Behavioral and Cortical Activation Changes in Children Following Auditory Training for Dichotic Deficits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Baseline Behavioral Tests
2.3. fMRI Scanning
2.4. ARIA Training
2.5. Post-ARIA Behavioral Measures
2.6. Behavioral Data Analysis
2.7. fMRI Analysis
3. Results
3.1. Behavioral Results Pre-ARIA
3.2. Behavioral Results Post-ARIA
3.3. fMRI Results Pre- and Post-ARIA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Speech-Language-Hearing Association (ASHA) 2005. (Central) Auditory Processing Disorders [Technical Report]. Available online: www.asha.org/policy (accessed on 13 December 2023).
- American Academy of Audiology (AAA) 2010. Practice Guidelines for the Diagnosis, Treatment, and Management of Children and Adults with Central Auditory Processing Disorder (CAPD). Available online: http://www.audiology.org/publications-resources/document-library/central-auditory-processingdisorder (accessed on 13 December 2023).
- Emanuel, D.C.; Ficca, K.N.; Korczak, P. Survey of the diagnosis and management of auditory processing disorder. Am. J. Audiol. 2011, 20, 48–60. [Google Scholar] [CrossRef]
- Ismen, K.; Emanuel, D.C. Auditory processing disorder: Protocols and controversy. Am. J. Audiol. 2023, 32, 614–639. [Google Scholar] [CrossRef]
- Kimura, D. Some effects of temporal-lobe damage on auditory perception. Can. J. Psychol. 1961, 15, 156–165. [Google Scholar] [CrossRef]
- Musiek, F.E. Assessment of central auditory dysfunction: The dichotic digit test revisited. Ear Hear. 1983, 4, 79–83. [Google Scholar] [CrossRef]
- Damasio, H.; Damasio, A. “Paradoxic” ear extinction in dichotic listening. Possible anatomic significance. Neurology 1979, 29, 644–653. [Google Scholar] [CrossRef]
- Moncrieff, D.; Keith, W.; Abramson, M.; Swann, A. Clinical evidence on the diagnosis of amblyaudia, a binaural integration type of auditory processing disorder. Int. J. Audiol. 2016, 55, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.B.; Kozin, E.D.; Remenschneider, A.; Eftekhari, K.; Jung, D.H.; Polley, D.B.; Lee, D.J. Amblyaudia: Review of pathophysiology, clinical presentation, and treatment of a new diagnosis. Otolaryngol. Head Neck Surg. 2016, 154, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, D. Amblyaudia: Evidence of indistinct processing of binaural information in children. In Proceedings of the American Auditory Society Annual Meeting, Phoenix, AZ, USA, 1–3 March 2010. [Google Scholar]
- Kimura, D. Functional asymmetry of the brain in dichotic listening. Cortex 1967, 3, 163–168. [Google Scholar] [CrossRef]
- Pujol, J.; Vendrell, P.; Junqué, C.; Marti-Vilalta, J.L.; Capdevila, A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann. Neurol. 1993, 34, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Von Plessen, K.; Lundervold, A.; Duta, N.; Heiervang, E.; Klauschen, F.; Smievoll, A.I.; Ersland, L.; Hugdahl, K. Less developed corpus callosum in dyslexic subjects-a structural MRI study. Neuropsychologia 2002, 40, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Choo, A.L.; Kraft, S.J.; Olivero, W.; Ambrose, N.G.; Sharma, H.; Chang, S.-E.; Loucks, T.M. Corpus callosum differences associated with persistent stuttering in adults. J. Commun. Disord. 2011, 44, 470–477. [Google Scholar] [CrossRef]
- Kronfeld-Duenias, V.; Civier, O.; Ezrati-Vinacour, O.A.R.; Ben-Shachar, M. White matter pathways in persistent developmental stuttering: Lessons from tractography. J. Fluency Dis. 2018, 55, 68–83. [Google Scholar] [CrossRef]
- Luders, E.; Kurth, F.; Pigdon, L.; Conti-Ramsden, G.; Reilly, S.; Morgan, A.T. Atypical callosal morphology in children with speech sound disorder. Neuroscience 2017, 367, 211–218. [Google Scholar] [CrossRef]
