A Dual Role for the Dorsolateral Prefrontal Cortex (DLPFC) in Auditory Deviance Detection
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Behavioral Task
2.3. EEG Recording and ERP Processing
2.4. Optical Recording and EROS Processing
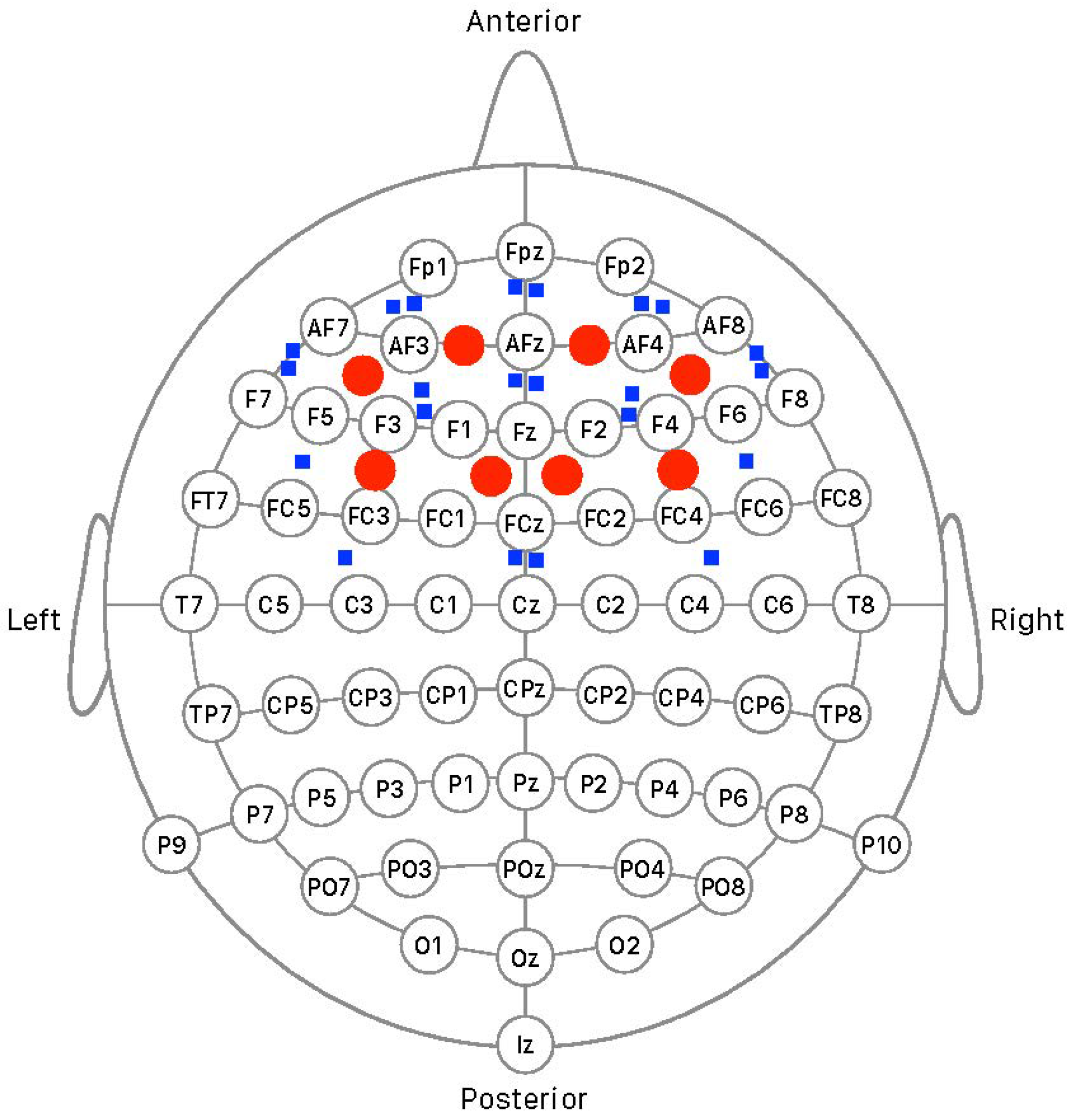
2.5. Brain Mapping and Regions of Interest
2.6. Statistical Analysis
3. Results
3.1. Analysis of the Local Peaks in the ERP Components
3.2. Differential Activations between Conditions in the ERPs
3.3. Differential Activations between Conditions in the Fast Optical Signals
4. Discussion
4.1. Early Activation of the Left DLPFC (BA 46) and the Predictive Coding Framework
4.2. Late Activation of the Right DLPFC (BA 8) and Attention-Switching Theories
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maturana, H.R.; Varela, F.J. Autopoiesis and Cognition: The Realization of the Living; D. Reidel Publishing Company: Dordrecht, Holland, 1980; Volume 42. [Google Scholar]
- Sokolov, E.N. The orienting response, and future directions of its development. Pavlov. J. Biol. Sci. 1990, 25, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Ritter, W.; Vaughan, H.G., Jr. Averaged evoked responses in vigilance and discrimination: A reassessment. Science 1969, 164, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Squires, N.K.; Squires, K.C.; Hillyard, S.A. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr. Clin. Neurophysiol. 1975, 38, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Carretta, D.; Hervé-Minvielle, A.; Bajo, V.M.; Villa, A.E.P.; Rouiller, E.M. c-Fos expression in the auditory pathways related to the significance of acoustic signals in rats performing a sensory-motor task. Brain Res. 1999, 841, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Romanski, L.M.; Bates, J.F.; Goldman-Rakic, P.S. Auditory belt and parabelt projections to the prefrontal cortex in the rhesus monkey. J. Comput. Neurol. 1999, 403, 141–157. [Google Scholar] [CrossRef]
- Brefczynski-Lewis, J.A.; Lewis, J.W. Auditory object perception: A neurobiological model and prospective review. Neuropsychologia 2017, 105, 223–242. [Google Scholar] [CrossRef]
- Banquet, J.P.; Renault, B.; Lesèvre, N. Effect of task and stimulus probability on evoked potentials. Biol. Psychol. 1981, 13, 203–214. [Google Scholar] [CrossRef]
- Polich, J. Attention, probability, and task demands as determinants of P300 latency from auditory stimuli. Electroencephalogr. Clin. Neurophysiol. 1986, 63, 251–259. [Google Scholar] [CrossRef]
- Näätänen, R.; Picton, T. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology 1987, 24, 375–425. [Google Scholar] [CrossRef]
- Friedman, D.; Cycowicz, Y.M.; Gaeta, H. The novelty P3: An event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci. Biobehav. Rev. 2001, 25, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Tomé, D.; Barbosa, F.; Nowak, K.; Marques-Teixeira, J. The development of the N1 and N2 components in auditory oddball paradigms: A systematic review with narrative analysis and suggested normative values. J. Neural. Transm. 2015, 122, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Woldorff, M.G.; Hillyard, S.A. Modulation of early auditory processing during selective listening to rapidly presented tones. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 170–191. [Google Scholar] [CrossRef] [PubMed]
