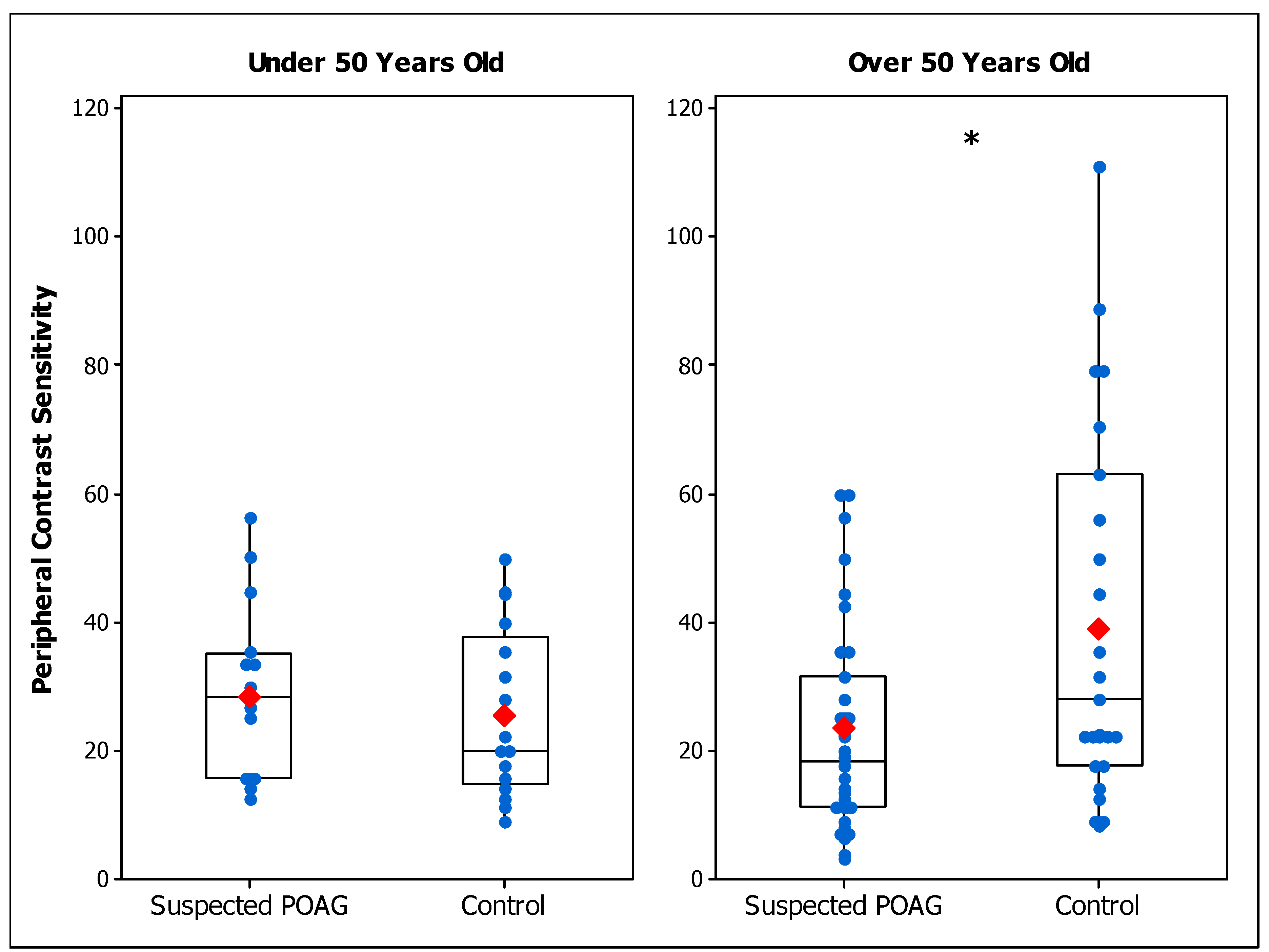
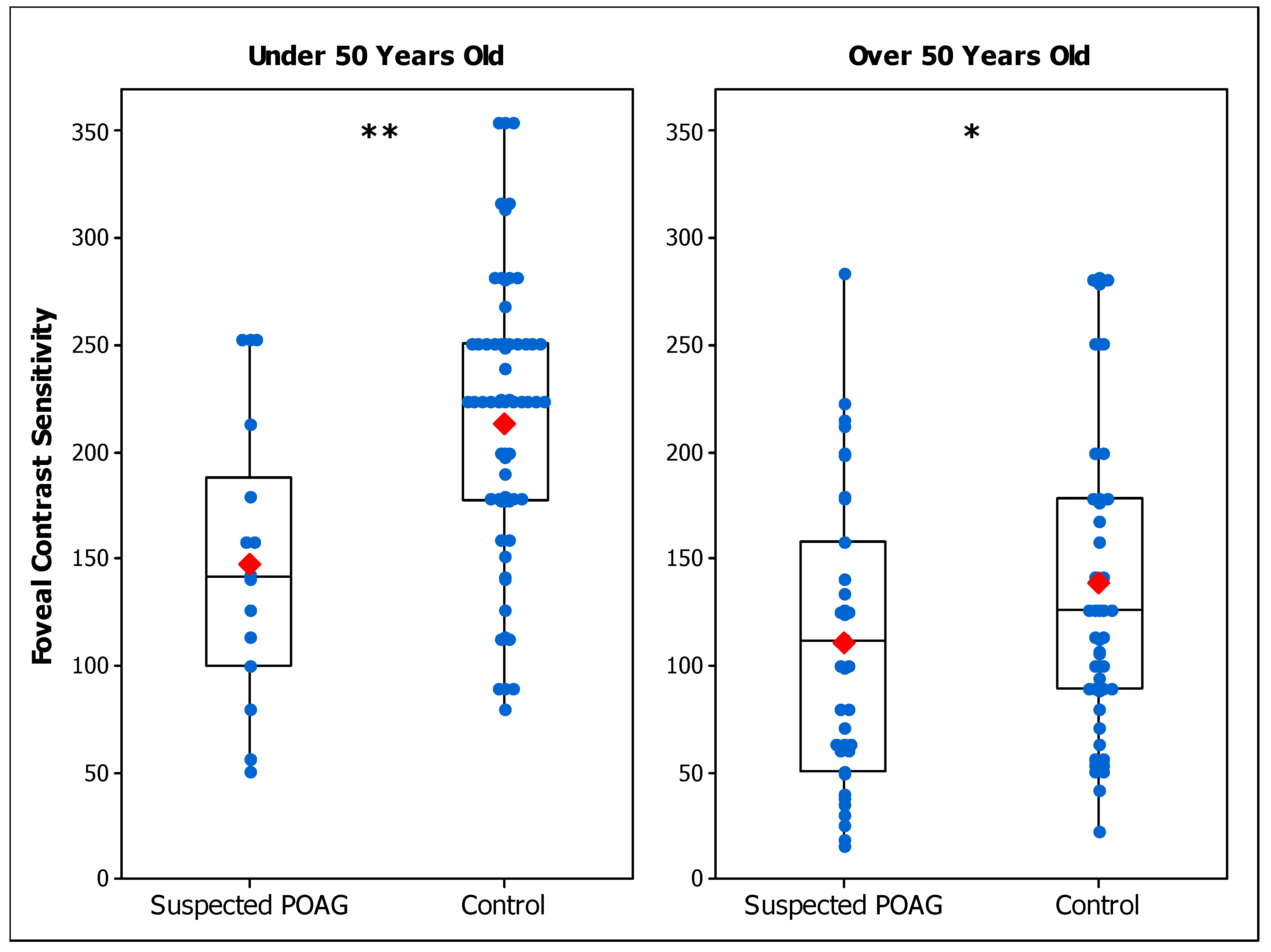
3.1. Experiment 1: Contrast Sensitivity in Suspected POAG Patients
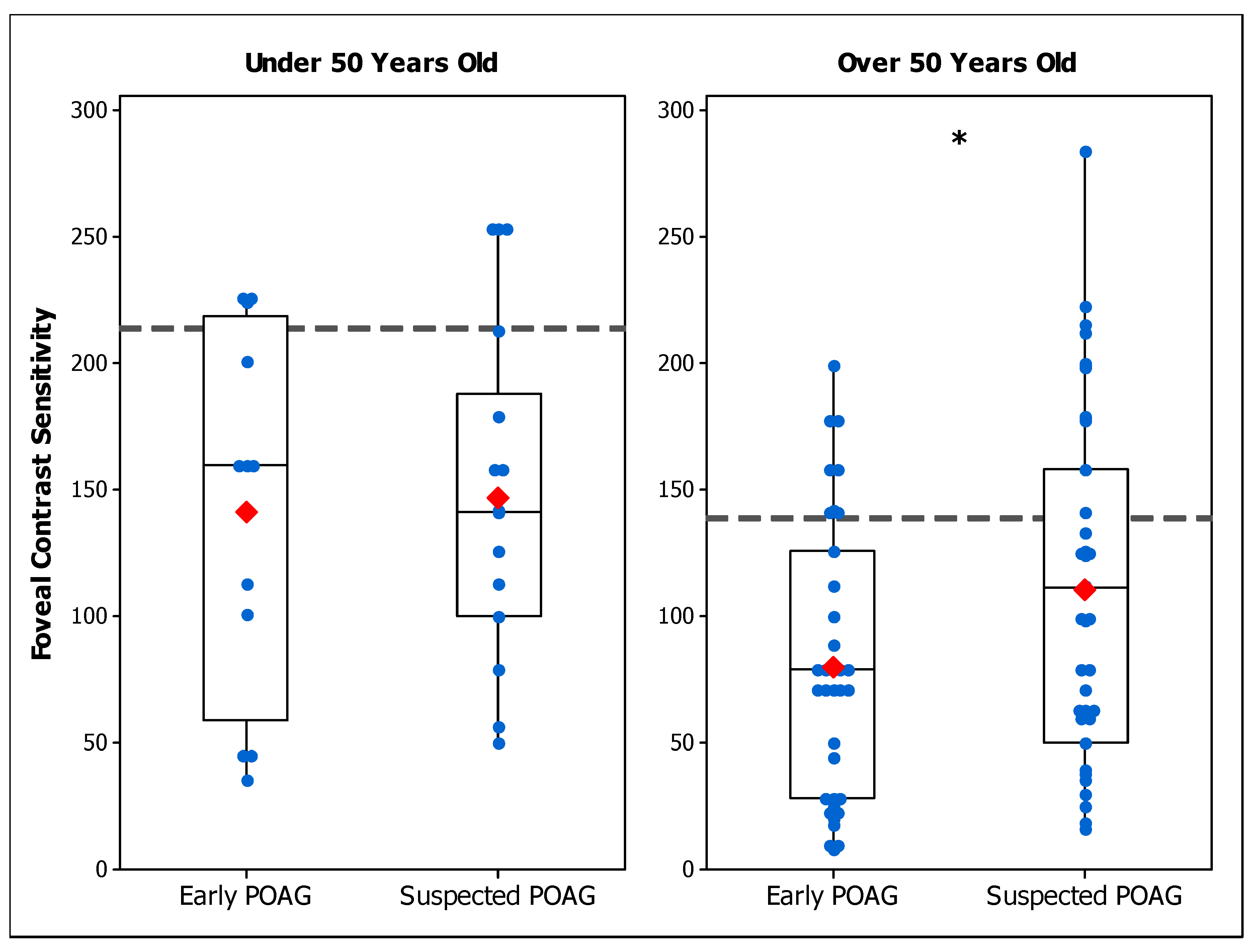
3.2. Experiment 2: Comparison with Early POAG Patients
3.2.1. Foveal CS in Early and Suspected POAG Groups
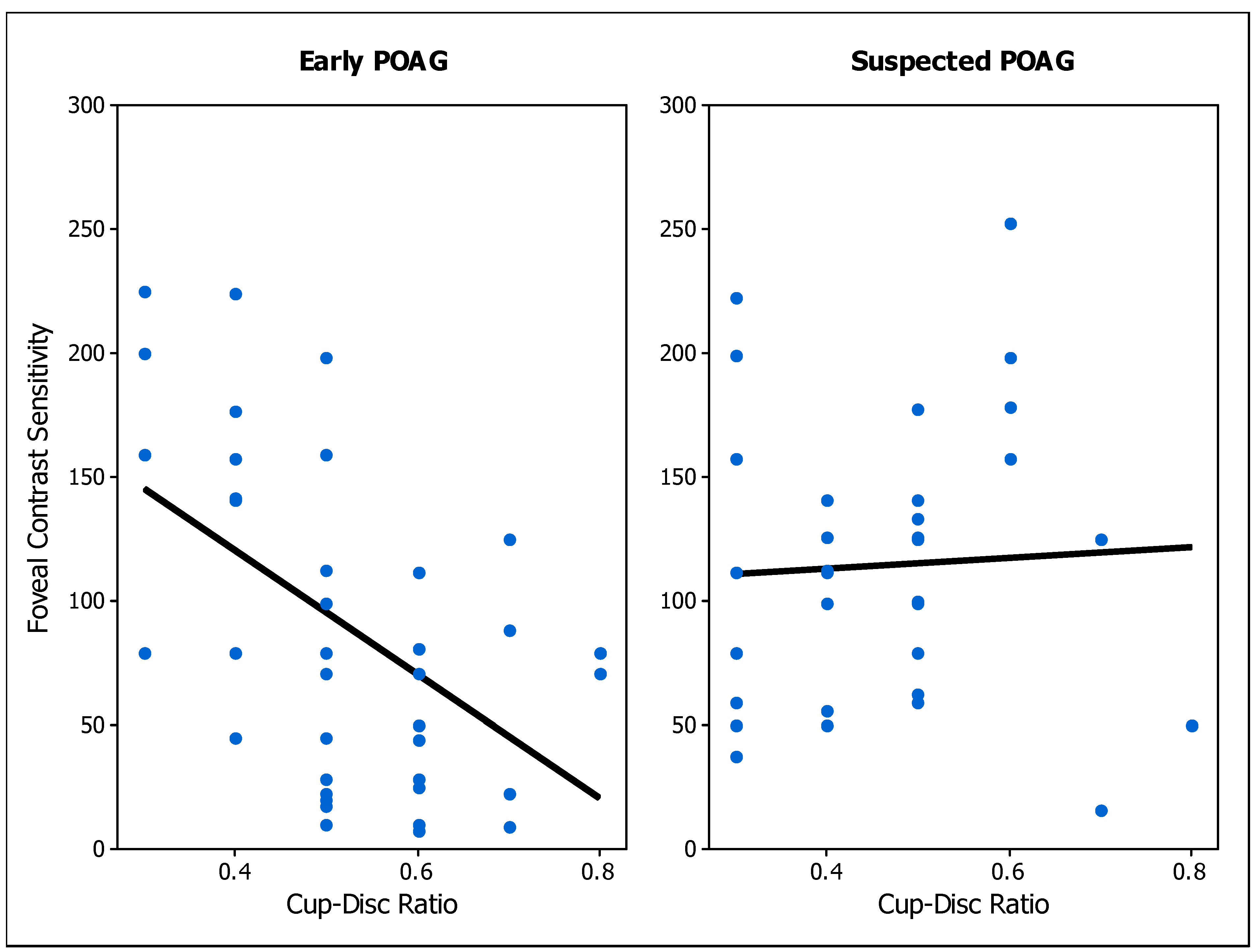
3.2.2. Correlation of Contrast Sensitivity with Clinical Parameters
3.3. Assessment of CS as a Screening Tool
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein, J.D.; Khawaja, A.P.; Weizer, J.S. Glaucoma in Adults—Screening, Diagnosis, and Management: A Review. JAMA 2021, 325, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, J.; Li, Y.; Jiang, B. Prevalence of primary open angle glaucoma in the last 20 years: A meta-analysis and systematic review. Sci. Rep. 2021, 11, 13762. [Google Scholar] [CrossRef] [PubMed]
- Mowatt, G.; Burr, J.M.; Cook, J.A.; Siddiqui, M.A.R.; Ramsay, C.; Fraser, C.; Azuara-Blanco, A.; Deeks, J.J. Screening Tests for Detecting Open-Angle Glaucoma: Systematic Review and Meta-analysis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5373–5385. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.; Weinreb, R.; Medeiros, F. Strategies for improving early detection of glaucoma: The combined structure-function index. Clin. Ophthalmol. Auckl. NZ 2014, 8, 611–621. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Gedde, S.J.; Vinod, K.; Wright, M.M.; Muir, K.W.; Lind, J.T.; Chen, P.P.; Li, T.; Mansberger, S.L. Primary Open-Angle Glaucoma Preferred Practice Pattern®. Ophthalmology 2021, 128, P71–P150. [Google Scholar] [CrossRef]
