Jugular Foramen Tumors: Surgical Strategies and Representative Cases
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Posterolateral Approaches
3.1.1. Retrosigmoid Approach
3.1.2. Far-Lateral Approach
3.2. Anterolateral Approaches
3.2.1. Postauricular Transtemporal Approach
3.2.2. Preauricular Subtemporal Infratemporal Approach
3.3. Combined Approach
Combined Transmastoid Retro- and Infralabyrinthine Transjugular Transcondylar Transtubercular Transcervical Approach
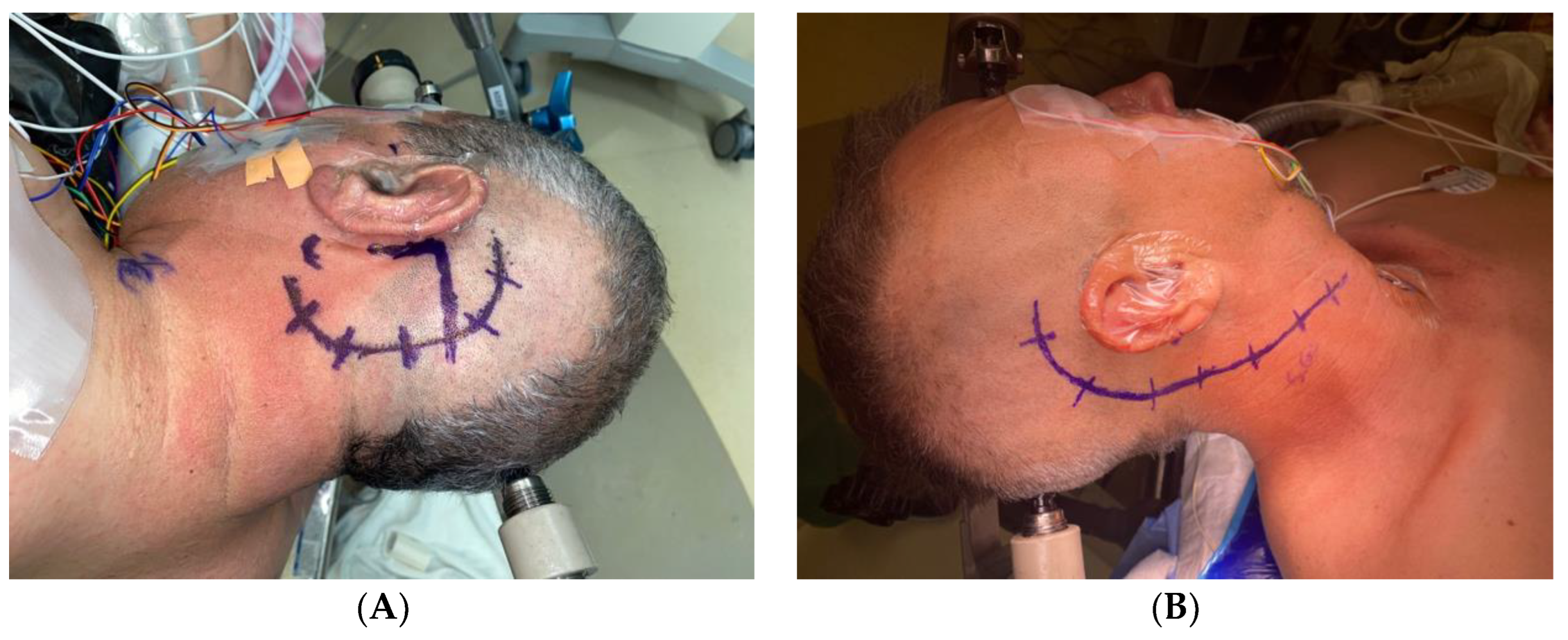
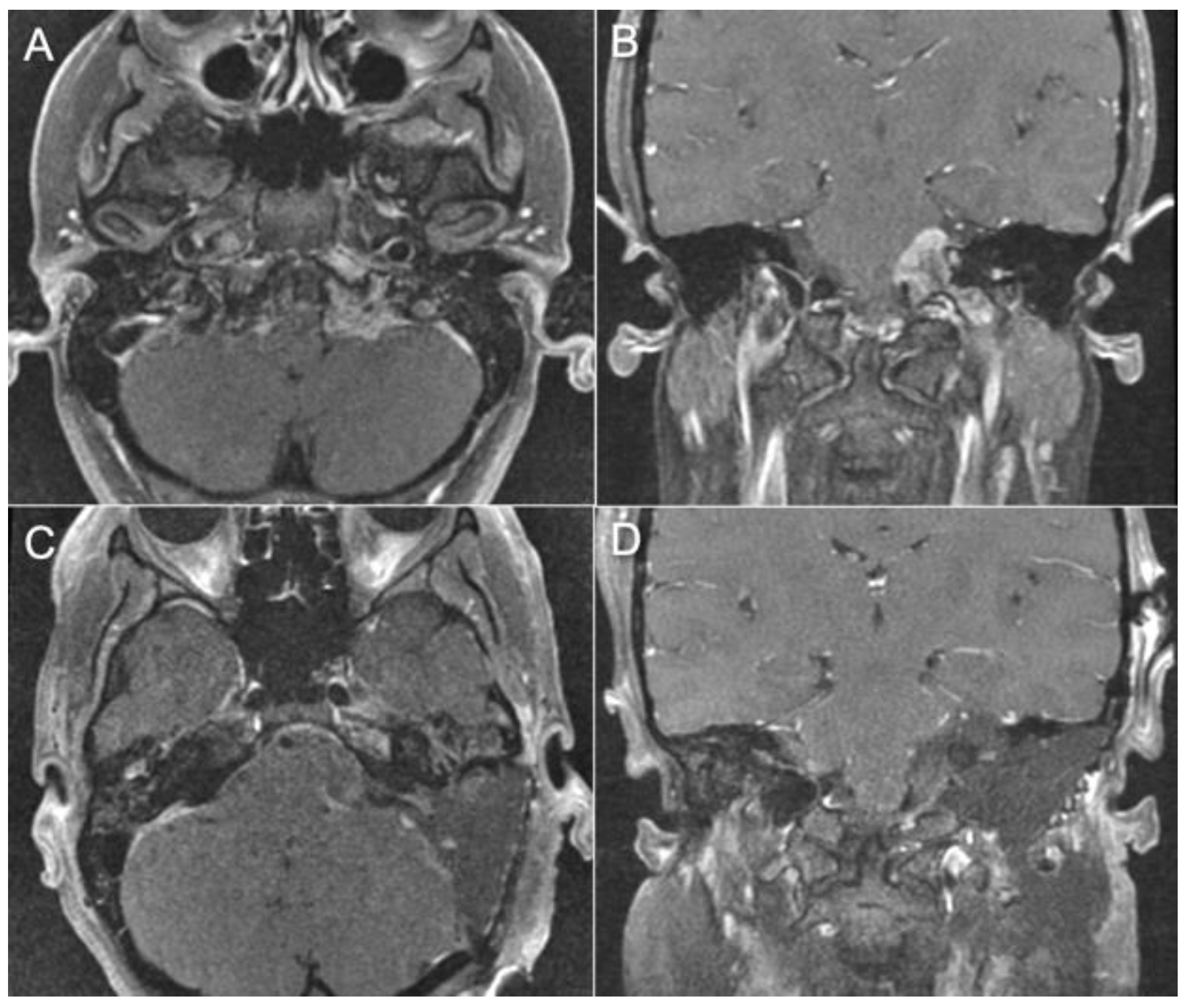
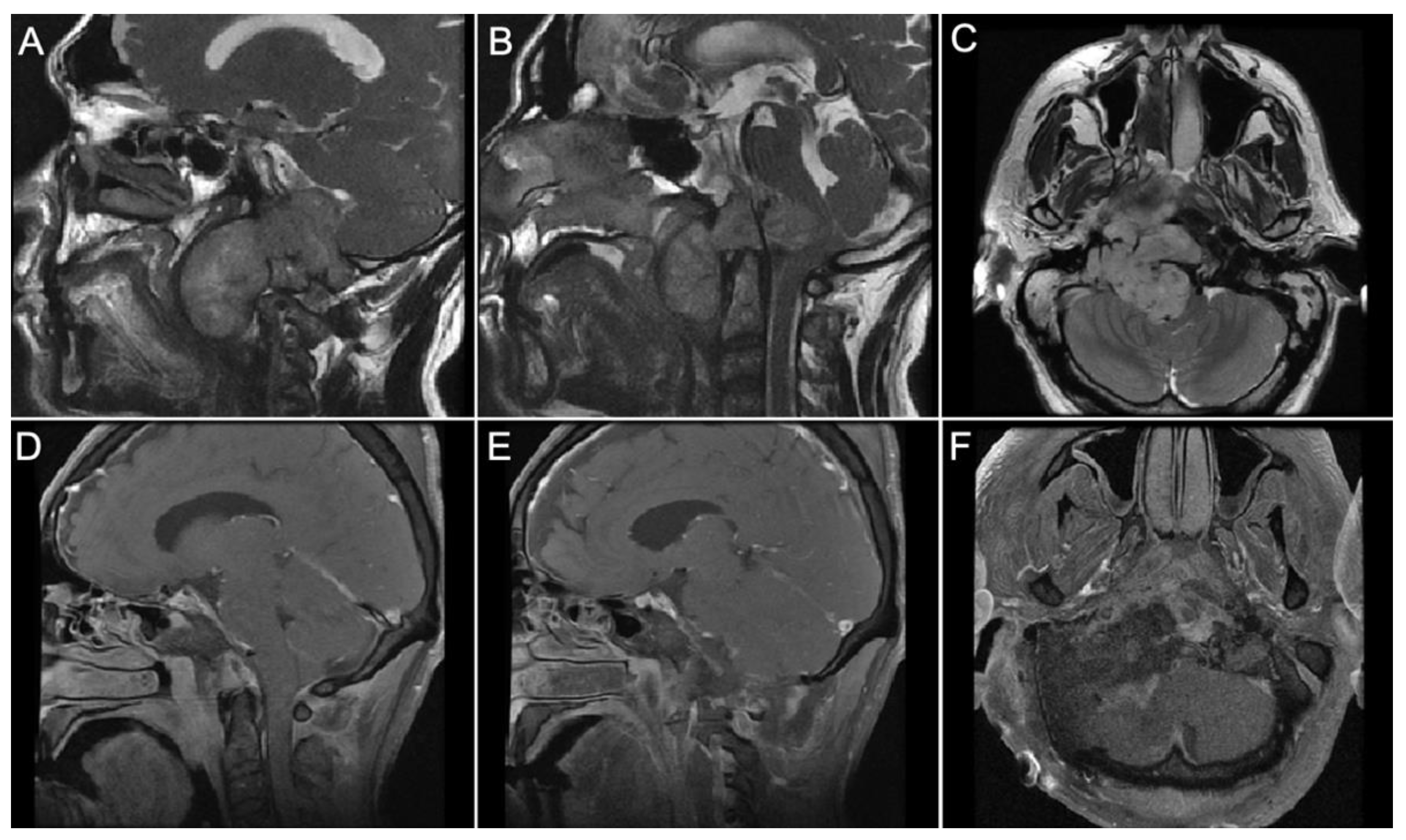
3.4. Representative Cases
- Case 1:
- Case 2:
- Case 3:
- Case 4:
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramina, R.; Maniglia, J.J.; Fernandes, Y.B.; Paschoal, J.R.; Pfeilsticker, L.N.; Neto, M.C.; Borges, G. Jugular foramen tumors: Diagnosis and treatment. Neurosurg. Focus 2004, 17, E5. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.L.; McKenna, M.J. Radiologic evaluation of the jugular foramen. Anatomy, vascular variants, anomalies, and tumors. Neuroimaging Clin. N. Am. 1994, 4, 579–598. [Google Scholar] [PubMed]
- Rhoton, A.L. Jugular Foramen. In Rhoton Cranial Anatomy and Surgical Approaches; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Inserra, M.M.; Pfister, M.; Jackler, R.K. Anatomy involved in the jugular foramen approach for jugulotympanic paraganglioma resection. Neurosurg. Focus 2004, 12, E6. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R. Compartmentation of the jugular foramen. J. Neurosurg. 1972, 36, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Tekdemir, I.; Tuccar, E.; Aslan, A.; Elhan, A.; Deda, H.; Ciftci, E.; Akyar, S. The Jugular Foramen A Comparative RadioAnatomic Study. Surg. Neurol. 1998, 50, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Ayeni, S.A.; Ohata, K.; Tanaka, K.; Hakuba, A. The microsurgical anatomy of the jugular foramen. J. Neurosurg. 1995, 83, 903–909. [Google Scholar] [CrossRef]