- Oades, R.D.; Dittmann-Balcar, A.; Zerbin, D. The topography of 4 subtraction ERP-waveforms derived from a 3-tone auditory oddball task in healthy young adults. Int. J. Neurosci. 1995, 81, 265–281. [Google Scholar] [CrossRef]
- Brattico, E.; Tervaniemi, M.; Picton, T.W. Effects of brief discrimination-training on the auditory N1 wave. Neuroreport 2003, 14, 2489–2492. [Google Scholar] [CrossRef]
- Reinke, K.S.; He, Y.; Wang, C.; Alain, C. Perceptual learning modulates sensory evoked response during vowel segregation. Brain Res. Cogn. Brain Res. 2003, 17, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Seppänen, M.; Hämäläinen, J.; Pesonen, A.K.; Tervaniemi, M. Music training enhances rapid neural plasticity of N1 and P2 source activation for unattended sounds. Front. Human Neurosci. 2012, 6, 43. [Google Scholar] [CrossRef]
- Heacock, R.M.; Pigeon, A.; Chermak, G.; Musiek, F.; Weihing, J. Enhancement of the Auditory Late Response (N1-P2) by Presentation of Stimuli From an Unexpected Location. J. Am. Acad. Audiol. 2019, 30, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Sams, M.; Alho, K.; Näätänen, R. Sequential effects on the ERP in discriminating two stimuli. Biol. Psychol. 1983, 17, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Paavilainen, P.; Rinne, T.; Alho, K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clin. Neurophysiol. 2007, 118, 2544–2590. [Google Scholar] [CrossRef]
- Pincze, Z.; Lakatos, P.; Rajkai, C.; Ulbert, I.; Karmos, G. Separation of mismatch negativity and the N1 wave in the auditory cortex of the cat: A topographic study. Clin. Neurophysiol. 2001, 112, 778–784. [Google Scholar] [CrossRef]
- Pincze, Z.; Lakatos, P.; Rajkai, C.; Ulbert, I.; Karmos, G. Effect of deviant probability and interstimulus/interdeviant interval on the auditory N1 and mismatch negativity in the cat auditory cortex. Brain Res. Cogn. Brain Res. 2002, 13, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.L.; Villa, A.E.P. Event-related potentials in an auditory oddball situation in the rat. BioSystems 2005, 79, 207–212. [Google Scholar] [CrossRef]
- Nelken, I.; Ulanovsky, N. Mismatch Negativity and Stimulus-Specific Adaptation in Animal Models. J. Psychophysiol. 2007, 21, 214–223. [Google Scholar] [CrossRef]
- Giard, M.H.; Perrin, F.; Pernier, J.; Bouchet, P. Brain generators implicated in the processing of auditory stimulus deviance: A topographic event-related potential study. Psychophysiology 1990, 27, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Rinne, T.; Alho, K.; Ilmoniemi, R.J.; Virtanen, J.; Näätänen, R. Separate time behaviors of the temporal and frontal mismatch negativity sources. Neuroimage 2000, 12, 14–19. [Google Scholar] [CrossRef]
- Näätänen, R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behav. Brain Sci. 1990, 13, 201–288. [Google Scholar] [CrossRef]
