Developmental Predictors of Suicidality in Schizophrenia: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Descriptive Findings
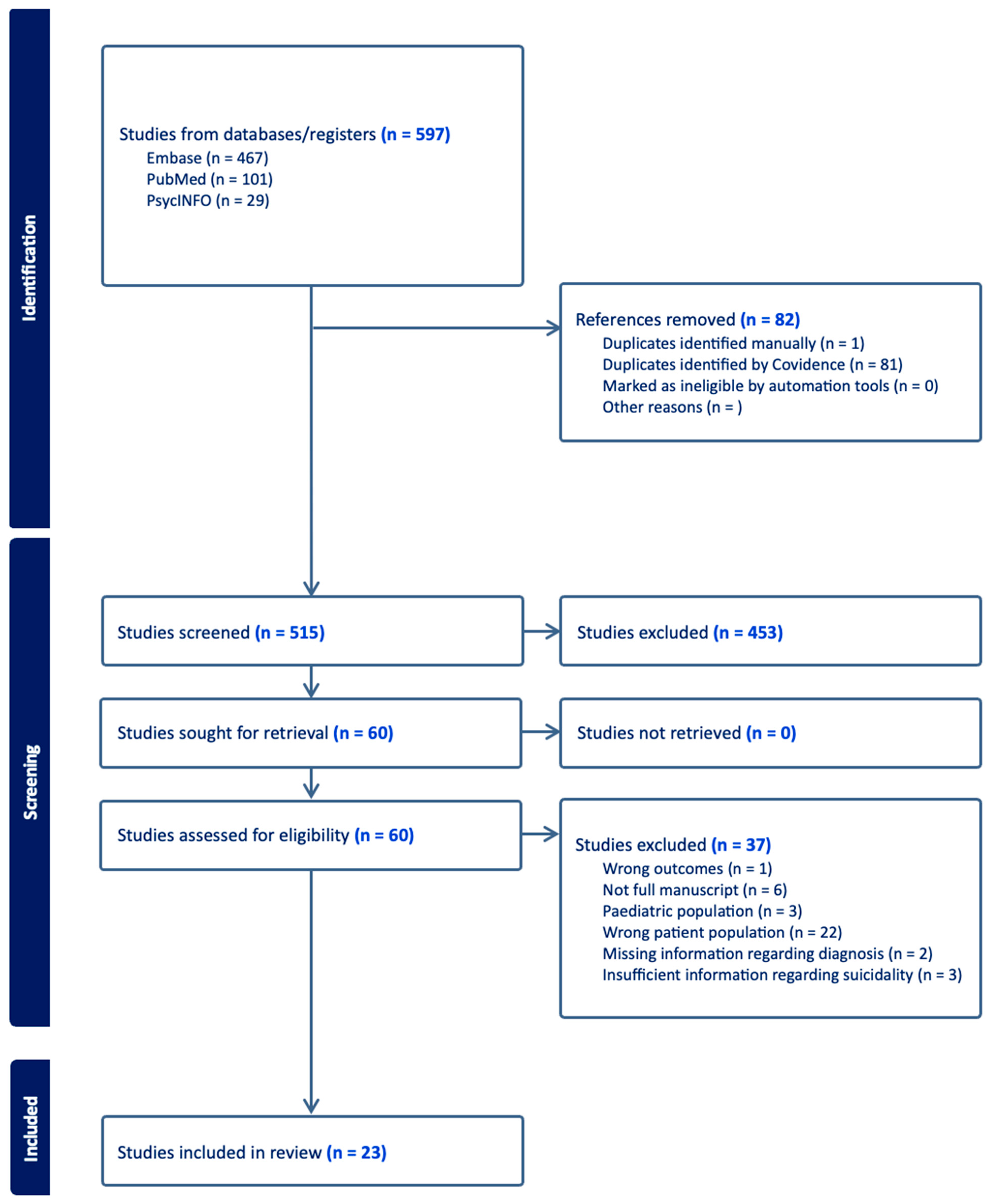
3.1.1. Study Selection
3.1.2. Sample Characteristics
3.1.3. Quality of Included Studies
3.1.4. Assessment of Suicide
3.1.5. Assessment of Developmental Factors
3.2. Key Findings
| Study | Country | Study Design | Sample Size | Population Characteristics | Developmental Predictors | SI Metric | Findings |
|---|---|---|---|---|---|---|---|
| Acosta 2020 [26] | Spain | Cross-sectional | 133 | Outpatients Age: 46.7 (10.3) Gender: 69.2% M | SES | CDS | SA and SI groups exhibited higher SES versus non-suicidal group. |
| Aydın 2019 [27] | Turkey | Retrospective | 223 | Inpatients Age and Gender of suicide group: 41.0 ± 10.6 46% M | Family history of psychotic disorder, Lifetime traumatic event | SA | No group difference in family history. Significant difference in trauma type between SA and non-SA groups |
| Bani-Fatemi 2016 [28] | Canada | Cross-sectional | 121 | Inpatients Age: 45.3 ± 11.7 Gender: 65.5% M | Ethnicity, CT; Genetics/trauma interaction | C-SSRS; BSSI | White Europeans higher likelihood of SA. General abuse strongly linked to suicidal behavior. GWAS suggests interaction between SNPs and early CT but no genome-wide significance for SA in SZ |
| Bani-Fatemi 2018 [29] | Canada | Cross-sectional | 123 | Outpatients Age: 44.7 ± 12.3 Gender: 70% M | Genetics | C-SSRS; BSSI | Methylation at various gene regions differed between SZ patients with and without a history of SA |
| Bani-Fatemi 2020 [30] | Canada | Cross-sectional | 107 | Outpatients Age: 44.58 ± 9.14 Gender: 63.2% M | Genetics | CDS | Increased methylation of the SMPD2 gene was observed among individuals with SI |
| Chang 2019 [31] | China | Retrospective | 263 | Inpatients Age: 64.11 ± 3.87 Gender: 19.1% M | SES/demographic; Other: Familry history of serious psychiatric problem; CT; Environmental factors | SA | SA in SZ linked to poor family relationships. No connection to other SES factors. Traumatic life events higher among SA patients. |
| Cheng 2022 [32] | China | Cross-sectional | 91 | Inpatients Age: 31.00 ± 7.78 Gender: 51.2% M | Childhood abuse/maltreatment; Early adversity | Beck’s suicide intent scale, nurses’ global assessment of suicide risk | Emotional neglect predicted suicide risk and SI, influenced by BMI linked to SMI. Relapse patients exhibit more CT and stress, uniquely associated with increased SI |
| Hu 2014 [33] | Canada | Case-control | 234 | Outpatients Age: 28.39 ± 9.1 Gender: 50% M | Genetics; SES/demographic | SA | TH gene TCAT(6) allele linked to 15-fold increased SA risk; TCAT(8) allele may be protective. |
| İngeç 2020 [34] | Turkey | Cross-sectional | 200 | Patients Age: 31.00 ± 7.78 Gender: 51.2% M | CT | MINI | All types of CT increase the risk of suicide |
| Kilicaslan 2017 [35] | Turkey | Cross-sectional | 200 | Inpatient and outpatient Age: 40.42 ± 11.20 Gender: 68.3% M | Childhood abuse/maltreatment; Early adversity; Family history of SI; Family history of SZ | MINI | CT predictive of SZ symptoms but not found significant predictor of SI |