- McGrew, B.; Matusz, P.; De Caro, R.; Loukas, M.; Tubbs, R.; Griessenauer, C. Surgical Approaches to the Jugular Foramen: A Comprehensive Review. J. Neurol. Surg. Part B Skull Base 2016, 77, 260–264. [Google Scholar] [CrossRef]
- Liu, J.K.; Sameshima, T.; Gottfried, O.N.; Couldwell, W.T.; Fukushima, T. The combined Transmastoid Retro- and Infralabyrinthine Transjugular Transcaondylar Transtubercular High Cervical approach for resection of Glomus jugulare Tumors. Neurosurgery 2006, 59 (Suppl. 1), ONS115–ONS125. [Google Scholar] [CrossRef]
- Matsushima, K.; Kohno, M.; Nakajima, N.; Izawa, H.; Ichimasu, N.; Tanaka, Y.; Sora, S. Retrosigmoid Intradural Suprajugular Approach to Jugular Foramen Tumors with Intraforaminal Extension: Surgical Series of 19 Cases. World Neurosurg. 2019, 125, e984–e991. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.G.; Kaylie, D.M.; Coppit, G.; Gardner, E.K. Glomus Jugulare Tumors with intracranial extension. Neurosurg. Focus 2004, 17, E7. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Michihiro, K.; Komune, N.; Miki, K.; Matsushima, T.; Rhoton, A.L. Suprajugular extension of the retrosigmoid approach: Microsurgical anatomy. J. Neurosurg. 2014, 121, 397–407. [Google Scholar] [CrossRef]
- Luzzi, S.; Lucifero, A.G.; Bruno, N.; Baldoncini, M.; Campero, A.; Galio, R. Far Lateral Approach. Acta Biomed. 2021, 92, e2021352. [Google Scholar] [CrossRef]
- Chaddad-Neto, F.; Doria-Netto, H.L.; Campos-Filho, J.M.; Reghin-Neto, M.; Rothon, A.L., Jr.; de Oliveria, E. The Far-lateral craniotomy: Tips ans Tricks. Arq. Neuropsiquiatr. 2014, 72, 699–705. [Google Scholar] [CrossRef]