- Gedde, S.J.; Lind, J.T.; Wright, M.M.; Chen, P.P.; Muir, K.W.; Vinod, K.; Li, T.; Mansberger, S.L. Primary Open-Angle Glaucoma Suspect Preferred Practice Pattern®. Ophthalmology 2021, 128, P151–P192. [Google Scholar] [CrossRef]
- Hood, D.C. Imaging Glaucoma. Annu. Rev. Vis. Sci. 2015, 1, 51–72. [Google Scholar] [CrossRef][Green Version]
- Hood, D.C. Improving our understanding, and detection, of glaucomatous damage: An approach based upon optical coherence tomography (OCT). Prog. Retin. Eye Res. 2017, 57, 46–75. [Google Scholar] [CrossRef]
- Hu, R.; Racette, L.; Chen, K.S.; Johnson, C.A. Functional assessment of glaucoma: Uncovering progression. Surv. Ophthalmol. 2020, 65, 639–661. [Google Scholar] [CrossRef]
- Ng, M.; Sample, P.A.; Pascual, J.P.; Zangwill, L.M.; Girkin, C.A.; Liebmann, J.M.; Weinreb, R.N.; Racette, L. Comparison of visual field severity classification systems for glaucoma. J. Glaucoma 2012, 21, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.P.; Budenz, D.L.; Lee, P.P.; Noecker, R.J.; Walt, J.G.; Siegartel, L.R.; Evans, S.J.; Doyle, J.J. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am. J. Ophthalmol. 2006, 141, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Dunkelberger, G.R.; Green, W.R. Retinal Ganglion Cell Atrophy Correlated With Automated Perimetry in Human Eyes With Glaucoma. Am. J. Ophthalmol. 1989, 107, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan-Baumrind, L.A.; Quigley, H.A.; Pease, M.E.; Kerrigan, D.F.; Mitchell, R.S. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Investig. Ophthalmol. Vis. Sci. 2000, 41, 741–748. [Google Scholar]
