Norepinephrine-Activated p38 MAPK Pathway Mediates Stress-Induced Cytotoxic Edema of Basolateral Amygdala Astrocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Animal Treatments and Experimental Procedure
2.3. Behavioral Experiments
2.4. Determination of Serum and BLA Levels of NE and Epinephrine (E)
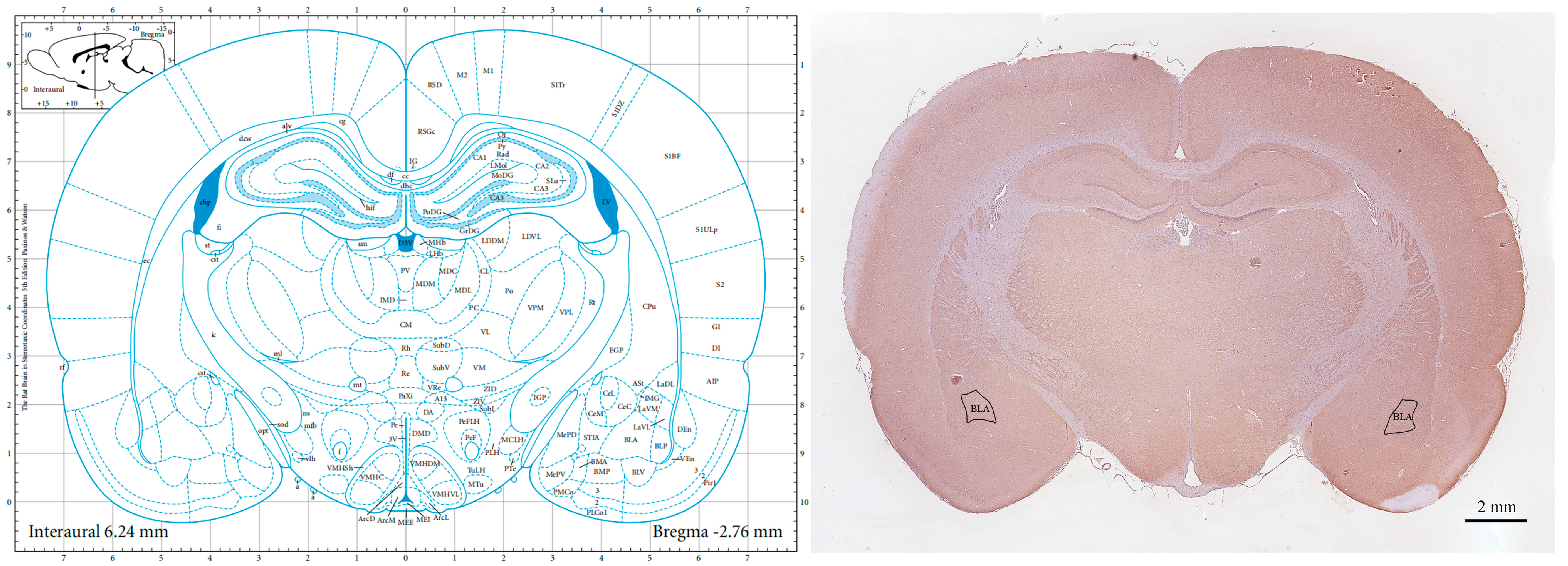
2.5. Tissue Preparation
2.6. Cells and Cell Culture
2.7. Hematoxylin and Eosin (HE) Staining
2.8. Transmission Electron Microscopy (TEM)
2.9. Immunohistochemistry Assay
2.10. Immunocytochemistry Assay
2.11. Western Blot Analysis
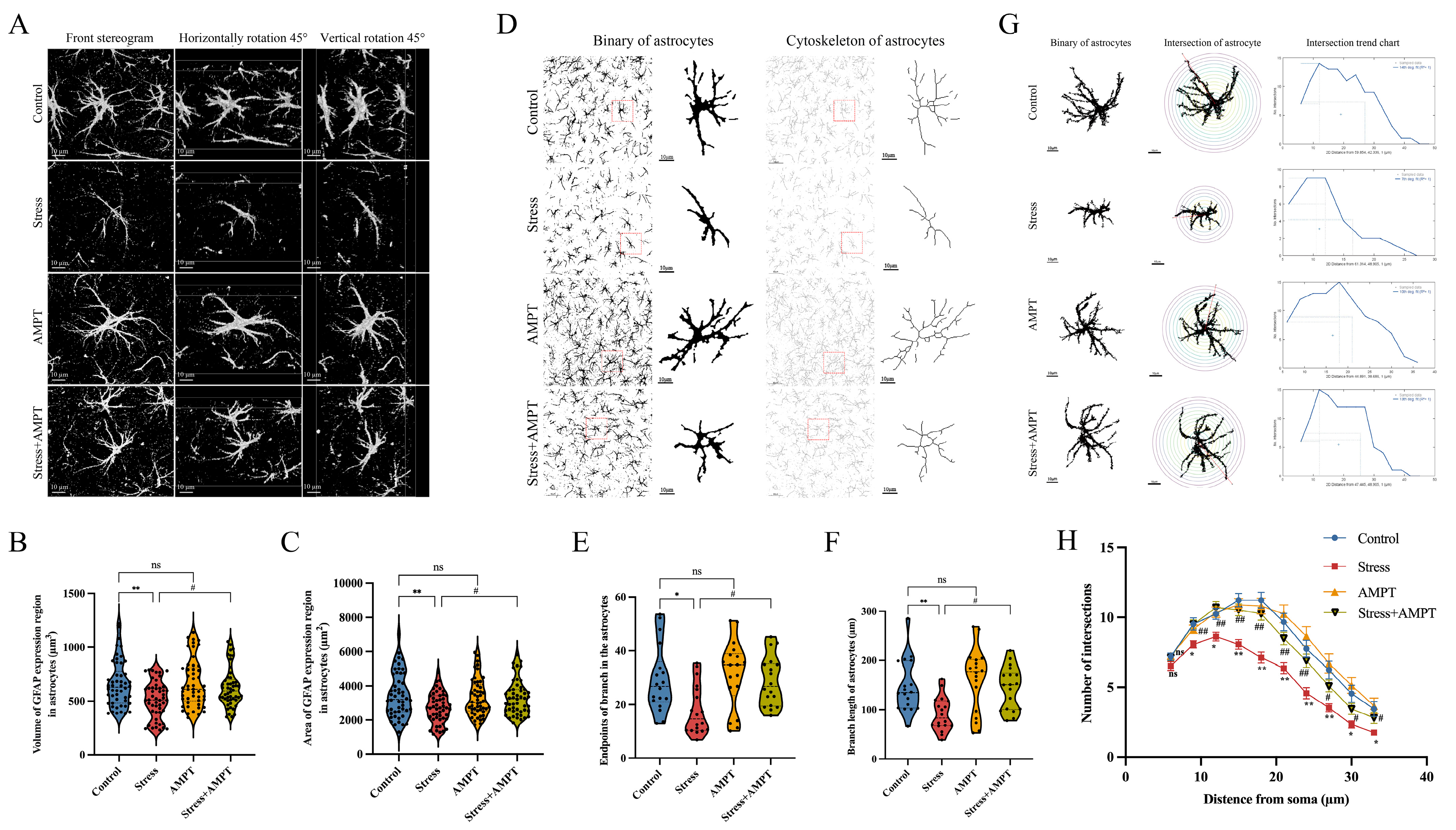
2.12. Astrocyte Morphometry
2.13. Statistical Analysis
3. Results
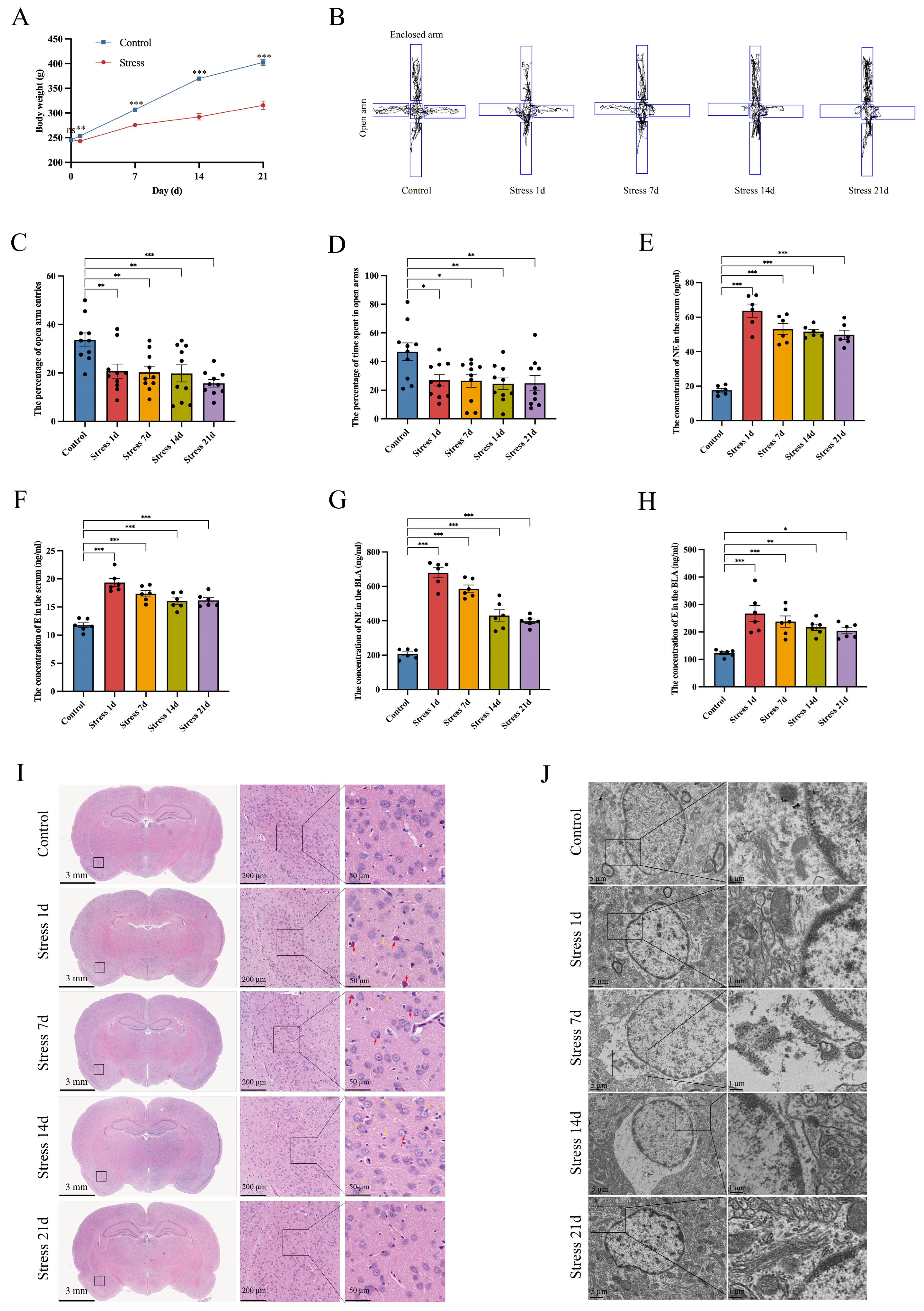
3.1. Stress Induces BLA Astrocyte Cytotoxic Edema in Rats
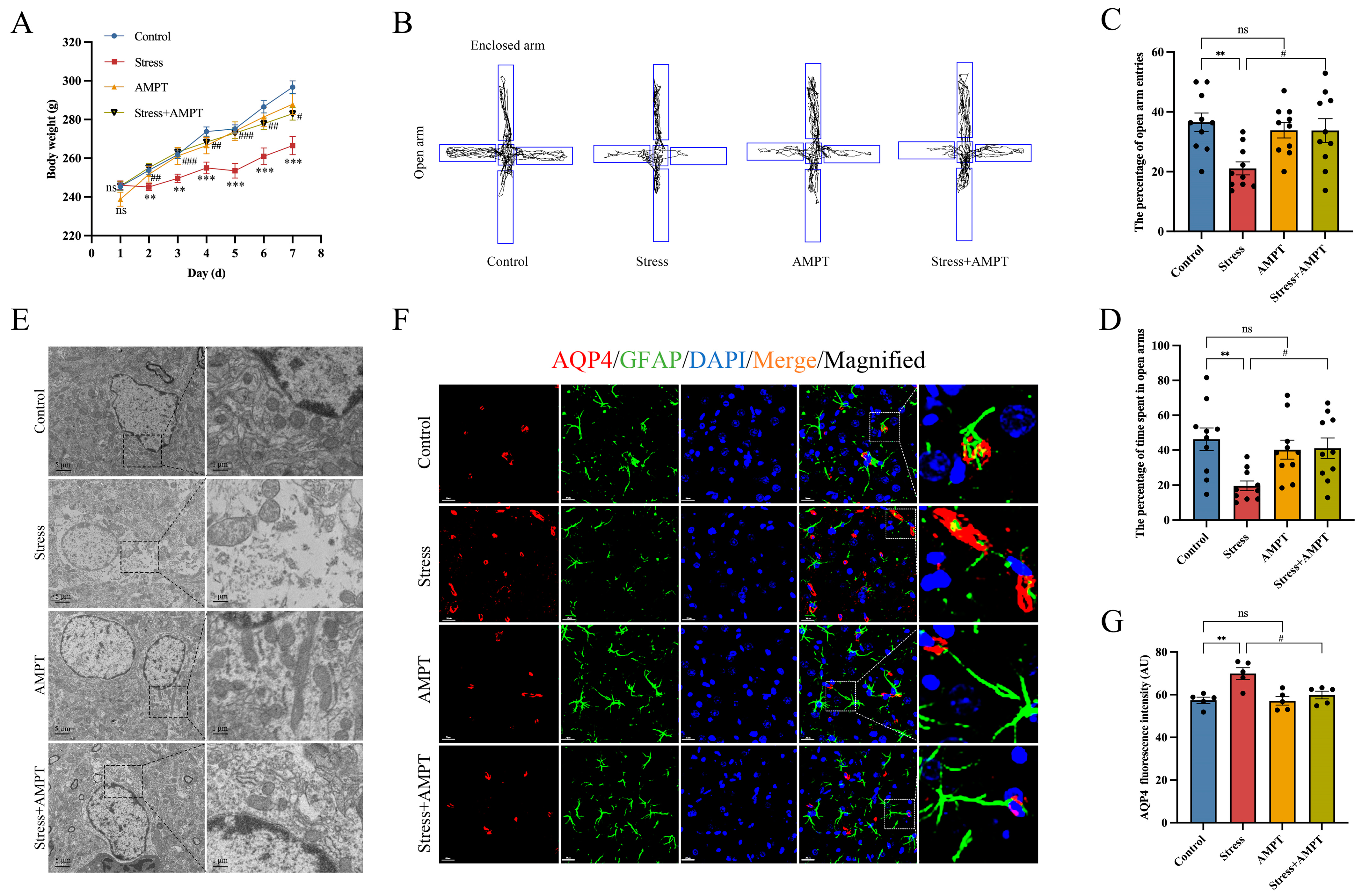
3.2. Stress Up-Regulates BLA AQP4 Expression in Rats
3.3. NE Down-Regulation Inhibits Stress-Induced BLA Astrocyte Cytotoxic Edema in Rats
3.4. NE Treatment Triggers Cytotoxic Edema and Enhances the Expression of AQP4 in Cultured Astrocytes
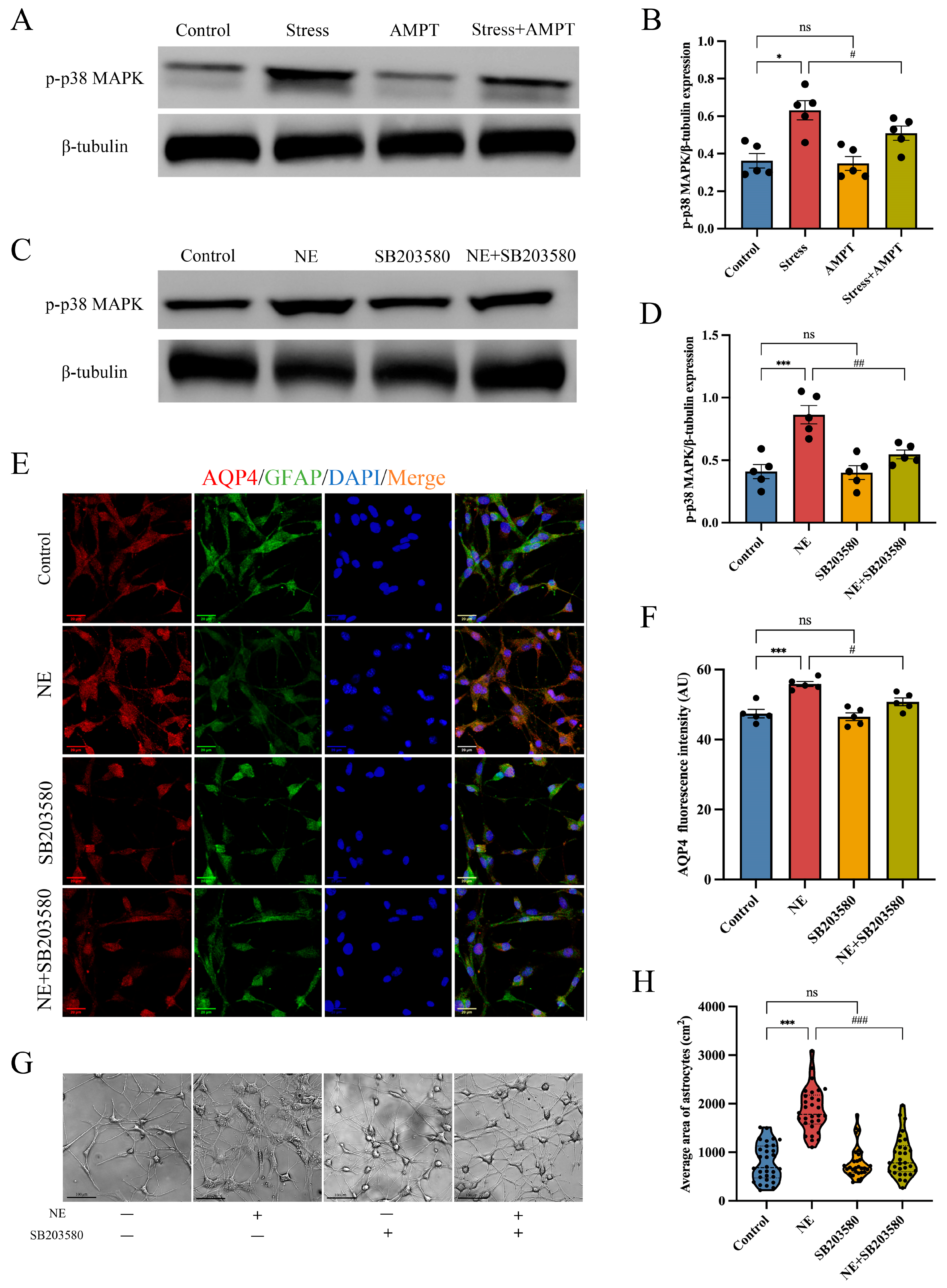
3.5. p38 MAPK Pathway Inactivation Weakens NE-Mediated Cytotoxic Edema in Astrocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galluzzi, L.; Yamazaki, T.; Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Timmer-Murillo, S.C.; Schramm, A.; deRoon-Cassini, T.A. Life threat during assaultive trauma: Critical posttraumatic stress disorder risk factors for injured patients. J. Trauma. Acute Care Surg. 2022, 92, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Liu, T.H.; He, Y.; Pan, H.Q.; Zhang, W.H.; Yin, X.P.; Tian, X.L.; Li, B.M.; Wang, X.D.; Holmes, A.; et al. Chronic Stress Remodels Synapses in an Amygdala Circuit-Specific Manner. Biol. Psychiatry 2019, 85, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Welcome, M.O.; Mastorakis, N.E. Stress-induced blood brain barrier disruption: Molecular mechanisms and signaling pathways. Pharmacol. Res. 2020, 157, 104769. [Google Scholar] [CrossRef]