- Rhoton, A.L., Jr. The Far-Lateral Approach and Its Transcondylar, Supracondular, and Paracondylar Extensions. Neurosurgery 2000, 47, S195–S209. [Google Scholar] [CrossRef]
- Wen, H.T.; Albert LRothon, A.L.; Katsuta, T.; de Oliveira, E. Microsurgical anatomy of the transcondylar, supracondylar, and paracondylar extensions of the far-lateral approach. J. Neurosurg. 1997, 87, 555–585. [Google Scholar] [CrossRef]
- Salas, E.; Sekhar, L.N.; Ziyal, I.M.; Caputy, A.J.; Wright, D.C. Variations of the extreme-lateral craniocervical approach: Anatomical study and clinical analysis of 69 patients. J. Neurosurg. Spine 1999, 90, 206–219. [Google Scholar] [CrossRef]
- Sen, C.N.; Sekhar, L.N. Surgical Management of Anteriorly Placed Lesions at the Craniocervical Junction-an Alternative Approach. Acta Neurochir. 1991, 108, 70–77. [Google Scholar] [CrossRef]
- Sato, A.; Hirai, S.; Obata, Y.; Maehara, T.; Aoyagi, M. Muscular-Stage Dissection during Far Lateral Approach and Its Transcondylar Extension. J. Neurol. Surg. Part B Skull Base 2018, 79 (Suppl. 4), S356–S361. [Google Scholar] [CrossRef]
- Spektor, S.; Anderson, G.J.; McMenomey, S.O.; Horgan, M.A.; Kellogg, J.X.; Delashaw, J.B. Quantitative description of the far-lateral transcondylar transtubercular approach to the foramen magnum and clivus. J. Neurosurg. 2000, 92, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.P.; Sekhar, L.N.; Wright, D.C. Extreme lateral transcondylar approach: Technical improvements and lessons learned. J. Neurosurg. 1994, 81, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Açikbaş, S.C.; Tuncer, R.; Demirez, I.; Rahat, Ö.; Kazan, S.; Sindel, M.; Saveren, M. The effect of condylectomy on extreme lateral transcondylar approach to the anterior foramen magnum. Acta Neurochir. 