- Harwerth, R.S.; Carter-Dawson, L.; Shen, F.; Smith, E.L., III; Crawford, M.L.J. Ganglion Cell Losses Underlying Visual Field Defects from Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2242–2250. [Google Scholar]
- Harwerth, R.S.; Quigley, H.A. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Arch. Ophthalmol. Chic. 2006, 124, 853–859. [Google Scholar] [CrossRef]
- De Valois, R.L.; De Valois, K.K. Spatial Vision; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Cornsweet, T.N. Visual Perception; Academic Press: Oxford, UK, 1970. [Google Scholar]
- Ichhpujani, P.; Thakur, S.; Spaeth, G.L. Contrast Sensitivity and Glaucoma. J. Glaucoma 2020, 29, 71. [Google Scholar] [CrossRef]
- Amanullah, S.; Okudolo, J.; Rahmatnejad, K.; Lin, S.-C.; Wizov, S.S.; Manzi Muhire, R.S.; Hark, L.A.; Zheng, C.X.; Zhan, T.; Spaeth, G.L. The relationship between contrast sensitivity and retinal nerve fiber layer thickness in patients with glaucoma. Graefes Arch Clin Exp Ophthalmol. 2017, 255, 2415–2422. [Google Scholar] [CrossRef]
- Ansari, E.A.; Morgan, J.E.; Snowden, R.J. Psychophysical characterisation of early functional loss in glaucoma and ocular hypertension. Br. J. Ophthalmol. 2002, 86, 1131–1135. [Google Scholar] [CrossRef]
- Bambo, M.P.; Ferrandez, B.; Güerri, N.; Fuertes, I.; Cameo, B.; Polo, V.; Larrosa, J.M.; Garcia-Martin, E. Evaluation of Contrast Sensitivity, Chromatic Vision, and Reading Ability in Patients with Primary Open Angle Glaucoma. J. Ophthalmol. 2016, 2016, 7074016. [Google Scholar] [CrossRef]
- Bierings, R.A.J.M.; de Boer, M.H.; Jansonius, N.M. Visual Performance as a Function of Luminance in Glaucoma: The De Vries-Rose, Weber’s, and Ferry-Porter’s Law. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3416–3423. [Google Scholar] [CrossRef] [PubMed]