- Turner, A.I.; Smyth, N.; Hall, S.J.; Torres, S.J.; Hussein, M.; Jayasinghe, S.U.; Ball, K.; Clow, A.J. Psychological stress reactivity and future health and disease outcomes: A systematic review of prospective evidence. Psychoneuroendocrinology 2020, 114, 104599. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Zhang, W.H.; Zheng, Z.H.; Zou, J.X.; Liu, X.X.; Huang, S.H.; You, W.J.; He, Y.; Zhang, J.Y.; Wang, X.D.; et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat. Commun. 2020, 11, 2221. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, C.; Wang, J.; Zhang, X.; Li, M.; Zhang, R.; Huang, Z.; Zhang, Y. Amygdala-hippocampal innervation modulates stress-induced depressive-like behaviors through AMPA receptors. Proc. Natl. Acad. Sci. USA 2021, 118, e2019409118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kim, J.; Tonegawa, S. Amygdala Reward Neurons Form and Store Fear Extinction Memory. Neuron 2020, 105, 1077–1093.e7. [Google Scholar] [CrossRef]
- Kim, J.; Kang, S.; Choi, T.Y.; Chang, K.A.; Koo, J.W. Metabotropic Glutamate Receptor 5 in Amygdala Target Neurons Regulates Susceptibility to Chronic Social Stress. Biol. Psychiatry 2022, 92, 104–115. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhan, J.; Cai, Q.; Xu, F.; Chai, R.; Lam, K.; Luan, Z.; Zhou, G.; Tsang, S.; Kipp, M.; et al. The Water Transport System in Astrocytes-Aquaporins. Cells 2022, 11, 2564. [Google Scholar] [CrossRef]