- Farah, M.J.; Shera, D.M.; Savage, J.H.; Betancourt, L.; Giannetta, J.M.; Brodsky, N.L.; Malmud, E.K.; Hurt, H. The corpus callosum in childhood poverty: Specific associations with neurocognitive development. Brain Res. 2006, 1110, 166–174. [Google Scholar] [CrossRef]
- Bryden, M.P.; Munhall, K.; Allard, F. Attentional biases and the right-ear effect in dichotic listening. Brain Lang. 1983, 18, 236–248. [Google Scholar] [CrossRef]
- Kinsbourne, M. The cerebral basis of lateral asymmetries in attention. Acta Psychol. 1970, 33, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, M.; Kinsbourne, M. Attention and the right-ear advantage: What is the connection? Brain Cogn. 2011, 76, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chang, W.-T.; Belliveau, J.W.; Hämäläinen, M.; Ahveninen, J. Lateralized parietotemporal oscillatory phase synchronization during auditory selective attention. Neuroimage 2014, 86, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.; Rogers, C.S.; Wingfield, A.; Sekuler, R. A right-ear bias of auditory selective attention is evident in alpha oscillations. Psychophysiology 2017, 54, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Pugh, K.R.; Shaywitz, B.A.; Shaywitz, S.E.; Fulbright, R.K.; Byrd, D.; Skudlarski, P.; Shankweiler, D.P.; Katz, L.; Constable, R.T.; Fletcher, J.; et al. Auditory selective attention: An fMRI investigation. Neuroimage 1996, 4, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Asbjørnsen, A.E.; Hugdahl, K. Attentional effects in dichotic listening. Brain Lang. 1995, 49, 189–201. [Google Scholar] [CrossRef]
- Kershner, J.R.; Morton, L.L. Directed attention dichotic listening in reading disabled children: A test of four models of maladaptive lateralization. Neuropsychologia 1990, 28, 181–198. [Google Scholar] [CrossRef]
- Obrzut, J.E.; Boliek, C.A.; Obrzut, A. The effect of stimulus type and directed attention on dichotic listening with children. J. Exp. Child. Psychol. 1986, 41, 198–209. [Google Scholar] [CrossRef]
- Blauert, J. Spatial Hearing: The Psychophysics of Human Sound Localization; MIT Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Middlebrooks, J.C.; Green, D.M. Sound localization by human listeners. Ann. Rev. Psychol. 1991, 42, 135–159. [Google Scholar] [CrossRef]
- Litovsky, R.Y. Development of binaural and spatial hearing. In Springer Handbook of Auditory Research; Werner, L.A., Popper, A., Fay, R., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Moncrieff, D.W. Dichotic listening in children: Age-related changes in direction and magnitude of ear advantage. Brain Cogn. 2011, 76, 316–322. [Google Scholar] [CrossRef]
- Moushegian, G.; Rupert, A.; Whitcomb, M.A. Brain-stem neuronal response patterns to monaural and binaural tones. J. Neurophysiol. 1964, 27, 1174–1191. [Google Scholar] [CrossRef]
- Westerhausen, R.; Luders, E.; Specht, K.; Ofte, S.H.; Toga, A.W.; Thompson, P.M.; Helland, T.; Hugdahl, K. Structural and functional reorganization of the corpus callosum between the age of 6 and 8 years. Cereb. Cortex 2011, 21, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Polley, D.B.; Thompson, J.H.; Guo, W. Brief hearing loss disrupts binaural integration during two early critical periods of auditory cortex development. Nat. Commun. 2013, 4, 2547. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.V.; Polley, D.B. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron 2010, 65, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, D.; Mendez, K.R.G. Prevalence and severity of dichotic deficits in adjudicated adolescents: Implications for language skills, educational success and competency within the juvenile justice system. Eur. Soc. Med. 2024; submitted. [Google Scholar]
