Contributions of the Primary Sensorimotor Cortex and Posterior Parietal Cortex to Motor Learning and Transfer
Abstract
1. Introduction
2. Methods
2.1. Participants
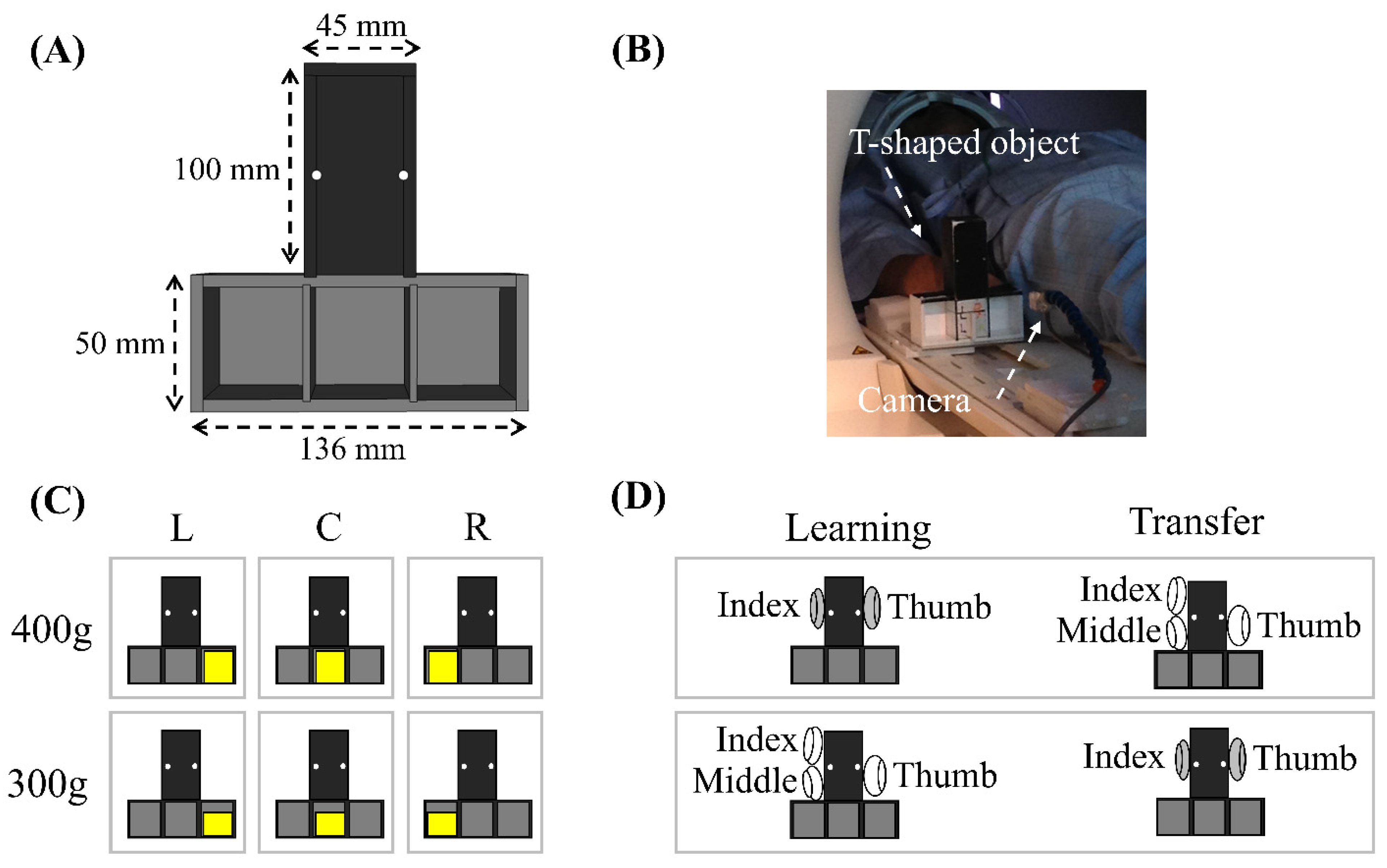
2.2. Grasp and Lift Manipulation Experiments
2.2.1. Apparatus
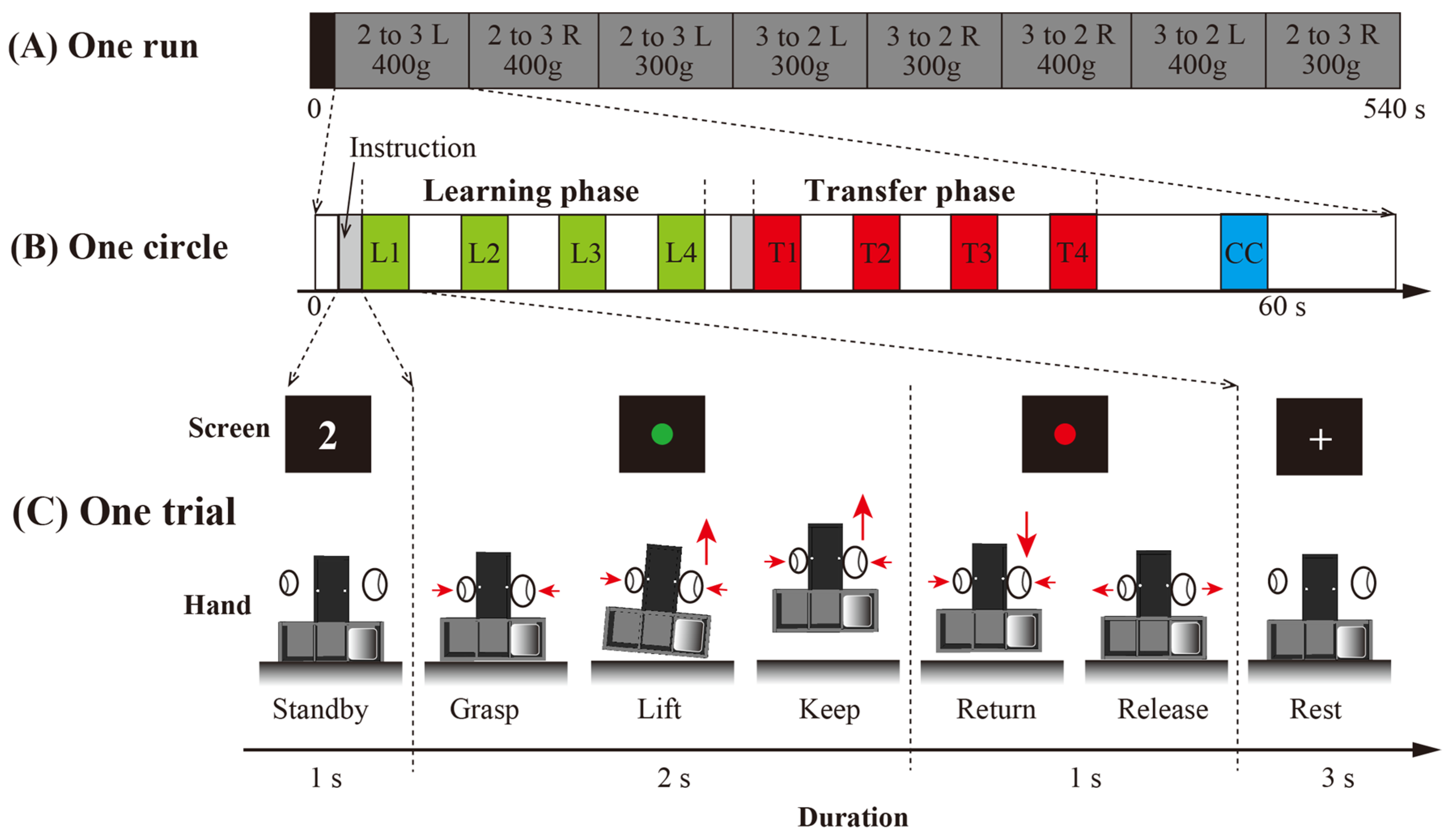
2.2.2. Task Procedure
2.3. fMRI Image Acquisition
2.4. fMRI Data Analysis
2.4.1. Initial Individual Analysis
2.4.2. Group Analysis
3. Results
3.1. Whole-Brain Neural Activity for Grasp and Lift Manipulations During the Learning Period Compared with the Baseline
3.2. Whole-Brain Neural Activity for Grasp and Lift Manipulations During the Transfer Period Compared with the Baseline
3.3. Brain Activities Associated with Transferred Motor Learning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Censor, N.; Sagi, D.; Cohen, L.G. Common Mechanisms of Human Perceptual and Motor Learning. Nat. Rev. Neurosci. 2012, 13, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Floyer-Lea, A.; Matthews, P.M. Distinguishable Brain Activation Networks for Short- and Long-Term Motor Skill Learning. J. Neurophysiol. 2005, 94, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.T.; Miller, L.M.; Rao, A.A.; D’Esposito, M. Functional Connectivity of Cortical Networks Involved in Bimanual Motor Sequence Learning. Cereb. Cortex 2007, 17, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.A.; Tanaka, S.; Wise, S.P.; Sadato, N.; Tanabe, H.C.; Willingham, D.T.; Cohen, L.G. Neural Substrates of Intermanual Transfer of a Newly Acquired Motor Skill. Curr. Biol. 2007, 17, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Hasan, Z.; Santello, M. Transfer of Learned Manipulation Following Changes in Degrees of Freedom. J. Neurosci. 2011, 31, 13576–13584. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, W.; Santello, M. Anticipatory Planning and Control of Grasp Positions and Forces for Dexterous Two-Digit Manipulation. J. Neurosci. 2010, 30, 9117–9126. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.A.; Bobich, L.R.; Santello, M. Coordination of Intrinsic and Extrinsic Hand Muscle Activity as a Function of Wrist Joint Angle during Two-Digit Grasping. Neurosci. Lett. 2010, 474, 104–108. [Google Scholar] [CrossRef]