- Lafrenaye, A.D.; Simard, J.M. Bursting at the Seams: Molecular Mechanisms Mediating Astrocyte Swelling. Int. J. Mol. Sci. 2019, 20, 330. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Wu, J.; Zhu, Z.; Feng, X.; Qin, L.; Zhu, Y.; Sun, L.; Liu, Y.; Qiu, Z.; et al. Activation of astrocytes in hippocampus decreases fear memory through adenosine A(1) receptors. Elife 2020, 9, e57155. [Google Scholar] [CrossRef] [PubMed]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Guo, F.; Mo, R.; Chen, L.Y.; Mo, J.W.; Lu, C.L.; Ren, J.; Zhong, Q.L.; Kuang, X.J.; Wen, Y.L.; et al. O-GlcNAc transferase in astrocytes modulates depression-related stress susceptibility through glutamatergic synaptic transmission. J. Clin. Investig. 2023, 133, e160016. [Google Scholar] [CrossRef]
- Yi, S.; Chen, K.; Zhang, L.; Shi, W.; Zhang, Y.; Niu, S.; Jia, M.; Cong, B.; Li, Y. Endoplasmic Reticulum Stress Is Involved in Stress-Induced Hypothalamic Neuronal Injury in Rats via the PERK-ATF4-CHOP and IRE1-ASK1-JNK Pathways. Front. Cell Neurosci. 2019, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, Y.; Ma, C.; Wang, C.; Sun, Z.; Shen, Y.; Liu, L.; Li, S.; Zhang, X.; Cong, B. Restraint Stress Induced Hyperpermeability and Damage of the Blood-Brain Barrier in the Amygdala of Adult Rats. Front. Mol. Neurosci. 2019, 12, 32. [Google Scholar] [CrossRef]
- Wang, S.; Shi, W.; Zhang, G.; Zhang, X.; Ma, C.; Zhao, K.; Cong, B.; Li, Y. Endoplasmic Reticulum Stress-Mediated Basolateral Amygdala GABAergic Neuron Injury Is Associated with Stress-Induced Mental Disorders in Rats. Front. Cell Neurosci. 2019, 13, 511. [Google Scholar] [CrossRef]
- Akter, S.; Sasaki, H.; Uddin, K.R.; Ikeda, Y.; Miyakawa, H.; Shibata, S. Anxiolytic effects of γ-oryzanol in chronically- stressed mice are related to monoamine levels in the brain. Life Sci. 2019, 216, 119–128. [Google Scholar] [CrossRef]
- Benton, K.C.; Wheeler, D.S.; Kurtoglu, B.; Ansari, M.B.Z.; Cibich, D.P.; Gonzalez, D.A.; Herbst, M.R.; Khursheed, S.; Knorr, R.C.; Lobner, D.; et al. Norepinephrine activates β(1)-adrenergic receptors at the inner nuclear membrane in astrocytes. Glia 2022, 70, 1777–1794. [Google Scholar] [CrossRef] [PubMed]