| Lang 2020 [36] | China | Cross-sectional | 1087 | Inpatient Age: 47.8 ± 10.2 Gender: 81.9% M | Genetics | SA | No significant association was found between TNF-alpha gene polymorphisms and SZ or SA. The -1031C>T polymorphism linked to the age of first SA |
| Liu 2020 [37] | China | Cross-sectional | 957 | Inpatients Age: 47.8 ± 10.2 Gender: 81.8% M | Genetics | SA | The MTHFR polymorphism showed a weak correlation with SA in SZ. The Val/Val genotype was more prevalent among attempters |
| Lopez-Morinigo 2014 [38] | UK | Retrospective | 54 | Outpatient Age: 39.3 ± 11.9 Gender: 70.3% M | Demographic info | Death by suicide | SZ suicide deaths were often younger, of Black origin, English as a first language, and more socially deprived compared to non-SZ suicide deaths |
| Lyu 2021 [39] | China | Psychological Autopsy (PA) | 38 | Survey Age: 29.03 ± 5.592 Gender: 39.5% M | SES; Environmental factors | Death by suicide | No significant differences in education, residence, marital status, living alone, or family size between SZ and non-SZ suicide groups, but significant differences in age and gender, with female patients more likely to die by suicide. Lower levels of social support in SA groups |
| Mohammadzadeh 2019 [40] | Iran | Cross-sectional | 82 | Inpatient Age: 34.78 ± 9.10 Gender: 41.5% M | CT | BDI; BSSI | High levels of CT linked to more severe SI. Genderual abuse was a unique predictor of lifetime SA, while physical neglect and depression unique predictors of current SI |
| Nath 2021 [41] | India | Cross-sectional | 140 | Inpatients Age: 31.17 ± 8.5 54.3% M | Family history, SES | ISST | SI was significantly associated with a family history of psychiatric illness (especially SZ), and suicide. No relationship with SES factors |
| Olfson 2021 [42] | United States | Retrospective | 668,836 | Medicare beneficiaries Gender: 52.5%M | Resources/medicare/SES | Death by suicide | Medicare patients with SZ had a 4.5-fold increased risk of suicide, with higher risk for men, Whites. |
| Prokopez 2020 [43] | Argentina | Cross-sectional | 100 | Inpatients Age: 45.82 (±12.68) Gender: 50% M | CT | C-SSRS | Women with ≥5 ACEs had higher death ideation, SA frequency, and SA median numbers. Emotional abuse most common ACE in SZ. |
| Taktak 2023 [44] | Turkey | Cross-sectional | 222 | Outpatients Age: 45.92 ± 12.31 Gender: 70.27% M | SES | SBQ-R | Higher SI associated with high school education and lower income |
| Xia 2018 [45] | China | Retrospective | 223 | Inpatient Age: 48.1 9.6 years) Gender: 84% M | SES/CT | SA | Marital status, gender, employment, education, family history of psychiatric illness, cohabitation, and living situation were not linked to SA. Traumatic life event history was higher in those with past SA. |
| Xie 2018 [46] | China | Cross-sectional | 216 | Outpatients Age: 27.78 ± 8.13 Gender: 55.5% M | CT | SIOSS | SI positively correlated with CT severity and variety; inversely related to social support |