- Poston, B.; Santos, A.D.D.; Jesunathadas, M.; Hamm, T.M.; Santello, M. Force-Independent Distribution of Correlated Neural Inputs to Hand Muscles during Three-Digit Grasping. J. Neurophysiol. 2010, 104, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Ehrsson, H.H.; Fagergren, A.; Jonsson, T.; Westling, G.; Johansson, R.S.; Forssberg, H. Cortical Activity in Precision- versus Power-Grip Tasks: An FMRI Study. J. Neurophysiol. 2000, 83, 528–536. [Google Scholar] [CrossRef]
- Castiello, U. The Neuroscience of Grasping. Nat. Rev. Neurosci. 2005, 6, 726–736. [Google Scholar] [CrossRef]
- Lemon, R.N. Descending Pathways in Motor Control. Annu. Rev. Neurosci. 2008, 31, 195–218. [Google Scholar] [CrossRef]
- Davare, M.; Kraskov, A.; Rothwell, J.C.; Lemon, R.N. Interactions between Areas of the Cortical Grasping Network. Curr. Opin. Neurobiol. 2011, 21, 565–570. [Google Scholar] [CrossRef]
- Casiraghi, L.; Alahmadi, A.A.S.; Monteverdi, A.; Palesi, F.; Castellazzi, G.; Savini, G.; Friston, K.; Gandini Wheeler-Kingshott, C.A.M.; D’Angelo, E. I See Your Effort: Force-Related BOLD Effects in an Extended Action Execution-Observation Network Involving the Cerebellum. Cereb. Cortex 2019, 29, 1351–1368. [Google Scholar] [CrossRef]
- Adams, R.A.; Shipp, S.; Friston, K.J. Predictions Not Commands: Active Inference in the Motor System. Brain Struct. Funct. 2013, 218, 611–643. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.F.; Simmons, W.K. Interoceptive Predictions in the Brain. Nat. Rev. Neurosci. 2015, 16, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Jbabdi, S.; Caspers, S.; Laird, A.R.; Fox, P.T.; Zilles, K.; Behrens, T.E.J. Anatomical and Functional Connectivity of Cytoarchitectonic Areas within the Human Parietal Operculum. J. Neurosci. 2010, 30, 6409–6421. [Google Scholar] [CrossRef] [PubMed]
- Rajaei, N.; Aoki, N.; Takahashi, H.K.; Miyaoka, T.; Kochiyama, T.; Ohka, M.; Sadato, N.; Kitada, R. Brain Networks Underlying Conscious Tactile Perception of Textures as Revealed Using the Velvet Hand Illusion. Hum. Brain Mapp. 2018, 39, 4787–4801. [Google Scholar] [CrossRef]
- Sathian, K.; Lacey, S.; Stilla, R.; Gibson, G.O.; Deshpande, G.; Hu, X.; LaConte, S.; Glielmi, C. Dual Pathways for Haptic and Visual Perception of Spatial and Texture Information. Neuroimage 2011, 57, 462–475. [Google Scholar] [CrossRef]