- Plastira, I.; Bernhart, E.; Joshi, L.; Koyani, C.N.; Strohmaier, H.; Reicher, H.; Malle, E.; Sattler, W. MAPK signaling determines lysophosphatidic acid (LPA)-induced inflammation in microglia. J. Neuroinflammation 2020, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Muslin, A.J. MAPK signalling in cardiovascular health and disease: Molecular mechanisms and therapeutic targets. Clin. Sci. 2008, 115, 203–218. [Google Scholar] [CrossRef]
- Tang, K.; Zhong, B.; Luo, Q.; Liu, Q.; Chen, X.; Cao, D.; Li, X.; Yang, S. Phillyrin attenuates norepinephrine-induced cardiac hypertrophy and inflammatory response by suppressing p38/ERK1/2 MAPK and AKT/NF-kappaB pathways. Eur. J. Pharmacol. 2022, 927, 175022. [Google Scholar] [CrossRef]
- Qi, J.; Li, R.J.; Fu, L.Y.; Liu, K.L.; Qiao, J.A.; Yang, Y.; Yu, X.J.; Yu, J.Y.; Li, Y.; Tan, H.; et al. Exercise Training Attenuates Hypertension via Suppressing ROS/MAPK/NF-κB/AT-1R Pathway in the Hypothalamic Paraventricular Nucleus. Nutrients 2022, 14, 3968. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.C.; Cao, X.; Das, M.; Zhu, X.H.; Gao, T.M. Behavioral animal models of depression. Neurosci. Bull. 2010, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, C.; Peng, J.; Liu, M.; Cong, B. Multi-omics analysis of pathological changes in the amygdala of rats subjected to chronic restraint stress. Behav. Brain Res. 2020, 392, 112735. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Elevated Plus Maze Test for Measuring Anxiety-Like Behavior in Rodents. Methods Mol. Biol. 2019, 1916, 69–74. [Google Scholar]