- Moncrieff, D. Identification and treatment of dichotic listening deficits in children. In Auditory Processing Disorders: Assessment, Management, and Treatment, 4th ed.; Geffner, D., Ross-Swain, D., Eds.; Plural Publishing, Inc.: San Diego, CA, USA, 2024. [Google Scholar]
- Bamiou, D.-E.; Musiek, F.E.; Luxon, L.M. Aetiology and clinical presentations of auditory processing disorders-a review. Arch. Dis. Child. 2001, 85, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.C. Neuroplasticity as a proposed mechanism for the efficacy of optometric vision therapy & rehabilitation. J. Behav. Optometry 2009, 20, 95–99. [Google Scholar]
- Hakkennes, S.; Keating, J. Constraint-induced movement therapy following stroke: A systematic review of randomized controlled trials. Aust. J. Physiother. 2005, 51, 221–231. [Google Scholar] [CrossRef]
- Hsin, Y.; Chen, F.-C.; Lin, K.; Kang, L.; Chen, C.; Chen, C. Efficacy of constraint-induced therapy on functional performance and health-related quality of life for children with cerebral palsy: A randomized controlled trial. J. Child. Neurol. 2012, 27, 992–999. [Google Scholar] [CrossRef]
- Moncrieff, D.W.; Wertz, D. Auditory rehabilitation for interaural asymmetry: Preliminary evidence of improved dichotic listening performance following intensive training. Int. J. Audiol. 2008, 47, 84–97. [Google Scholar] [CrossRef]
- Moncrieff, D.; Keith, W.; Abramson, M.; Swann, A. Evidence of binaural integration benefits following ARIA training in children and adolescents diagnosed with amblyaudia. Int. J. Audiol. 2017, 56, 580–588. [Google Scholar] [CrossRef]
- Hoshino, O. Neuronal bases of perceptual learning revealed by a synaptic balance scheme. Neural Comput. 2004, 16, 563–594. [Google Scholar] [CrossRef] [PubMed]
- Wamsley, E.J. Memory Consolidation during Waking Rest. Trends Cogn. Sci. 2019, 23, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.H.; Johnsrude, I.S. Hierarchical processing in spoken language comprehension. J. Neurosci. 2003, 23, 3423–3431. [Google Scholar] [CrossRef]
- Hickok, G.; Poeppel, D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007, 8, 393–402. [Google Scholar] [CrossRef]
- Obleser, J.; Zimmermann, J.; Van Meter, J.; Rauschecker, J.P. Multiple stages of auditory speech perception reflected in event-related FMRI. Cereb. Cortex 2007, 17, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language, and reading. Neuroimage 2012, 62, 816–847. [Google Scholar] [CrossRef]
- Scott, S.K.; Blank, C.C.; Rosen, S.; Wise, R.J. Identification of a pathway for intelligible speech in the left temporal lobe. Brain 2000, 123, 2400–2406. [Google Scholar] [CrossRef]
- Hugdahl, K.; Brøonnick, K.; Kyllingsbæk, S.; Law, I.; Gade, A.; Paulson, O.B. Brain activation during dichotic presentations of consonant-vowel and musical instrument stimuli: A 15O-PET study. Neuropsychologia 1999, 37, 431–440. [Google Scholar] [CrossRef]
- Stefanatos, G.A.; Joe, W.Q.; Aguirre, G.K.; Detre, J.A.; Wetmore, G. Activation of human auditory cortex during speech perception: Effects of monaural, binaural, and dichotic presentation. Neuropsychologia 2008, 46, 301–315. [Google Scholar] [CrossRef]
- Van den Noort, M.; Specht, K.; Rimol, L.M.; Ersland, L.; Hugdahl, K. A new verbal reports fMRI dichotic listening paradigm for studies of hemispheric asymmetry. Neuroimage 2008, 40, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Westerhausen, R.; Kompus, K.; Hugdahl, K. Mapping hemispheric asymmetries, relative asymmetries, and absolute asymmetries underlying the auditory laterality effect. Neuroimage 2014, 84, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Zatorre, R.J. Neural substrates for dividing and focusing attention between simultaneous auditory and visual events. Neuroimage 2006, 31, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, S. A unified structural-attentional framework for dichotic listening. In The Two Halves of the Brain: Information Processing in the Cerebral Hemispheres; Hugdahl, K., Westerhausen, R., Eds.; MIT Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Bellis, T.J. Assessment and Management of Central Auditory Processing Disorders in the Educational Setting: From Science to Practice; Thompson Learning: Delmar, NY, USA, 2003. [Google Scholar]