- Bierings, R.A.J.M.; Overkempe, T.; van Berkel, C.M.; Kuiper, M.; Jansonius, N.M. Spatial contrast sensitivity from star- to sunlight in healthy subjects and patients with glaucoma. Vision Res. 2019, 158, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Falcão-Reis, F.; O’Donoghue, E.; Buceti, R.; Hitchings, R.A.; Arden, G.B. Peripheral contrast sensitivity in glaucoma and ocular hypertension. Br. J. Ophthalmol. 1990, 74, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Lahav, K.; Levkovitch-Verbin, H.; Belkin, M.; Glovinsky, Y.; Polat, U. Reduced mesopic and photopic foveal contrast sensitivity in glaucoma. Arch Ophthalmol. 2011, 129, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Ichhpujani, P.; Kumar, S.; Kaur, R.; Sood, S. Assessment of contrast sensitivity by Spaeth Richman Contrast Sensitivity Test and Pelli Robson Chart Test in patients with varying severity of glaucoma. Eye 2018, 32, 1392–1400. [Google Scholar] [CrossRef]
- Fatehi, N.; Nowroozizadeh, S.; Henry, S.; Coleman, A.L.; Caprioli, J.; Nouri-Mahdavi, K. Association of Structural and Functional Measures With Contrast Sensitivity in Glaucoma. Am. J. Ophthalmol. 2017, 178, 129–139. [Google Scholar] [CrossRef]
- McKendrick, A.M.; Sampson, G.P.; Walland, M.J.; Badcock, D.R. Contrast sensitivity changes due to glaucoma and normal aging: Low-spatial-frequency losses in both magnocellular and parvocellular pathways. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2115–2122. [Google Scholar] [CrossRef]