- Wronka, E.; Kaiser, J.; Coenen, A.M.L. The auditory P3 from passive and active three-stimulus oddball paradigm. Acta Neurobiol. Exp. 2008, 68, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Justen, C.; Herbert, C. The spatio-temporal dynamics of deviance and target detection in the passive and active auditory oddball paradigm: A sLORETA study. BMC Neurosci. 2018, 19, 25. [Google Scholar] [CrossRef]
- Polich, J.; McIsaac, H.K. Comparison of auditory P300 habituation from active and passive conditions. Int. J. Psychophysiol. 1994, 17, 25–34. [Google Scholar] [CrossRef]
- Verleger, R.; Grauhan, N.; Śmigasiewicz, K. Is P3 a strategic or a tactical component? Relationships of P3 sub-components to response times in oddball tasks with go, no-go and choice responses. Neuroimage 2016, 143, 223–234. [Google Scholar] [CrossRef]
- Romanski, L.M.; Tian, B.; Fritz, J.; Mishkin, M.; Goldman-Rakic, P.S.; Rauschecker, J.P. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat. Neurosci. 1999, 2, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Alain, C.; Arnott, S.R.; Hevenor, S.; Graham, S.; Grady, C.L. “What” and “where” in the human auditory system. Proc. Natl. Acad. Sci. USA 2001, 98, 12301–12306. [Google Scholar] [CrossRef] [PubMed]
- Ahveninen, J.; Jääskeläinen, I.P.; Raij, T.; Bonmassar, G.; Devore, S.; Hämäläinen, M.; Levänen, S.; Lin, F.H.; Sams, M.; Shinn-Cunningham, B.G.; et al. Task-modulated “what” and “where” pathways in human auditory cortex. Proc. Natl. Acad. Sci. USA 2006, 103, 14608–14613. [Google Scholar] [CrossRef] [PubMed]
- Plakke, B.; Romanski, L.M. Neural circuits in auditory and audiovisual memory. Brain Res. 2016, 1640, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Camalier, C.R.; Scarim, K.; Mishkin, M.; Averbeck, B.B. A Comparison of Auditory Oddball Responses in Dorsolateral Prefrontal Cortex, Basolateral Amygdala, and Auditory Cortex of Macaque. J. Cogn. Neurosci. 2019, 31, 1054–1064. [Google Scholar] [CrossRef]
- Kiehl, K.A.; Stevens, M.C.; Laurens, K.R.; Pearlson, G.; Calhoun, V.D.; Liddle, P.F. An adaptive reflexive processing model of neurocognitive function: Supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. Neuroimage 2005, 25, 899–915. [Google Scholar] [CrossRef]
- Casey, B.J.; Forman, S.D.; Franzen, P.; Berkowitz, A.; Braver, T.S.; Nystrom, L.E.; Thomas, K.M.; Noll, D.C. Sensitivity of prefrontal cortex to changes in target probability: A functional MRI study. Human Brain Mapp. 2001, 13, 26–33. [Google Scholar] [CrossRef]
- Gratton, G.; Fabiani, M. Fast optical imaging of human brain function. Front. Human Neurosci. 2010, 4, e00052. [Google Scholar] [CrossRef] [PubMed]
- Gratton, G.; Corballis, P.M.; Cho, E.; Fabiani, M.; Hood, D.C. Shades of gray matter: Noninvasive optical images of human brain responses during visual stimulation. Psychophysiology 1995, 32, 505–509. [Google Scholar] [CrossRef]
- Gratton, G.; Fabiani, M. Shedding light on brain function: The event-related optical signal. Trends Cogn. Sci. 2001, 5, 357–363. [Google Scholar] [CrossRef]