1997, 139, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, K.; Fujita, S.; Kawaguchi, T.; Yamada, H. A transcondylar approach to the arteriovenous malformation at the ventral cervicomedullary junction: Report of three cases. Neurosurgery 1994, 34, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Nonaka, Y. Fukushima Manual of Skull Base Dissection, 3rd ed.; AF-Neuro Video: Raleigh, NC, USA, 2010. [Google Scholar]
- Sanna, M.; Saleh, E.; Khrais, T.; Mancini, F.; Piazza, P.; Russo, A.; Taibah, A. Atlas of Microsurgery of the Lateral Skull Base, 2nd ed.; Thieme: New York, NY, USA, 2008. [Google Scholar]
- Shane Tubbs, R.; Griessenauer, C.; Loukas, M.; Shaheryar FAnsari, S.F.; Fritsch, M.H.; Cohen-Gadol, A. Trautmann’s Triangle Anatomy With Application to Posterior Transpetrosal and Other Related Skull Base Procedures. Clin. Anat. 2014, 27, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Al-Mefty, O.; Fox, J.L.; Rifai, A.; Smith, R.R. A Combined Infratemporal and Posterior Fossa Approach for the Removal of Giant Glomus Tumors and Chondrosarcomas. Surg. Neurol. 1987, 28, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Fisch, U. Infratemporal fossa approach to tumours of the temporal bone and base of the skull. J. Laryngol. Otol. 1978, 92, 949–967. [Google Scholar] [CrossRef] [PubMed]
- Fisch, U.; Pillsbury, H.C. Infratemporal Fossa Approach to Lesions in the Temporal Bone and Base of the Skull. Arch. Otolaryngol. 1979, 105, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.; Zhao, K.; Baredes, S.; Jyung, R.W. Extended Anterolateral Infralabyrinthine Transjugular Approach for Microsurgical Resection of Giant Glomus Vagale Tumor: Operative Video and Technical Nuances. J. Neurol. Surg. Part B Skull Base 2021, 82 (Suppl. 1), S59–S60. [Google Scholar] [CrossRef]