- Johansson, R.S.; Flanagan, J.R. Coding and Use of Tactile Signals from the Fingertips in Object Manipulation Tasks. Nat. Rev. Neurosci. 2009, 10, 345–359. [Google Scholar] [CrossRef]
- Goodwin, A.W.; Jenmalm, P.; Johansson, R.S. Control of Grip Force When Tilting Objects: Effect of Curvature of Grasped Surfaces and Applied Tangential Torque. J. Neurosci. 1998, 18, 10724–10734. [Google Scholar] [CrossRef]
- Salimi, I.; Hollender, I.; Frazier, W.; Gordon, A.M. Specificity of Internal Representations Underlying Grasping. J. Neurophysiol. 2000, 84, 2390–2397. [Google Scholar] [CrossRef] [PubMed]
- Salimi, I.; Frazier, W.; Reilmann, R.; Gordon, A.M. Selective Use of Visual Information Signaling Objects’ Center of Mass for Anticipatory Control of Manipulative Fingertip Forces. Exp. Brain Res. 2003, 150, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Lukos, J.R.; Ansuini, C.; Santello, M. Anticipatory Control of Grasping: Independence of Sensorimotor Memories for Kinematics and Kinetics. J. Neurosci. 2008, 28, 12765–12774. [Google Scholar] [CrossRef]
- Westling, G.; Johansson, R.S. Factors Influencing the Force Control during Precision Grip. Exp. Brain Res. 1984, 53, 277–284. [Google Scholar] [CrossRef]
- Johansson, R.S.; Westling, G. Programmed and Triggered Actions to Rapid Load Changes during Precision Grip. Exp. Brain Res. 1988, 71, 72–86. [Google Scholar] [CrossRef]
- Dimitriou, M.; Edin, B.B. Human Muscle Spindles Act as Forward Sensory Models. Curr. Biol. 2010, 20, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Westling, G.; Cole, K.J.; Johansson, R.S. Memory Representations Underlying Motor Commands Used during Manipulation of Common and Novel Objects. J. Neurophysiol. 1993, 69, 1789–1796. [Google Scholar] [CrossRef]
- Mojtahedi, K.; Fu, Q.; Santello, M. Extraction of Time and Frequency Features from Grip Force Rates during Dexterous Manipulation. IEEE Trans. Biomed. Eng. 2015, 62, 1363–1375. [Google Scholar] [CrossRef]
- de Lange, F.P.; Heilbron, M.; Kok, P. How Do Expectations Shape Perception? Trends Cogn. Sci. 2018, 22, 764–779. [Google Scholar] [CrossRef]
- Yu, Y.; Huber, L.; Yang, J.; Jangraw, D.C.; Handwerker, D.A.; Molfese, P.J.; Chen, G.; Ejima, Y.; Wu, J.; Bandettini, P.A. Layer-Specific Activation of Sensory Input and Predictive Feedback in the Human Primary Somatosensory Cortex. Sci. Adv. 2019, 5, eaav9053. [Google Scholar] [CrossRef]