- Wolf, M.; Wolf, U.; Choi, J.H.; Gupta, R.; Safonova, L.P.; Paunescu, L.A.; Michalos, A.; Gratton, E. Functional frequency-domain near-infrared spectroscopy detects fast neuronal signal in the motor cortex. Neuroimage 2002, 17, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Gratton, G.; Chiarelli, A.M.; Fabiani, M. From brain to blood vessels and back: A noninvasive optical imaging approach. Neurophotonics 2017, 4, 031208. [Google Scholar] [CrossRef] [PubMed]
- Villringer, A.; Chance, B. Non-invasive optical spectroscopy and imaging of human brain function. Trends Neurosci. 1997, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Rector, D.M.; Poe, G.R.; Kristensen, M.P.; Harper, R.M. Light scattering changes follow evoked potentials from hippocampal Schaeffer collateral stimulation. J. Neurophysiol. 1997, 78, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Steinbrink, J.; Kohl, M.; Obrig, H.; Curio, G.; Syré, F.; Thomas, F.; Wabnitz, H.; Rinneberg, H.; Villringer, A. Somatosensory evoked fast optical intensity changes detected non-invasively in the adult human head. Neurosci. Lett. 2000, 291, 105–108. [Google Scholar] [CrossRef]
- Wabnitz, H.; Moeller, M.; Liebert, A.; Obrig, H.; Steinbrink, J.; Macdonald, R. Time-resolved near-infrared spectroscopy and imaging of the adult human brain. Adv. Exp. Med. Biol. 2010, 662, 143–148. [Google Scholar] [CrossRef]
- Tse, C.Y.; Tien, K.R.; Penney, T.B. Event-related optical imaging reveals the temporal dynamics of right temporal and frontal cortex activation in pre-attentive change detection. Neuroimage 2006, 29, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Sable, J.J.; Low, K.A.; Whalen, C.J.; Maclin, E.L.; Fabiani, M.; Gratton, G. Optical imaging of temporal integration in human auditory cortex. Eur. J. Neurosci. 2007, 25, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.V.; Kainerstorfer, J.M.; Borisov, S.V.; Gandjbakhche, A.H.; Vanmeter, J. “Seeing” electroencephalogram through the skull: Imaging prefrontal cortex with fast optical signal. J. Biomed. Opt. 2010, 15, 061702. [Google Scholar] [CrossRef]
- Parks, N.A. Concurrent application of TMS and near-infrared optical imaging: Methodological considerations and potential artifacts. Front. Human Neurosci. 2013, 7, 592. [Google Scholar] [CrossRef]
- Fabiani, M.; Gordon, B.A.; Maclin, E.L.; Pearson, M.A.; Brumback-Peltz, C.R.; Low, K.A.; McAuley, E.; Sutton, B.P.; Kramer, A.F.; Gratton, G. Neurovascular coupling in normal aging: A combined optical, ERP and fMRI study. Neuroimage 2014, 85 Pt 1, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Manoochehri, M.; Mahmoudzadeh, M.; Bourel-Ponchel, E.; Wallois, F. Cortical light scattering during interictal epileptic spikes in frontal lobe epilepsy in children: A fast optical signal and electroencephalographic study. Epilepsia 2017, 58, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.Y.; Yip, L.Y.; Lui, T.K.Y.; Xiao, X.Z.; Wang, Y.; Chu, W.C.W.; Parks, N.A.; Chan, S.S.M.; Neggers, S.F.W. Establishing the functional connectivity of the frontotemporal network in pre-attentive change detection with Transcranial Magnetic Stimulation and event-related optical signal. Neuroimage 2018, 179, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.Y.; Low, K.A.; Fabiani, M.; Gratton, G. Rules rule! Brain activity dissociates the representations of stimulus contingencies with varying levels of complexity. J. Cogn. Neurosci. 2012, 24, 1941–1959. [Google Scholar] [CrossRef]