- Santillán, J.E.; Issolio, L.A.; Colombo, E.M. A Statistical Criterion to Establish Normal Ranges for Age in a Contrast Sensitivity Function Test. Opt. Appl. 2014, 44, 213–225. [Google Scholar]
- Derefeldt, G.; Lennerstrand, G.; Lundh, B. Age variations in normal human contrast sensitivity. Acta Ophthalmol. 1979, 57, 679–690. [Google Scholar] [CrossRef]
- Zhuang, X.; Tran, T.; Jin, D.; Philip, R.; Wu, C. Aging effects on contrast sensitivity in visual pathways: A pilot study on flicker adaptation. PLoS ONE 2021, 16, e0261927. [Google Scholar] [CrossRef]
- Ahmad, S.S. Glaucoma suspects: A practical approach. Taiwan J. Ophthalmol. 2018, 8, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.T.; Singh, K. Glaucoma Suspect: Diagnosis and Management. Asia-Pac. J. Ophthalmol. 2016, 5, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Issolio, L.; Santillán, J.; Aguirre, R. What characteristics a clinical CSF system has to have? Opt. Appl. 2009, 39, 415–428. [Google Scholar]
- Pelli, D.G.; Zhang, L. Accurate control of contrast on microcomputer displays. Vision Res. 1991, 31, 1337–1350. [Google Scholar] [CrossRef]
- Watson, A.B.; Pelli, D.G. QUEST: A Bayesian adaptive psychometric method. Percept. Psychophys. 1983, 33, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Tripolone, M.C.; Issolio, L.; Silva, B.; Filgueira, C.P.; Perez, D.; Barrionuevo, P. Sensibilidad al contraste en pacientes con glaucoma temprano: Efectos del nivel de iluminación y la excentricidad. In An AFA; Número Especial inVisionT; 2018; pp. 62–66. Available online: https://anales.fisica.org.ar/index.php/analesafa/article/view/2200 (accessed on 20 September 2024).