| Yu 2024 [47] | China | Cross-sectional | 281 | Inpatients Gender: 58.7% M | CT/environmental factors | PHQ-9; SIOSS | CT and psychological resilience influenced the onset of SI in SZ |
| Zhang 2021 [48] | Canada | Cross-sectional | 83 | Inpatients Age: 38.39 ± 10.23 Gender: 54% M | CT/physical neglect | BSSI | SI was related to insomnia and CT, with physical neglect identified as an independent risk factor |
3.2.1. Sociodemographic Factors
3.2.2. Genetic Associations
3.2.3. Childhood Adversity/Trauma
3.2.4. Family History
3.2.5. Environmental Factors
4. Discussion
4.1. Limitations
4.2. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Habtewold, T.D.; Rodijk, L.H.; Liemburg, E.J.; Sidorenkov, G.; Boezen, H.M.; Bruggeman, R.; Alizadeh, B.Z. A systematic review and narrative synthesis of data-driven studies in schizophrenia symptoms and cognitive deficits. Transl. Psychiatry 2020, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Solmi, M.; Seitidis, G.; Mavridis, D.; Correll, C.U.; Dragioti, E.; Guimond, S.; Tuominen, L.; Dargél, A.; Carvalho, A.F.; Fornaro, M.; et al. Incidence, prevalence, and global burden of schizophrenia—Data, with critical appraisal, from the Global Burden of Disease (GBD). Mol. Psychiatry 2023, 28, 5319–5327. [Google Scholar] [CrossRef] [PubMed]
- Kendhari, J.; Shankar, R.; Young-Walker, L. A Review of Childhood—Onset Schizophrenia. FOCUS 2016, 14, 328–332. [Google Scholar] [CrossRef]
- Harvey, P.D.; Strassing, M. Predicting the severity of everyday functional disability in people with schizophrenia: Cognitive deficits, functional capacity, symptoms, and health status. World Psychiatry 2012, 11, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Buckley, P.F.; Miller, B.J.; Lehrer, D.S.; Castle, D.J. Psychiatric Comorbidities and Schizophrenia. Schizophr. Bull. 2009, 35, 383–402. [Google Scholar] [CrossRef]
- Abdullah, H.M.; Azeb Shahul, H.; Hwang, M.Y.; Ferrando, S. Comorbidity in Schizophrenia: Conceptual Issues and Clinical Management. FOCUS 2020, 18, 386–390. [Google Scholar] [CrossRef]
- Hor, K.; Taylor, M. Suicide and schizophrenia: A systematic review of rates and risk factors. J. Psychopharmacol. 2010, 24 (Suppl. S4), 81–90. [Google Scholar] [CrossRef]
- Bushe, C.J.; Taylor, M.; Haukka, J. Mortality in schizophrenia: A measurable clinical endpoint. J. Psychopharmacol. 2010, 24 (Suppl. S4), 17–25. [Google Scholar] [CrossRef]
- Pompili, M.; Amador, X.F.; Girardi, P.; Harkavy-Friedman, J.; Harrow, M.; Kaplan, K.; Krausz, M.; Lester, D.; Meltzer, H.Y.; Modestin, J.; et al. Suicide risk in schizophrenia: Learning from the past to change the future. Ann. Gen. Psychiatry 2007, 6, 10. [Google Scholar] [CrossRef]
- Bai, W.; Liu, Z.H.; Jiang, Y.Y.; Zhang, Q.E.; Rao, W.W.; Cheung, T.; Hall, B.J.; Xiang, Y.T. Worldwide prevalence of suicidal ideation and suicide plan among people with schizophrenia: A meta-analysis and systematic review of epidemiological surveys. Transl. Psychiatry 2021, 11, 552. [Google Scholar] [CrossRef]
- Sher, L.; Kahn, R.S. Suicide in Schizophrenia: An Educational Overview. Medicina 2019, 55, 361. [Google Scholar] [CrossRef]