- Rinne, T.; Gratton, G.; Fabiani, M.; Cowan, N.; Maclin, E.; Stinard, A.; Sinkkonen, J.; Alho, K.; Näätänen, R. Scalp-recorded optical signals make sound processing in the auditory cortex visible? Neuroimage 1999, 10, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Tse, C.Y.; Rinne, T.; Ng, K.K.; Penney, T.B. The functional role of the frontal cortex in pre-attentive auditory change detection. Neuroimage 2013, 83, 870–879. [Google Scholar] [CrossRef]
- Low, K.A.; Leaver, E.; Kramer, A.F.; Fabiani, M.; Gratton, G. Fast optical imaging of frontal cortex during active and passive oddball tasks. Psychophysiology 2006, 43, 127–136. [Google Scholar] [CrossRef]
- Huettel, S.A.; McCarthy, G. What is odd in the oddball task? Prefrontal cortex is activated by dynamic changes in response strategy. Neuropsychologia 2004, 42, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.C.; Calhoun, V.D.; Kiehl, K.A. fMRI in an oddball task: Effects of target-to-target interval. Psychophysiology 2005, 42, 636–642. [Google Scholar] [CrossRef]
- Sininger, Y.S.; Bhatara, A. Laterality of basic auditory perception. Laterality 2012, 17, 129–149. [Google Scholar] [CrossRef]
- Jaquerod, M.E.; Knight, R.; Villa, A.E.P.; Lintas, A. Event-Related Potentials and Fast Optical Imaging of Cortical Activity During An Auditory Oddball Task. In Advances in Cognitive Neurodynamics (VII); Lintas, A., Enrico, P., Pan, X., Wang, R., Villa, A., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Lucker, J.R. Auditory Processing Testing: In the Booth versus Outside the Booth. J. Am. Acad. Audiol. 2017, 28, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Calderon, J.; Luck, S.J. ERPLAB: An open-source toolbox for the analysis of event-related potentials. Front. Human Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J.; Stewart, A.X.; Simmons, A.M.; Rhemtulla, M. Standardized measurement error: A universal metric of data quality for averaged event-related potentials. Psychophysiology 2021, 58, e13793. [Google Scholar] [CrossRef] [PubMed]
- Gratton, G.; Brumback, C.R.; Gordon, B.A.; Pearson, M.A.; Low, K.A.; Fabiani, M. Effects of measurement method, wavelength, and source-detector distance on the fast optical signal. NeuroImage 2006, 32, 1576–1590. [Google Scholar] [CrossRef]
- Fantini, S.; Sassaroli, A. Frequency-Domain Techniques for Cerebral and Functional Near-Infrared Spectroscopy. Front. Neurosci. 2020, 14, 300. [Google Scholar] [CrossRef]
- Chiarelli, A.M.; Maclin, E.L.; Low, K.A.; Fabiani, M.; Gratton, G. Comparison of procedures for co-registering scalp-recording locations to anatomical magnetic resonance images. J. Biomed. Opt. 2015, 20, 016009. [Google Scholar] [CrossRef]
- Gratton, G. “Opt-cont” and “Opt-3D”: A software suite for the analysis and 3D reconstruction of the event-related optical signal (EROS). Psychophysiology 2000, 37, S44. [Google Scholar]