- Curcio, C.A.; Allen, K.A. Topography of ganglion cells in human retina. J. Comp. Neurol. 1990, 300, 5–25. [Google Scholar] [CrossRef]
- Rodieck, R.W. The First Steps in Seeing; Sinauer Associates: Sunderland, MA, USA, 1998; Volume 1. [Google Scholar]
- Hood, D.C.; Kardon, R.H. A framework for comparing structural and functional measures of glaucomatous damage. Prog. Retin. Eye Res. 2007, 26, 688–710. [Google Scholar] [CrossRef]
- Curcio, C.A.; Sloan, K.R.; Kalina, R.E.; Hendrickson, A.E. Human photoreceptor topography. J. Comp. Neurol. 1990, 292, 497–523. [Google Scholar] [CrossRef]
- Ray, W.A.; O’Day, D.M. Statistical analysis of multi-eye data in ophthalmic research. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1186–1188. [Google Scholar]
- Rosner, B. Statistical methods in ophthalmology: An adjustment for the intraclass correlation between eyes. Biometrics 1982, 38, 105–114. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Lundh, B.L.; Gottvall, E. Peripheral contrast sensitivity for dynamic sinusoidal gratings in early glaucoma. Acta Ophthalmol. Scand. 1995, 73, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.C.; Raza, A.S.; de Moraes, C.G.V.; Liebmann, J.M.; Ritch, R. Glaucomatous damage of the macula. Prog. Retin. Eye Res. 2013, 32, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, H.; Sanvicente, C.T.; Gogte, P.; Waisbourd, M.; Lee, D.; Manzi, R.R.S.; Leiby, B.E.; Richman, J.; Wizov, S.S.; Spaeth, G.L. Measuring Contrast Sensitivity in Specific Areas of Vision–A Meaningful Way to Assess Quality of Life and Ability to Perform Daily Activities in Glaucoma. Ophthalmic Epidemiol. 2019, 26, 301–310. [Google Scholar] [CrossRef]
- Onal, S.; Yenice, O.; Cakir, S.; Temel, A. FACT contrast sensitivity as a diagnostic tool in glaucoma: FACT contrast sensitivity in glaucoma. Int. Ophthalmol. 2008, 28, 407–412. [Google Scholar] [CrossRef]
- Richman, J.; Zangalli, C.; Lu, L.; Wizov, S.S.; Spaeth, E.; Spaeth, G.L. The Spaeth/Richman contrast sensitivity test (SPARCS): Design, reproducibility and ability to identify patients with glaucoma. Br. J. Ophthalmol. 2015, 99, 16–20. [Google Scholar] [CrossRef]
- Wood, J.M.; Lovie-Kitchin, J.E. Evaluation of the efficacy of contrast sensitivity measures for the detection of early primary open-angle glaucoma. Optom Vis Sci. 1992, 69, 175–181. [Google Scholar] [CrossRef]
- Kurysheva, N.I.; Lepeshkina, L.V. Detection of Primary Angle Closure Glaucoma Progression by Optical Coherence Tomography. J. Glaucoma 2021, 30, 410–420. [Google Scholar] [CrossRef]
- Fan, R.; Bowd, C.; Christopher, M.; Brye, N.; Proudfoot, J.A.; Rezapour, J.; Belghith, A.; Goldbaum, M.H.; Chuter, B.; Girkin, C.A.; et al. Detecting Glaucoma in the Ocular Hypertension Study Using Deep Learning. JAMA Ophthalmol. 2022, 140, 383–391. [Google Scholar] [CrossRef]
- Stagg, B.C.; Medeiros, F.A. A Comparison of OCT Parameters in Identifying Glaucoma Damage in Eyes Suspected of Having Glaucoma. Ophthalmol. Glaucoma 2020, 3, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A.; Zangwill, L.M.; Bowd, C.; Mansouri, K.; Weinreb, R.N. The Structure and Function Relationship in Glaucoma: Implications for Detection of Progression and Measurement of Rates of Change. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6939–6946. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-López, C.; Gleitze, S.; Madariaga, S.; Plaza-Rosales, I. Pupillary response to chromatic light stimuli as a possible biomarker at the early stage of glaucoma: A review. Int. Ophthalmol. 2023, 43, 343–356. [Google Scholar] [CrossRef]