- Bornheimer, L.A. Suicidal Ideation in First-Episode Psychosis (FEP): Examination of Symptoms of Depression and Psychosis Among Individuals in an Early Phase of Treatment. Suicide Life-Threat. Behav. 2019, 49, 423–431. [Google Scholar] [CrossRef]
- Carlborg, A.; Winnerbäck, K.; Jönsson, E.G.; Jokinen, J.; Nordström, P. Suicide in schizophrenia. Expert. Rev. Neurother. 2010, 10, 1153–1164. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D. The Neurodevelopmental Hypothesis of Schizophrenia, Revisited. Schizophr. Bull. 2009, 35, 528–548. [Google Scholar] [CrossRef]
- Bornheimer, L.A.; Nguyen, D. Suicide among individuals with schizophrenia: A risk factor model. Soc. Work Ment. Health 2016, 14, 112–132. [Google Scholar] [CrossRef]
- Brown, A.S. Prenatal Infection as a Risk Factor for Schizophrenia. Schizophr. Bull. 2006, 32, 200–202. [Google Scholar] [CrossRef]
- Varese, F.; Smeets, F.; Drukker, M.; Lieverse, R.; Lataster, T.; Viechtbauer, W.; Read, J.; van Os, J.; Bentall, R.P. Childhood adversities increase the risk of psychosis: A meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr. Bull. 2012, 38, 661–671. [Google Scholar] [CrossRef]
- Arseneault, L.; Cannon, M.; Fisher, H.L.; Polanczyk, G.; Moffitt, T.E.; Caspi, A. Childhood trauma and children’s emerging psychotic symptoms: A genetically sensitive longitudinal cohort study. Am. J. Psychiatry 2011, 168, 65–72. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Borgwardt, S.; Bechdolf, A.; Addington, J.; Riecher-Rössler, A.; Schultze-Lutter, F.; Keshavan, M.; Wood, S.; Ruhrmann, S.; Seidman, L.J.; et al. The psychosis high-risk state: A comprehensive state-of-the-art review. JAMA Psychiatry 2013, 70, 107–120. [Google Scholar] [CrossRef]
- Weinberger, D.R. Future of Days Past: Neurodevelopment and Schizophrenia. Schizophr Bull. 2017, 43, 1164–1168. [Google Scholar] [CrossRef]
- Velthorst, E.; Fett, A.K.J.; Reichenberg, A.; Perlman, G.; van Os, J.; Bromet, E.J.; Kotov, R. The 20-Year Longitudinal Trajectories of Social Functioning in Individuals With Psychotic Disorders. Am. J. Psychiatry 2017, 174, 1075–1085. [Google Scholar] [CrossRef]
- Mathalon, D.H.; Hoffman, R.E.; Watson, T.D.; Miller, R.M.; Roach, B.J.; Ford, J.M. Neurophysiological Distinction between Schizophrenia and Schizoaffective Disorder. Front. Hum. Neurosci. 2010, 3, 70. [Google Scholar] [CrossRef]
- Amann, B.L.; Canales-Rodríguez, E.J.; Madre, M.; Radua, J.; Monte, G.; Alonso-Lana, S.; Landin-Romero, A.; Moreno-Alcázar, C.M.; Bonnin, S.; Sarró, J.; et al. Brain structural changes in schizoaffective disorder compared to schizophrenia and bipolar disorder. Acta. Psychiatr. Scand. 2016, 133, 23–33. [Google Scholar] [CrossRef]
- Rostom, A.; Dubé, C.; Cranney, A.; Saloojee, N.; Sy, R.; Garritty, C.; Mack, D. Appendix D. Quality Assessment Forms. In Celiac Disease; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2004. Available online: https://www.ncbi.nlm.nih.gov/books/NBK35156/ (accessed on 19 April 2024).
- Liang, M.; Guo, L.; Huo, J.; Zhou, G. Prevalence of sleep disturbances in Chinese adolescents: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0247333. [Google Scholar] [CrossRef]