- Lancaster, J.L.; Woldorff, M.G.; Parsons, L.M.; Liotti, M.; Freitas, C.S.; Rainey, L.; Kochunov, P.V.; Nickerson, D.; Mikiten, S.A.; Fox, P.T. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapp. 2000, 10, 120–131. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Champely, S. pwr: Basic Functions for Power Analysis. R package version 1.3-0. R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Moore, M.; Maclin, E.L.; Iordan, A.D.; Katsumi, Y.; Larsen, R.J.; Bagshaw, A.P.; Mayhew, S.; Shafer, A.T.; Sutton, B.P.; Fabiani, M.; et al. Proof-of-concept evidence for trimodal simultaneous investigation of human brain function. Human Brain Mapp. 2021, 42, 4102–4121. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, D.; Günal, M.; Chiou, N.; Koyejo, S.; Mathewson, K.; Low, K.A.; Fabiani, M.; Gratton, G.; Chiarelli, A.M. Fast Optical Signals for Real-Time Retinotopy and Brain Computer Interface. Bioengineering 2023, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, D.; Fang, F.; Hong, K.S.; Reiss, A.L.; Zhang, Y. Concurrent fNIRS and EEG for Brain Function Investigation: A Systematic, Methodology-Focused Review. Sensors 2022, 22, 5865. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.Z.; Wang, Y.; Wong, G.C.S.; Zhao, K.; Tse, C.Y. Frontotemporal network in automatic / pre-attentive detection of abstract change: An event-related optical signal (EROS) study. Neuropsychologia 2022, 164, 108093. [Google Scholar] [CrossRef]
- Muñoz-Caracuel, M.; Muñoz, V.; Ruiz-Martínez, F.J.; Vázquez Morejón, A.J.; Gómez, C.M. Systemic neurophysiological signals of auditory predictive coding. Psychophysiology 2024, 61, e14544. [Google Scholar] [CrossRef]
- Daffner, K.R.; Mesulam, M.M.; Scinto, L.F.; Acar, D.; Calvo, V.; Faust, R.; Chabrerie, A.; Kennedy, B.; Holcomb, P. The central role of the prefrontal cortex in directing attention to novel events. Brain 2000, 123 Pt 5, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Böckermann, I.; Nyhuis, P.W. The impact of transcranial magnetic stimulation on cognitive processing: An event-related potential study. Neuroreport 2001, 12, 2915–2918. [Google Scholar] [CrossRef] [PubMed]
- Soder, H.E.; de Dios, C.; Potts, G.F. The role of the neural reward system in attention selection. Neuroreport 2016, 27, 787–790. [Google Scholar] [CrossRef]
- Weigl, M.; Mecklinger, A.; Rosburg, T. Transcranial direct current stimulation over the left dorsolateral prefrontal cortex modulates auditory mismatch negativity. Clin. Neurophysiol. 2016, 127, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, K.; Melkonian, D.; Spooner, C.J.; Scher, S.; Meares, R. Cortical arousal in children and adolescents with functional neurological symptoms during the auditory oddball task. Neuroimage Clin. 2017, 13, 228–236. [Google Scholar] [CrossRef]
- Dubreuil-Vall, L.; Chau, P.; Ruffini, G.; Widge, A.S.; Camprodon, J.A. tDCS to the left DLPFC modulates cognitive and physiological correlates of executive function in a state-dependent manner. Brain Stimul. 2019, 12, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, Z.; Cheng, L.; Tian, R.; Zhao, W.; Du, J.; Zhang, Y.; Wang, C. Effect of Transcranial Direct Current Stimulation on the Mismatch Negativity Features of Deviated Stimuli in Children With Autism Spectrum Disorder. Front. Neurosci. 2022, 16, 721987. [Google Scholar] [CrossRef]