- Al-Mefty, O.; Teixeira, A. Complex tumors of the glomus jugulare: Criteria, treatment, and outcome. J. Neurosurg. 2002, 97, 1356–1366. [Google Scholar] [CrossRef]
- Sanna, M.; Bacciu, A.; Falcioni, M.; Taibah, A. Surgical management of jugular foramen schwannomas with hearing and facial nerve function preservation: A series of 23 cases and review of the literature. Laryngoscope 2006, 116, 2191–2204. [Google Scholar] [CrossRef]
- Jackson, G.G.; McGrew, B.M.; Forest, J.A.; Netterville, J.L.; Hampf, C.F.; Glasscock, M.E. Lateral Skull Base Surgery for Glomus Tumors: Long-Term Control. Otol. Neurotol. 2001, 22, 377–382. [Google Scholar] [CrossRef]
- Makiese, O.; Chibbaro, S.; Marsella, M.; Tran Ba Huy, P.; George, B. Jugular Foramen Paragangliomas: Manegement, outcome and avoidance of complications in a series of 75 cases. Neurosurg. Rev. 2012, 35, 185–194. [Google Scholar] [CrossRef]
- Goodard, J.C.; Oliver, E.R.; Lambert, P.L. Prevention of cerebroespinal fluid leak after translabyrinthine resection of vesticular schwannoma. Otol. Neurotol. 2010, 31, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Selesnick, S.H.; Liu, J.C.; Jen, A.; Newman, J. The Incidence of cerebrospinal fluid leak after vestibular schwannoma surgery. Otol. Neurotol. 2004, 25, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.; Patel, S.K.; Podolski, A.J.; Jyung, R.W. Fascial sling technique for dural reconstruction after translabyrinthine resection of acoustic neuroma: Technical note. Neurosurg. Focus 2016, 33, E17. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.; D’Souza, A.R.; Chavda, S.V.; Irving, R.M. Dissemination of fat in CSF: A common finding following translabyrinthine acoustic neuroma surgery. Clin. Otolaryngol. 2005, 30, 405–408. [Google Scholar] [CrossRef]