- Acosta, F.J.; Navarro, S.; Cabrera, B.; Ramallo-Fariña, Y.; Martínez, N. Painful insight vs. usable insight in schizophrenia. Do they have different influences on suicidal behavior? Schizophr. Res. 2020, 220, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Aydın, M.; İlhan, B.Ç.; Tekdemir, R.; Çokünlü, Y.; Erbasan, V.; Altınbaş, K. Suicide attempts and related factors in schizophrenia patients. Saudi. Med. J. 2019, 40, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Bani-Fatemi, A.; Graff, A.; Zai, C.; Strauss, J.; De Luca, V. GWAS analysis of suicide attempt in schizophrenia: Main genetic effect and interaction with early life trauma. Neurosci. Lett. 2016, 622, 102–106. [Google Scholar] [CrossRef]
- Bani-Fatemi, A.; Jeremian, R.; Wang, K.Z.; Silveira, J.; Zai, C.; Kolla, N.J.; Graff, A.; Gerretsen, P.; Strauss, J.; De Luca, V. Epigenome-wide association study of suicide attempt in schizophrenia. J. Psychiatr. Res. 2018, 104, 192–197. [Google Scholar] [CrossRef]
- Bani-Fatemi, A.; Adanty, C.; Dai, N.; Dada, O.; Strauss, J.; Zai, C.; Gerretsen, P.; Graff, A.; De Luca, V. Genome-wide methylation association with current suicidal ideation in schizophrenia. J. Neural Transm. (Vienna) 2020, 127, 1315–1322. [Google Scholar] [CrossRef]
- Chang, Q.; Wu, D.; Rong, H.; Wu, Z.; Tao, W.; Liu, H.; Zhou, P.; Luo, G.; Xie, G.; Huang, S.; et al. Suicide ideation, suicide attempts, their sociodemographic and clinical associates among the elderly Chinese patients with schizophrenia spectrum disorders. J. Affect Disord. 2019, 256, 611–617. [Google Scholar] [CrossRef]
- Cheng, P.; Ju, P.; Xia, Q.; Chen, Y.; Li, J.; Gao, J.; Zhang, L.; Yan, F.; Cheng, X.; Pei, W.; et al. Childhood maltreatment increases the suicidal risk in Chinese schizophrenia patients. Front. Psychiatry 2022, 13, 927540. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chan, L.F.; Souza, R.P.; Tampakeras, M.; Kennedy, J.L.; Zai, C.; De Luca, V. The role of tyrosine hydroxylase gene variants in suicide attempt in schizophrenia. Neurosci. Lett. 2014, 559, 39–43. [Google Scholar] [CrossRef]
- İngeç, C.; Evren Kılıçaslan, E. The effect of childhood trauma on age of onset in patients with schizophrenia. Int. J. Soc. Psychiatry 2020, 66, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Kilicaslan, E.E.; Esen, A.T.; Kasal, M.I.; Ozelci, E.; Boysan, M.; Gulec, M. Childhood trauma, depression, and sleep quality and their association with psychotic symptoms and suicidality in schizophrenia. Psychiatry Res. 2017, 258, 557–564. [Google Scholar] [CrossRef]
- Lang, X.; Trihn, T.H.; Wu, H.E.; Tong, Y.; Xiu, M.; Zhang, X.Y. Association between TNF-alpha polymorphism and the age of first suicide attempt in chronic patients with schizophrenia. Aging 2020, 12, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Zhu, C.; Zheng, K.; Tang, W.; Gao, L.L.; Trihn, T.H.; Wu, H.E.; Chen, D.C.; Xiu, M.H.; Zhang, X.Y. MTHFR Ala222Val polymorphism and clinical characteristics confer susceptibility to suicide attempt in chronic patients with schizophrenia. Sci. Rep. 2020, 10, 5008. [Google Scholar] [CrossRef]