- Voegtle, A.; Reichert, C.; Hinrichs, H.; Sweeney-Reed, C.M. Repetitive Anodal TDCS to the Frontal Cortex Increases the P300 during Working Memory Processing. Brain Sci. 2022, 12, 1545. [Google Scholar] [CrossRef] [PubMed]
- Doeller, C.F.; Opitz, B.; Mecklinger, A.; Krick, C.; Reith, W.; Schröger, E. Prefrontal cortex involvement in preattentive auditory deviance detection: Neuroimaging and electrophysiological evidence. Neuroimage 2003, 20, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Deouell, L.Y. The frontal generator of the mismatch negativity revisited. J. Psychophysiol. 2007, 21, 188–203. [Google Scholar] [CrossRef]
- Garrido, M.I.; Kilner, J.M.; Stephan, K.E.; Friston, K.J. The mismatch negativity: A review of underlying mechanisms. Clin. Neurophysiol. 2009, 120, 453–463. [Google Scholar] [CrossRef]
- Quintana, J.; Fuster, J.M. Mnemonic and predictive functions of cortical neurons in a memory task. Neuroreport 1992, 3, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Friston, K. A theory of cortical responses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 815–836. [Google Scholar] [CrossRef]
- Shipp, S. Neural Elements for Predictive Coding. Front. Psychol. 2016, 7, 1792. [Google Scholar] [CrossRef]
- Postle, B.R.; Berger, J.S.; D’Esposito, M. Functional neuroanatomical double dissociation of mnemonic and executive control processes contributing to working memory performance. Proc. Natl. Acad. Sci. USA 1999, 96, 12959–12964. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.B.; Passingham, R.E. Working memory for location and time: Activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage 2001, 14, 77–86. [Google Scholar] [CrossRef]
- Petrides, M. Lateral prefrontal cortex: Architectonic and functional organization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Badre, D.; D’Esposito, M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J. Cogn. Neurosci. 2007, 19, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Minamoto, T.; Yaoi, K.; Osaka, M.; Osaka, N. The rostral prefrontal cortex underlies individual differences in working memory capacity: An approach from the hierarchical model of the cognitive control. Cortex 2015, 71, 277–290. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Dadario, N.B.; Tanglay, O.; Sughrue, M.E. Deconvoluting human Brodmann area 8 based on its unique structural and functional connectivity. Front. Neuroanat. 2023, 17, 1127143. [Google Scholar] [CrossRef]
- Zink, N.; Stock, A.K.; Colzato, L.; Beste, C. Evidence for a neural dual-process account for adverse effects of cognitive control. Brain Struct. Funct. 2018, 223, 3347–3363. [Google Scholar] [CrossRef]
- Yago, E.; Duarte, A.; Wong, T.; Barceló, F.; Knight, R.T. Temporal kinetics of prefrontal modulation of the extrastriate cortex during visual attention. Cogn. Affect. Behav. Neurosci. 2004, 4, 609–617. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Egner, T. Principles of cognitive control over task focus and task switching. Nat. Rev. Psychol. 2023, 2, 702–714. [Google Scholar] [CrossRef]