- Foo, L.C.; Allen, N.J.; Bushong, E.A.; Ventura, P.B.; Chung, W.S.; Zhou, L.; Cahoy, J.D.; Daneman, R.; Zong, H.; Ellisman, M.H.; et al. Development of a method for the purification and culture of rodent astrocytes. Neuron 2011, 71, 799–811. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Hu, Y.Y.; Zhao, C.C.; Qi, J.; Su, A.C.; Lou, N.; Zhang, M.Y.; Li, L.; Xian, X.H.; Gong, J.X.; et al. The mechanism of GLT-1 mediating cerebral ischemic injury depends on the activation of p38 MAPK. Brain Res. Bull. 2019, 147, 1–13. [Google Scholar] [CrossRef]
- Young, K.; Morrison, H. Quantifying Microglia Morphology from Photomicrographs of Immunohistochemistry Prepared Tissue Using ImageJ. J. Vis. Exp. 2018, 136, e57648. [Google Scholar]
- Rai, D.; Dey, S.; Ray, K. A method for estimating relative changes in the synaptic density in Drosophila central nervous system. BMC Neurosci. 2018, 19, 30. [Google Scholar] [CrossRef]
- Popov, A.; Brazhe, A.; Denisov, P.; Sutyagina, O.; Li, L.; Lazareva, N.; Verkhratsky, A.; Semyanov, A. Astrocyte dystrophy in ageing brain parallels impaired synaptic plasticity. Aging Cell 2021, 20, e13334. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Luo, Y.; Wang, H.; Kuang, S.; Liang, G.; Yang, Y.; Mai, S.; Yang, J. Re-evaluation of the interrelationships among the behavioral tests in rats exposed to chronic unpredictable mild stress. PLoS ONE 2017, 12, e0185129. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Guo, H.; Cui, J.; Shi, Y.; Su, R.; Xie, Q.; Chang, J.; Wang, Y.; Lai, J. The basolateral amygdala regulation of complex cognitive behaviours in the five-choice serial reaction time task. Psychopharmacology 2019, 236, 3135–3146. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.O.; Zhang, E.T.; Piantadosi, S.C.; Marcus, D.J.; Motard, L.E.; Kan, B.K.; Gomez, A.M.; Nguyen, T.K.; Xia, L.; Bruchas, M.R. A locus coeruleus to dentate gyrus noradrenergic circuit modulates aversive contextual processing. Neuron 2021, 109, 2116–2130.e6. [Google Scholar] [CrossRef] [PubMed]