- Alexander, W.H.; Brown, J.W. Frontal Cortex Function as Derived from Hierarchical Predictive Coding. Sci. Rep. 2018, 8, 3843. [Google Scholar] [CrossRef]
- Cope, T.E.; Sohoglu, E.; Sedley, W.; Patterson, K.; Jones, P.S.; Wiggins, J.; Dawson, C.; Grube, M.; Carlyon, R.P.; Griffiths, T.D.; et al. Evidence for Causal Top-down Frontal Contributions to Predictive Processes in Speech Perception. Nat. Commun. 2017, 8, 2154. [Google Scholar] [CrossRef]
- Desmurget, M.; Reilly, K.T.; Richard, N.; Szathmari, A.; Mottolese, C.; Sirigu, A. Movement Intention after Parietal Cortex Stimulation in Humans. Science 2009, 324, 811–813. [Google Scholar] [CrossRef]
- Zhang, W.; Gordon, A.M.; Fu, Q.; Santello, M. Manipulation after Object Rotation Reveals Independent Sensorimotor Memory Representations of Digit Positions and Forces. J. Neurophysiol. 2010, 103, 2953–2964. [Google Scholar] [CrossRef] [PubMed]
- Penny, W.; Friston, K.; Ashburner, J.; Kiebel, S.; Nichols, T. Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Friston, K.J.; Glaser, D.E.; Henson, R.N.A.; Kiebel, S.; Phillips, C.; Ashburner, J. Classical and Bayesian Inference in Neuroimaging: Applications. Neuroimage 2002, 16, 484–512. [Google Scholar] [CrossRef] [PubMed]
- R Studio Team. R Studio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2016; Available online: http://www.rstudio.com/ (accessed on 1 July 2022).
- Wolpert, D.M.; Pearson, K.G.; Ghez, C.P.J. The Organization and Planning of Movement. Princ. Neurosci. 2013, 5, 743–767. [Google Scholar]
- Hummelsheim, H.; Bianchetti, M.; Wiesendanger, M.; Wiesendanger, R. Sensory Inputs to the Agranular Motor Fields: A Comparison between Precentral, Supplementary-Motor and Premotor Areas in the Monkey. Exp. Brain Res. 1988, 69, 289–298. [Google Scholar] [CrossRef]
- Serences, J.T.; Ester, E.F.; Vogel, E.K.; Awh, E. Stimulus-Specific Delay Activity in Human Primary Visual Cortex. Psychol. Sci. 2009, 20, 207–214. [Google Scholar] [CrossRef]
- Harrison, S.A.; Tong, F. Decoding Reveals the Contents of Visual Working Memory in Early Visual Areas. Nature 2009, 458, 632–635. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Kay, K.N.; Tian, Y.; Ku, Y. Sensory Recruitment Revisited: Ipsilateral V1 Involved in Visual Working Memory. Cereb. Cortex 2022, 32, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhou, Y.D.; Bodner, M.; Ku, Y. The Causal Role of the Prefrontal Cortex and Somatosensory Cortex in Tactile Working Memory. Cereb. Cortex 2018, 28, 3468–3477. [Google Scholar] [CrossRef] [PubMed]