- Barrionuevo, P.A.; Issolio, L.A.; Tripolone, C. Photoreceptor contributions to the human pupil light reflex. J. Photochem. Photobiol. 2023, 15, 100178. [Google Scholar] [CrossRef]
- Al-Nosairy, K.O.; Hoffmann, M.B.; Bach, M. Non-invasive electrophysiology in glaucoma, structure and function—A review. Eye 2021, 35, 2374–2385. [Google Scholar] [CrossRef] [PubMed]
- Senger, C.; Moreto, R.; Watanabe, S.E.S.; Matos, A.G.; Paula, J.S. Electrophysiology in Glaucoma. J. Glaucoma 2020, 29, 147. [Google Scholar] [CrossRef]
- Adhikari, P.; Zele, A.J.; Thomas, R.; Feigl, B. Quadrant Field Pupillometry Detects Melanopsin Dysfunction in Glaucoma Suspects and Early Glaucoma. Sci. Rep. 2016, 6, 33373. [Google Scholar] [CrossRef]
- Tirsi, A.; Orshan, D.; Wong, B.; Gliagias, V.; Tsai, J.; Obstbaum, S.A.; Tello, C. Associations between steady-state pattern electroretinography and estimated retinal ganglion cell count in glaucoma suspects. Doc. Ophthalmol. Adv. Ophthalmol. 2022, 145, 11–25. [Google Scholar] [CrossRef]
- Banitt, M.R.; Ventura, L.M.; Feuer, W.J.; Savatovsky, E.; Luna, G.; Shif, O.; Bosse, B.; Porciatti, V. Progressive Loss of Retinal Ganglion Cell Function Precedes Structural Loss by Several Years in Glaucoma Suspects. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2346–2352. [Google Scholar] [CrossRef] [PubMed]
- Paz-Filgueira, C.; Colombo, E.M. Quantifying the effect of straylight on photopic contrast sensitivity. JOSA A 2018, 35, 1124–1130. [Google Scholar] [CrossRef]
- Ridder, W.H.; Comer, G.; Oquindo, C.; Yoshinaga, P.; Engles, M.; Burke, J. Contrast Sensitivity in Early to Intermediate Age-Related Macular Degeneration (AMD). Curr. Eye Res. 2022, 47, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Silva-Viguera, M.-C.; García-Romera, M.-C.; López-Izquierdo, I.; De-Hita-Cantalejo, C.; Sánchez-González, M.C.; Bautista-Llamas, M.-J. Contrast Sensitivity Assessment in Early Diagnosis of Diabetic Retinopathy: A Systematic Review. Semin. Ophthalmol. 2023, 38, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.R.; Taylor, H.R.B.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.G.; et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef] [PubMed]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.T.L.; Legge, G.E. Comparing the Shape of Contrast Sensitivity Functions for Normal and Low Vision. Investig. Ophthalmol. Vis. Sci. 2016, 57, 198–207. [Google Scholar] [CrossRef]
- Cicinelli, M.V.; Marmamula, S.; Khanna, R.C. Comprehensive eye care-Issues, challenges, and way forward. Indian J. Ophthalmol. 2020, 68, 316–323. [Google Scholar] [CrossRef]

| Suspected POAG Group | Early POAG Group | Peripheral Control Group | Foveal Control Group | |
|---|---|---|---|---|
| N° Participants, Gender | 34 20 F, 14 M | 34 21 F; 13 M | 28 12 F; 16 M | 71 34 F; 37 M |
| Age, mean ± SD (years old) | 52 ± 13 | 53 ± 13 | 50 ± 13 | 46 ± 14 |