- Desimone, R.; Duncan, J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995, 18, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Beck, D.M.; Kastner, S. Top-down and bottom-up mechanisms in biasing competition in the human brain. Vision Res. 2009, 49, 1154–1165. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Cohen, J.D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef]
- Posner, M.I.; Petersen, S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990, 13, 25–42. [Google Scholar] [CrossRef]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The origin of extracellular fields and currents–EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012, 13, 407–420. [Google Scholar] [CrossRef]
- Foust, A.J.; Rector, D.M. Optically teasing apart neural swelling and depolarization. Neuroscience 2007, 145, 887–899. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.J. Spectrum measurement of fast optical signal of neural activity in brain tissue and its theoretical origin. Neuroimage 2010, 51, 713–722. [Google Scholar] [CrossRef]
- Boas, D.A.; Elwell, C.E.; Ferrari, M.; Taga, G. Twenty years of functional near-infrared spectroscopy: Introduction for the special issue. Neuroimage 2014, 85 Pt 1, 1–5. [Google Scholar] [CrossRef]

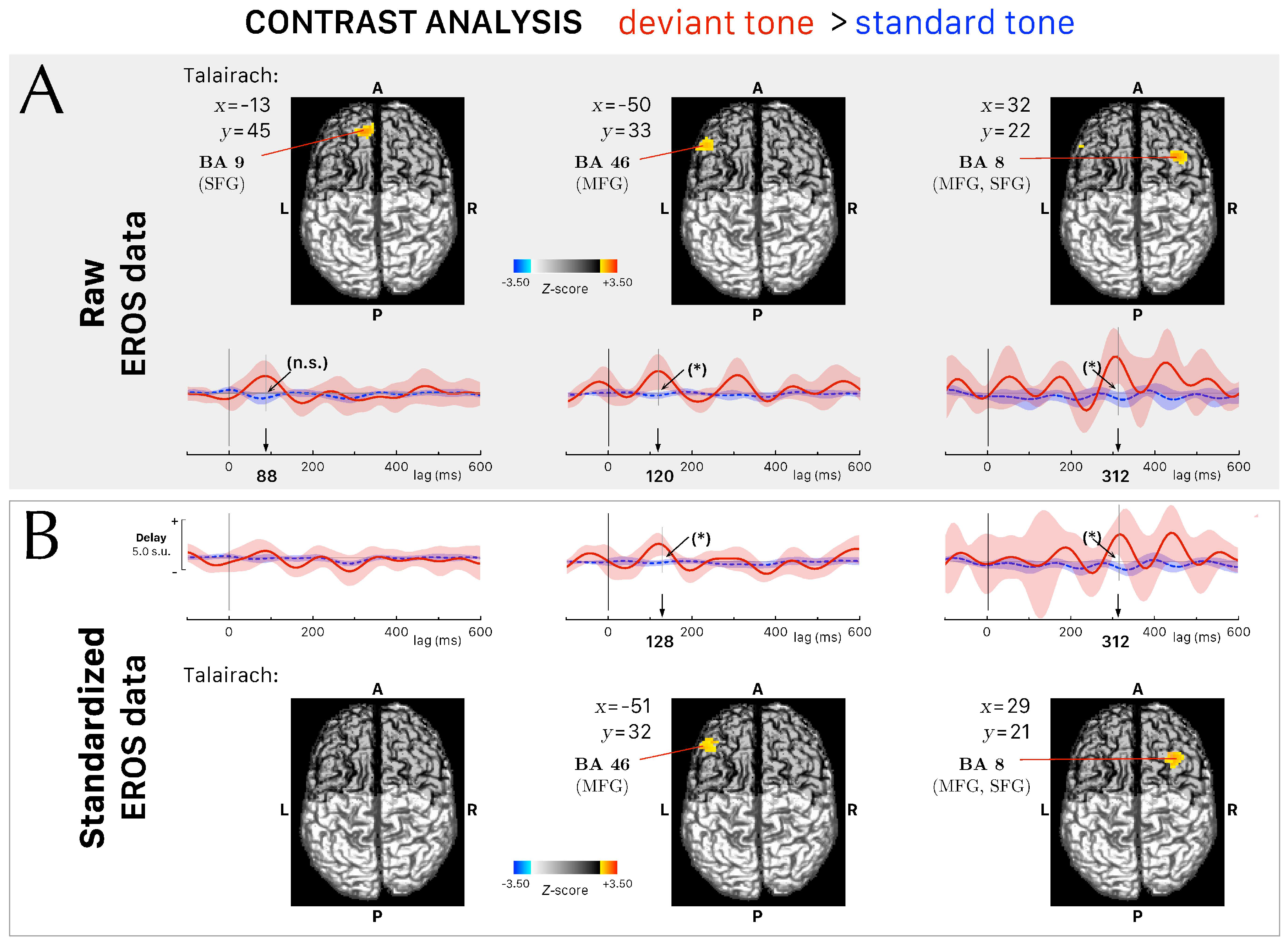
| Peak Latency (ms) | Talairach Coordinates (Peak Voxel) | Interval of Interest (ms) | Talairach Coordinates (Range of Interest) | Z-Score | ROI | ||
|---|---|---|---|---|---|---|---|
| Raw data | |||||||
| 88 | x: −13 | 72–104 | x: [−18,−5] | 2.95 | < | 3.11 | BA 9 |
| y: 45 | y: [ 43,50] | SFG | |||||
| 120 | x: −50 | 112–136 | x: [−53,−45] | 2.76 * | > | 2.67 | BA 46 |
| y: 33 | y: [ 33,35] | MFG | |||||
| 312 | x: 32 | 296–320 | x: [ 30,40] | 2.91 * | > | 2.81 | BA 8 |
| y: 22 | y: [ 20,28] | MFG, SFG | |||||
| Standardized data | |||||||
| 128 | x: −51 | 112–136 | x: [−53,−40] | 2.81 * | > | 2.69 | BA 46 |
| y: 32 | y: [ 33,40] | MFG | |||||
| 312 | x: 29 | 296–320 | x: [ 28,38] | 2.88 * | > | 2.65 | BA 8 |
| y: 21 | y: [ 18,28] | MFG, SFG | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaquerod, M.E.; Knight, R.S.; Lintas, A.; Villa, A.E.P. A Dual Role for the Dorsolateral Prefrontal Cortex (DLPFC) in Auditory Deviance Detection. Brain Sci. 2024, 14, 994. https://doi.org/10.3390/brainsci14100994
Jaquerod ME, Knight RS, Lintas A, Villa AEP. A Dual Role for the Dorsolateral Prefrontal Cortex (DLPFC) in Auditory Deviance Detection. Brain Sciences. 2024; 14(10):994. https://doi.org/10.3390/brainsci14100994
Chicago/Turabian StyleJaquerod, Manon E., Ramisha S. Knight, Alessandra Lintas, and Alessandro E. P. Villa. 2024. "A Dual Role for the Dorsolateral Prefrontal Cortex (DLPFC) in Auditory Deviance Detection" Brain Sciences 14, no. 10: 994. https://doi.org/10.3390/brainsci14100994
APA StyleJaquerod, M. E., Knight, R. S., Lintas, A., & Villa, A. E. P. (2024). A Dual Role for the Dorsolateral Prefrontal Cortex (DLPFC) in Auditory Deviance Detection. Brain Sciences, 14(10), 994. https://doi.org/10.3390/brainsci14100994