- Kaas, A.L.; Van Mier, H.; Goebel, R. The Neural Correlates of Human Working Memory for Haptically Explored Object Orientations. Cereb. Cortex 2007, 17, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Jenmalm, P.; Schmitz, C.; Forssberg, H.; Ehrsson, H.H. Lighter or Heavier than Predicted: Neural Correlates of Corrective Mechanisms during Erroneously Programmed Lifts. J. Neurosci. 2006, 26, 9015–9021. [Google Scholar] [CrossRef]
- Chouinard, P.A.; Leonard, G.; Paus, T. Role of the Primary Motor and Dorsal Premotor Cortices in the Anticipation of Forces during Object Lifting. J. Neurosci. 2005, 25, 2277–2284. [Google Scholar] [CrossRef]
- Chouinard, P.A. Different Roles of PMv and PMd during Object Lifting. J. Neurosci. 2006, 26, 6397–6398. [Google Scholar] [CrossRef]
- Schabrun, S.M.; Ridding, M.C.; Miles, T.S. Role of the Primary Motor and Sensory Cortex in Precision Grasping: A Transcranial Magnetic Stimulation Study. Eur. J. Neurosci. 2008, 27, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Davare, M.; Parikh, P.J.; Santello, M. Sensorimotor Uncertainty Modulates Corticospinal Excitability during Skilled Object Manipulation. J. Neurophysiol. 2019, 121, 1162–1170. [Google Scholar] [CrossRef]
- Parikh, P.J.; Fine, J.M.; Santello, M. Dexterous Object Manipulation Requires Context-Dependent Sensorimotor Cortical Interactions in Humans. Cereb. Cortex 2020, 30, 3087–3101. [Google Scholar] [CrossRef] [PubMed]
- Weilke, F.; Spiegel, S.; Boecker, H.; Von Einsiedel, H.G.; Conrad, B.; Schwaiger, M.; Erhard, P. Time-Resolved FMRI of Activation Patterns in M1 and SMA during Complex Voluntary Movement. J. Neurophysiol. 2001, 85, 1858–1863. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Breakspear, M.; Cunnington, R. Reciprocal Interactions of the SMA and Cingulate Cortex Sustain Premovement Activity for Voluntary Actions. J. Neurosci. 2014, 34, 16397–16407. [Google Scholar] [CrossRef]