- Wyrofsky, R.R.; Reyes, B.A.S.; Zhang, X.Y.; Bhatnagar, S.; Kirby, L.G.; Van Bockstaele, E.J. Endocannabinoids, stress signaling, and the locus coeruleus-norepinephrine system. Neurobiol. Stress. 2019, 11, 100176. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Shi, W.; Qi, Q.; Zhang, G.; Li, Y.; Cong, B.; Zuo, M. Mechanism of Chronic Stress-Induced Glutamatergic Neuronal Damage in the Basolateral Amygdaloid Nucleus. Anal. Cell Pathol. 2021, 2021, 8388527. [Google Scholar] [CrossRef]
- Filippidis, A.S.; Carozza, R.B.; Rekate, H.L. Aquaporins in Brain Edema and Neuropathological Conditions. Int. J. Mol. Sci. 2016, 18, 55. [Google Scholar] [CrossRef]
- Kim, K.Y.; Shin, K.Y.; Chang, K.A. GFAP as a Potential Biomarker for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Cells 2023, 12, 1309. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Huang, W.; Chen, Y.; Li, G.; Liu, N.; Wu, Y.; Wang, G.; Li, Q.; Kong, D.; Xue, T.; et al. Chronic restraint stress promotes the mobilization and recruitment of myeloid-derived suppressor cells through β-adrenergic-activated CXCL5-CXCR2-Erk signaling cascades. Int. J. Cancer 2021, 149, 460–472. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Gong, J.; Mei, Z.; Gao, X.; Zhao, Y.; Ma, J.; Qian, L. The stress-related hormone norepinephrine induced upregulation of Nix, contributing to ECM protein expression. Cell Stress. Chaperones 2014, 19, 903–912. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Z.L.; Yuan, X.; Dong, H.Y.; Xu, X.; Xin, H.; Wang, Y.H.; Zhang, J.B.; Chen, L.; Li, H.L.; et al. Bilobetin induces kidney injury by influencing cGMP-mediated AQP-2 trafficking and podocyte cell cycle arrest. Phytomedicine 2019, 64, 153073. [Google Scholar] [CrossRef]