- Ricaurte, J.C.; Murali, R.; Mandell, W. Uncomplicated Postoperative lipoid Meningitis secondary to autologous fat graft necrosis. Clin. Infect. Dis. 2000, 30, 613–615. [Google Scholar] [CrossRef]
- Taha, A.N.; Almefty, R.; Pravdenkova, S.; Al-Mefty, O. Sequelae of autologous fat graft used for reconstruction in skull base surgery. World Neurosurg. 2011, 75, 692–695. [Google Scholar] [CrossRef]





| Anterolateral | Posterolateral | Combined |
|---|---|---|
| Postauricular transtemporal approach | Retrosigmoid approach | Combined transmastoid retro- and infralabyrinthine transjugular transcondylar trans tubercular high cervical approach |
| Preauricular Subtemporal infratemporal approach | Far-Lateral approach -Transcondylar approach -Supracondylar approach -Paracondylar approach |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, A.L.; Meybodi, A.T.; Liu, J.K. Jugular Foramen Tumors: Surgical Strategies and Representative Cases. Brain Sci. 2024, 14, 182. https://doi.org/10.3390/brainsci14020182
Castillo AL, Meybodi AT, Liu JK. Jugular Foramen Tumors: Surgical Strategies and Representative Cases. Brain Sciences. 2024; 14(2):182. https://doi.org/10.3390/brainsci14020182
Chicago/Turabian StyleCastillo, Andrea L., Ali Tayebi Meybodi, and James K. Liu. 2024. "Jugular Foramen Tumors: Surgical Strategies and Representative Cases" Brain Sciences 14, no. 2: 182. https://doi.org/10.3390/brainsci14020182
APA StyleCastillo, A. L., Meybodi, A. T., & Liu, J. K. (2024). Jugular Foramen Tumors: Surgical Strategies and Representative Cases. Brain Sciences, 14(2), 182. https://doi.org/10.3390/brainsci14020182