- Lopez-Morinigo, J.D.; Fernandes, A.C.; Chang, C.K.; Hayes, R.D.; Broadbent, M.; Stewart, R.; David, A.S.; Dutta, R. Suicide completion in secondary mental healthcare: A comparison study between schizophrenia spectrum disorders and all other diagnoses. BMC Psychiatry 2014, 14, 213. [Google Scholar] [CrossRef]
- Lyu, J.; Zhang, J.; Hennessy, D.A. Characteristics and Risk Factors for Suicide in People with Schizophrenia in Comparison to Those without Schizophrenia. Psychiatry Res. 2021, 304, 114166. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Azadi, S.; King, S.; Khosravani, V.; Sharifi Bastan, F. Childhood trauma and the likelihood of increased suicidal risk in schizophrenia. Psychiatry Res. 2019, 275, 100–107. [Google Scholar] [CrossRef]
- Nath, S.; Kalita, K.N.; Baruah, A.; Saraf, A.S.; Mukherjee, D.; Singh, P.K. Suicidal ideation in schizophrenia: A cross-sectional study in a tertiary mental hospital in North-East India. Indian J. Psychiatry 2021, 63, 179–183. [Google Scholar] [CrossRef]
- Olfson, M.; Stroup, T.S.; Huang, C.; Wall, M.M.; Crystal, S.; Gerhard, T. Suicide Risk in Medicare Patients With Schizophrenia Across the Life Span. JAMA Psychiatry 2021, 78, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Prokopez, C.R.; Vallejos, M.; Farinola, R.; Alberio, G.; Caporusso, G.B.; Cozzarin, L.G.; Chiapella, L.C.; Fuentes, P.; Daray, F.M. The history of multiple adverse childhood experiences in patients with schizophrenia is associated with more severe symptomatology and suicidal behavior with gender-specific characteristics. Psychiatry Res. 2020, 293, 113411. [Google Scholar] [CrossRef] [PubMed]
- Taktak, Ş.; Öz, H.S. The Relationship Between Depression, Anxiety and Stress Levels on Suicidal Behavior in Patients with Schizophrenia. Psychiatry Clin. Psychopharmacol. 2023, 33, 108–116. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, G.; Du, X.; Zhang, Y.; Yin, G.; Dai, J.; He, M.-X.; Soares, J.C.; Li, X.; Zhang, X.Y. Suicide attempt, clinical correlates, and BDNF Val66Met polymorphism in chronic patients with schizophrenia. Neuropsychology 2018, 32, 199–205. [Google Scholar] [CrossRef]
- Xie, P.; Wu, K.; Zheng, Y.; Guo, Y.; Yang, Y.; He, J.; Ding, Y.; Peng, H. Prevalence of childhood trauma and correlations between childhood trauma, suicidal ideation, and social support in patients with depression, bipolar disorder, and schizophrenia in southern China. J. Affect Disord. 2018, 228, 41–48. [Google Scholar] [CrossRef]
- Yu, C.L.; Kao, Y.C.; Thompson, T.; Brunoni, A.R.; Hsu, C.W.; Carvalho, A.F.; Chu, C.-S.; Tseng, P.-T.; Tu, Y.-K.; Yang, F.-C.; et al. The association of total pulses with the efficacy of repetitive transcranial magnetic stimulation for treatment-resistant major depression: A dose-response meta-analysis. Asian J. Psychiatry 2024, 92, 103891. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, X.; Tang, B.; Fan, K.; Wen, N.; Zhao, K.; Xu, W.; Tang, W.; Chen, Y. Childhood Trauma and Insomnia Increase Suicidal Ideation in Schizophrenia Patients: A Cross-Sectional Study. Front. Psychiatry 2021, 12, 769743. [Google Scholar] [CrossRef]