- Wang, C.; Yan, M.; Jiang, H.; Wang, Q.; He, S.; Chen, J.; Wang, C. Mechanism of aquaporin 4 (AQP 4) up-regulation in rat cerebral edema under hypobaric hypoxia and the preventative effect of puerarin. Life Sci. 2018, 193, 270–281. [Google Scholar] [CrossRef]
- Preininger, M.K.; Kaufer, D. Blood-Brain Barrier Dysfunction and Astrocyte Senescence as Reciprocal Drivers of Neuropathology in Aging. Int. J. Mol. Sci. 2022, 23, 6217. [Google Scholar] [CrossRef]
- Nutma, E.; van Gent, D.; Amor, S.; Peferoen, L.A.N. Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells 2020, 9, 600. [Google Scholar] [CrossRef]
- Paumier, A.; Boisseau, S.; Jacquier-Sarlin, M.; Pernet-Gallay, K.; Buisson, A.; Albrieux, M. Astrocyte-neuron interplay is critical for Alzheimer’s disease pathogenesis and is rescued by TRPA1 channel blockade. Brain 2022, 145, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.D.; Shetty, A.K. Treating Parkinson’s disease by astrocyte reprogramming: Progress and challenges. Sci. Adv. 2021, 7, eabg3198. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Castro, B.; Gangwani, M.R.; Yu, X.; Coppola, G.; Khakh, B.S. Astrocyte molecular signatures in Huntington’s disease. Sci. Transl. Med. 2019, 11, eaaw8546. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Hwang, H.J.; Hwang, Y.J.; Kim, Y.J. Mustard Leaf Extract Suppresses Psychological Stress in Chronic Restraint Stress-Subjected Mice by Regulation of Stress Hormone, Neurotransmitters, and Apoptosis. Nutrients 2020, 12, 3640. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, M.D.; Rao, K.V.; Jayakumar, A.R. Mechanisms of ammonia-induced astrocyte swelling. Metab. Brain Dis. 2005, 20, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Day, R.E.; Kitchen, P.; Owen, D.S.; Bland, C.; Marshall, L.; Conner, A.C.; Bill, R.M.; Conner, M.T. Human aquaporins: Regulators of transcellular water flow. Biochim. Biophys. Acta 2014, 1840, 1492–1506. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, A.; Tsunoda, A.; Tada, M.; Nishizawa, M.; Ugawa, Y.; Kakita, A. Expression of Aquaporin 1 and Aquaporin 4 in the Temporal Neocortex of Patients with Parkinson’s Disease. Brain Pathol. 2017, 27, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Michinaga, S.; Koyama, Y. Pathophysiological Responses and Roles of Astrocytes in Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 6418. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Zhou, D.; Guo, Y.; Zhang, S.; Guo, Y.M.; Guo, T.T.; Chen, X.Y.; Gong, Y.N.; Tang, H.L.; Xu, Z.F. Bloodletting Puncture at Hand Twelve Jing-Well Points Relieves Brain Edema after Severe Traumatic Brain Injury in Rats via Inhibiting MAPK Signaling Pathway. Chin. J. Integr. Med. 2021, 27, 291–299. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Zhang, X.; Dong, Y.; Liu, Y.; Wang, C.; Li, Y.; Ma, C.; Xu, G.; Wang, S.; Yang, C.; et al. Norepinephrine-Activated p38 MAPK Pathway Mediates Stress-Induced Cytotoxic Edema of Basolateral Amygdala Astrocytes. Brain Sci. 2024, 14, 161. https://doi.org/10.3390/brainsci14020161
Sun Z, Zhang X, Dong Y, Liu Y, Wang C, Li Y, Ma C, Xu G, Wang S, Yang C, et al. Norepinephrine-Activated p38 MAPK Pathway Mediates Stress-Induced Cytotoxic Edema of Basolateral Amygdala Astrocytes. Brain Sciences. 2024; 14(2):161. https://doi.org/10.3390/brainsci14020161
Chicago/Turabian StyleSun, Zhaoling, Xiaojing Zhang, Yiming Dong, Yichang Liu, Chuan Wang, Yingmin Li, Chunling Ma, Guangming Xu, Songjun Wang, Chenteng Yang, and et al. 2024. "Norepinephrine-Activated p38 MAPK Pathway Mediates Stress-Induced Cytotoxic Edema of Basolateral Amygdala Astrocytes" Brain Sciences 14, no. 2: 161. https://doi.org/10.3390/brainsci14020161