| N° Under 50 y.o. (n° of eyes) | 10 (16 PA, 18 FA) | 9 (12 FA) | 10 (17 PA) | 40 (71 FA) |
| Age, mean ± SD | 35 ± 9 | 35 ± 12 | 35 ± 8 | 35 ± 8 |
| N° Over 50 y.o. (n° of eyes) | 24 (44 PA, 47 FA) | 25 (39 FA) | 18 (29 PA) | 31 (58 FA) |
| Age, mean ± SD | 59 ± 6 | 60 ± 5 | 58 ± 5 | 60 ± 6 |
| VA, mean ± SD (logMAR) | −0.09 ± 0.10 a | −0.09 ± 0.10 a | 0.05 ± 0.10 | 0.05 ± 0.10 |
| IOP, mean ± SD (mmHg) | 15 ± 3 | 14 ± 3 | 15 ± 2 | 15 ± 2 |
| MD, mean ± SD | 0.01 ± 1.7 | 0.2 ± 1.6 a | −1.2 ± 2.0 | −1.2 ± 2.0 |
| CDR, mean ± SD | 0.4 ± 0.1 a,b | 0.5 ± 0.1 a | 0.2 ± 0.1 | 0.2 ± 0.1 |
| Suspected POAG Group | Early POAG | Control Group | |
|---|---|---|---|
| Peripheral CS Under 50 | 28.3 ± 13.7 | - | 25.6 ± 13.1 |
| Peripheral CS Over 50 | 23.4 ± 16.1 a | - | 39.1 ± 28.2 |
| Foveal CS Under 50 | 146.8 ± 63.3 a | 141.2 ± 72.6 a,b | 213.5 ± 66.2 |
| Foveal CS Under 50 | 110.5 ± 65.0 a | 80.2 ± 54.5 a,b | 138.6 ± 71.7 |
| Age | VA | IOP | CDR | MD | |
|---|---|---|---|---|---|
| Early POAG Foveal CS | r = −0.45 (p = 0.001) | r = 0.21 (p = 0.178) | r = 0.24 (p = 0.129) | r = −0.53 (p < 0.001) | r = 0.09 (p = 0.674) |
| POAG Suspect Foveal CS | r = −0.37 (p = 0.002) | r = 0.24 (p = 0.076) | r = −0.11 (p = 0.446) | r = 0.05 (p = 0.766) | r = −0.14 (p = 0.527) |
| POAG Suspect Peripheral CS | r = −0.21 (p = 0.106) | not applicable | r = −0.23 (p = 0.116) | r = −0.12 (p = 0.515) | r = −0.10 (p = 0.649) |
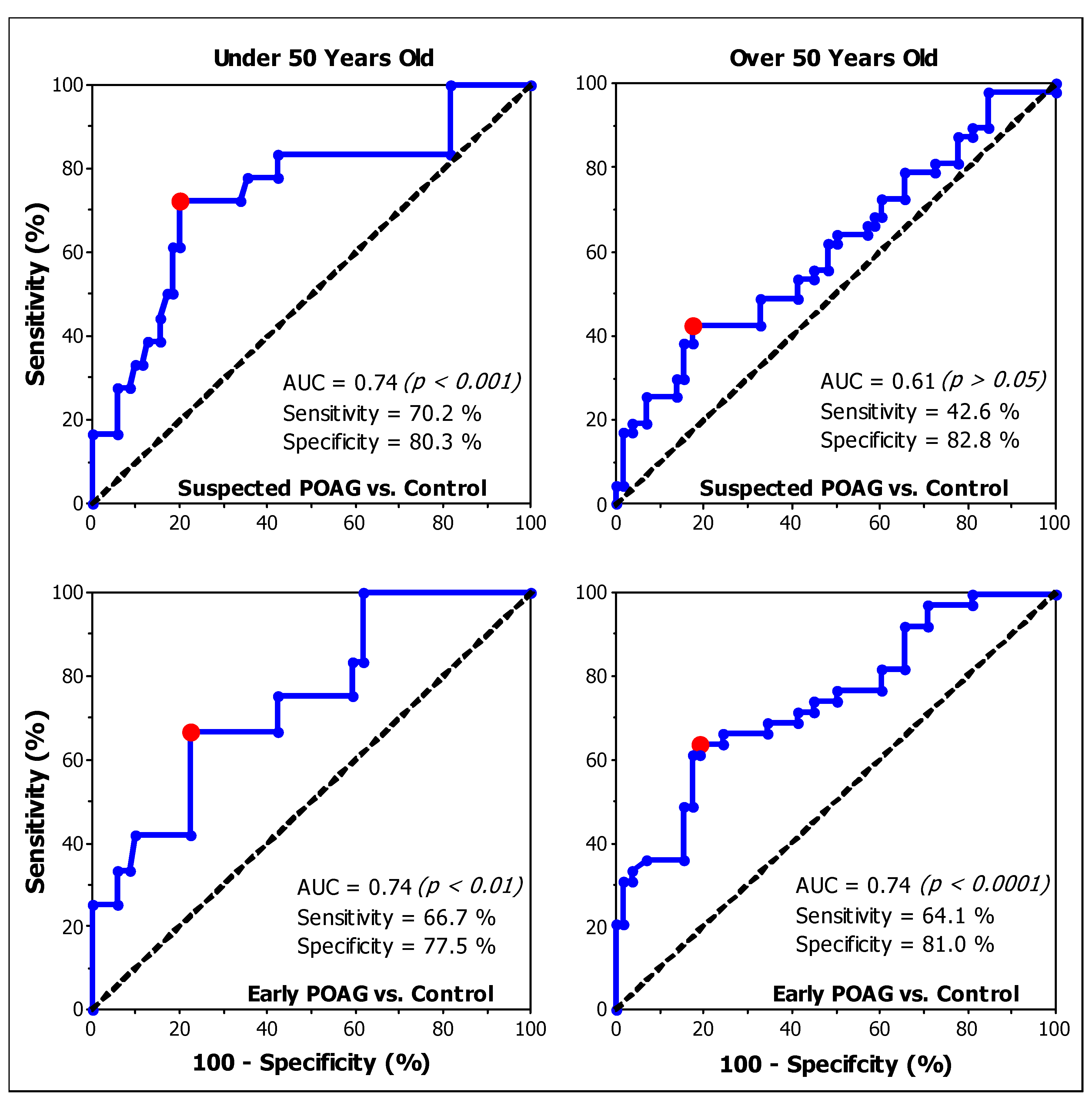
| SC | Cut Off Value | Sensitivity (%) | Specificity (%) | AUC | AUC 95% CI | Interpretation | |
|---|---|---|---|---|---|---|---|
| Suspected POAG vs. Control | Peripheral (under 50) | 22.3 | 62.5 | 58.8 | 0.55 | 0.37–0.73 | Fail |
| Peripheral (over 50) | 22.3 | 56.8 | 72.4 | 0.67 | 0.55–0.78 | Poor | |
| Foveal (under 50) | 158.1 | 72.2 | 80.3 | 0.74 | 0.64–0.83 | Fair | |
| Foveal (over 50) | 79.3 | 42.6 | 82.8 | 0.61 | 0.52–0.70 | Fail | |
| Early POAG vs. Control | Foveal (under 50) | 159.6 | 66.7 | 77.5 | 0.74 | 0.64–0.83 | Fair |
| Foveal (over 50) | 80.9 | 64.1 | 81.0 | 0.74 | 0.65–0.83 | Fair | |
| Early vs. Suspected POAG | Foveal (under 50) | 158.1 | 58.3 | 72.2 | 0.50 | 0.32–0.69 | Fail |
| Foveal (over 50) | 28.1 | 30.8 | 93.6 | 0.63 | 0.52–0.73 | Poor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripolone, M.C.; Issolio, L.A.; Perez, D.O.; Barrionuevo, P.A. Contrast Sensitivity Is Impaired in Suspected Primary Open-Angle Glaucoma Patients. Brain Sci. 2024, 14, 993. https://doi.org/10.3390/brainsci14100993
Tripolone MC, Issolio LA, Perez DO, Barrionuevo PA. Contrast Sensitivity Is Impaired in Suspected Primary Open-Angle Glaucoma Patients. Brain Sciences. 2024; 14(10):993. https://doi.org/10.3390/brainsci14100993
Chicago/Turabian StyleTripolone, María Constanza, Luis Alberto Issolio, Daniel Osvaldo Perez, and Pablo Alejandro Barrionuevo. 2024. "Contrast Sensitivity Is Impaired in Suspected Primary Open-Angle Glaucoma Patients" Brain Sciences 14, no. 10: 993. https://doi.org/10.3390/brainsci14100993
APA StyleTripolone, M. C., Issolio, L. A., Perez, D. O., & Barrionuevo, P. A. (2024). Contrast Sensitivity Is Impaired in Suspected Primary Open-Angle Glaucoma Patients. Brain Sciences, 14(10), 993. https://doi.org/10.3390/brainsci14100993