- Etchecopar-Etchart, D.; Korchia, T.; Loundou, A.; Llorca, P.M.; Auquier, P.; Lançon, C.; Boyer, L.; Fond, G. Comorbid Major Depressive Disorder in Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr. Bull. 2020, 47, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.D.; Huang, X.; Fox, K.R.; Franklin, J.C. Depression and hopelessness as risk factors for suicide ideation, attempts and death: Meta-analysis of longitudinal studies. Br. J. Psychiatry 2018, 212, 279–286. [Google Scholar] [CrossRef]
- Taylor, P.J.; Gooding, P.A.; Wood, A.M.; Johnson, J.; Pratt, D.; Tarrier, N. Defeat and entrapment in schizophrenia: The relationship with suicidal ideation and positive psychotic symptoms. Psychiatry Res. 2010, 178, 244–248. [Google Scholar] [CrossRef]
- Qin, P.; Agerbo, E.; Mortensen, P.B. Suicide risk in relation to family history of completed suicide and psychiatric disorders: A nested case-control study based on longitudinal registers. Lancet 2002, 360, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Janiri, D.; Rossi, P.D.; Kotzalidis, G.D.; Girardi, P.; Koukopoulos, A.E.; Reginaldi, D.; Dotto, F.; Manfredi, G.; Jollant, F.; Gorwood, P.; et al. Psychopathological characteristics and adverse childhood events are differentially associated with suicidal ideation and suicidal acts in mood disorders. Eur. Psychiatry 2018, 53, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Sheffler, J.L.; Stanley, I.; Sachs-Ericsson, N. Chapter 4—ACEs and mental health outcomes. In Adverse Childhood Experiences; Asmundson, G.J.G., Afifi, T.O., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 47–69. [Google Scholar] [CrossRef]
- Teicher, M.H.; Samson, J.A. Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. J. Child. Psychol. Psychiatry 2016, 57, 241–266. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benster, L.L.; Stapper, N.; Rodriguez, K.; Daniels, H.; Villodas, M.; Weissman, C.R.; Daskalakis, Z.J.; Appelbaum, L.G. Developmental Predictors of Suicidality in Schizophrenia: A Systematic Review. Brain Sci. 2024, 14, 995. https://doi.org/10.3390/brainsci14100995
Benster LL, Stapper N, Rodriguez K, Daniels H, Villodas M, Weissman CR, Daskalakis ZJ, Appelbaum LG. Developmental Predictors of Suicidality in Schizophrenia: A Systematic Review. Brain Sciences. 2024; 14(10):995. https://doi.org/10.3390/brainsci14100995
Chicago/Turabian StyleBenster, Lindsay L., Noah Stapper, Katie Rodriguez, Hadley Daniels, Miguel Villodas, Cory R. Weissman, Zafiris J. Daskalakis, and Lawrence G. Appelbaum. 2024. "Developmental Predictors of Suicidality in Schizophrenia: A Systematic Review" Brain Sciences 14, no. 10: 995. https://doi.org/10.3390/brainsci14100995
APA StyleBenster, L. L., Stapper, N., Rodriguez, K., Daniels, H., Villodas, M., Weissman, C. R., Daskalakis, Z. J., & Appelbaum, L. G. (2024). Developmental Predictors of Suicidality in Schizophrenia: A Systematic Review. Brain Sciences, 14(10), 995. https://doi.org/10.3390/brainsci14100995