- Shirinbayan, S.I.; Dreyer, A.M.; Rieger, J.W. Cortical and Subcortical Areas Involved in the Regulation of Reach Movement Speed in the Human Brain: An FMRI Study. Hum. Brain Mapp. 2019, 40, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Randerath, J.; Valyear, K.F.; Philip, B.A.; Frey, S.H. Contributions of the Parietal Cortex to Increased Efficiency of Planning-Based Action Selection. Neuropsychologia 2017, 105, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Ehrsson, H.H.; Fagergren, A.; Johansson, R.S.; Forssberg, H. Evidence for the Involvement of the Posterior Parietal Cortex in Coordination of Fingertip Forces for Grasp Stability in Manipulation. J. Neurophysiol. 2003, 90, 2978–2986. [Google Scholar] [CrossRef]
- Leib, R.; Mawase, F.; Karniel, A.; Donchin, O.; Rothwell, J.; Nisky, I.; Davare, M. Stimulation of PPC Affects the Mapping between Motion and Force Signals for Stiffness Perception but Not Motion Control. J. Neurosci. 2016, 36, 10545–10559. [Google Scholar] [CrossRef] [PubMed]
- Van Der Burgh, H.K.; Westeneng, H.J.; Walhout, R.; Van Veenhuijzen, K.; Tan, H.H.G.; Meier, J.M.; Bakker, L.A.; Hendrikse, J.; Van Es, M.A.; Veldink, J.H.; et al. Multimodal Longitudinal Study of Structural Brain Involvement in Amyotrophic Lateral Sclerosis. Neurology 2020, 94, e2592–e2604. [Google Scholar] [CrossRef]
- Castelnovo, V.; Canu, E.; Calderaro, D.; Riva, N.; Poletti, B.; Basaia, S.; Solca, F.; Silani, V.; Filippi, M.; Agosta, F. Progression of Brain Functional Connectivity and Frontal Cognitive Dysfunction in ALS. Neuroimage Clin. 2020, 28, 102509. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Yu, Y.; Yang, J. Contributions of the Primary Sensorimotor Cortex and Posterior Parietal Cortex to Motor Learning and Transfer. Brain Sci. 2024, 14, 1184. https://doi.org/10.3390/brainsci14121184
Wang C, Yu Y, Yang J. Contributions of the Primary Sensorimotor Cortex and Posterior Parietal Cortex to Motor Learning and Transfer. Brain Sciences. 2024; 14(12):1184. https://doi.org/10.3390/brainsci14121184
Chicago/Turabian StyleWang, Chenyu, Yinghua Yu, and Jiajia Yang. 2024. "Contributions of the Primary Sensorimotor Cortex and Posterior Parietal Cortex to Motor Learning and Transfer" Brain Sciences 14, no. 12: 1184. https://doi.org/10.3390/brainsci14121184
APA StyleWang, C., Yu, Y., & Yang, J. (2024). Contributions of the Primary Sensorimotor Cortex and Posterior Parietal Cortex to Motor Learning and Transfer. Brain Sciences, 14(12), 1184. https://doi.org/10.3390/brainsci14121184