- Bellis, T.J. Interpretation of APD test results. In An Introduction to Auditory Processing Disorders in Children; Parthasarathy, T.K., Ed.; Erlbaum: Mahwah, NJ, USA, 2006; pp. 145–160. [Google Scholar]
- Moncrieff, D.W.; Wilson, R.H. Recognition of one-, two-, and three-pair dichotic digits by children and young adults. J. Am. Acad. Audiol. 2009, 20, 58–70. [Google Scholar]
- Moncrieff, D. Age- and gender-specific normative information from children assessed with a dichotic words test. J. Am. Acad. Audiol. 2015, 26, 632–644. [Google Scholar] [CrossRef]
- Keith, R. SCAN-C: Test for Auditory Processing Disorders in Children-Revised; The Psychological Corporation: San Antonio, TX, USA, 2000. [Google Scholar]
- White, S.; Moncrieff, D. ARIA treatment benefits are related to severity of dichotic listening deficits in children. Int. J. Pediatr. Otorhinolaryngol. 2023, 168, 111551. [Google Scholar] [CrossRef]
- Wilson, R.H.; Farmer, N.M.; Gandhi AShelburne, E.; Weaver, J. Normative data for the words-in-noise test for 6- to 12-year-old children. J. Speech Lang. Hear. Res. 2010, 53, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Noffsinger, D.; Wilson, R.H.; Musiek, F.E. Department of Veterans Affairs compact disc recording for auditory perceptual assessment: Background and introduction. J. Am. Acad. Audiol. 1994, 5, 231–235. [Google Scholar] [PubMed]
- Myklebust, H.R. Auditory Disorders in Children; Grune & Stratton: New York, NY, USA, 1954. [Google Scholar]
- Celesia, G.G. Hearing disorders in brainstem lesions. In Handbook of Clinical Neurology, Volume 129: The Human Auditory System: Fundamental Organization and Clinical Disorders; Celesia, G.G., Hickok, G., Eds.; Elsevier: Edinburgh, UK, 2015; p. 531. [Google Scholar]
- Chermak, G.D.; Musiek, F.E. Managing central auditory processing disorders in youth. Am. J. Audiol. 1992, 1, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Chermak, G.D.; Musiek, F.E. Auditory training: Principles and approaches for managing auditory processing disorders. Semin. Hear. 2002, 23, 297–308. [Google Scholar] [CrossRef]
- Ahmmed, A.U.; Ahmmed, A.A.; Bath, J.R.; Ferguson, M.A.; Plack, C.J.; Moore, D.R. Assessment of children with suspected auditory processing disorder: A factor analysis study. Ear Hear. 2014, 35, 295–305. [Google Scholar] [CrossRef]
- Vermiglio, A.J. On the clinical entity in audiology: (Central) auditory processing and speech recognition in noise disorders. J. Am. Acad. Audiol. 2014, 25, 904–917. [Google Scholar] [CrossRef]
- Loo, J.H.Y.; Bamiou, D.-E.; Campbell, N.; Luxon, L.M. Computer-based auditory training (CBAT): Benefits for children with language- and reading-related learning difficulties. Dev. Med. Child. Neurol. 2010, 52, 708–717. [Google Scholar] [CrossRef]
- Delphi, M.; Abdollahi, F.Z. Dichotic training in children with auditory processing disorder. Int. J. Pediatr. Otorhinolaryngol. 2018, 110, 114–117. [Google Scholar] [CrossRef]
- Helland, T.; Morken, F.; Bless, J.J.; Valderhaug, H.V.; Eiken, M.; Helland, W.A.; Torkildsen, J.V.K. Auditive training effects from a dichotic listening app in children with dyslexia. Dyslexia 2018, 24, 336–356. [Google Scholar] [CrossRef]
- Denman, I.; Banajee, M.; Hurley, A. Dichotic listening training in children with autism spectrum disorder: A single subject design. Int. J. Audiol. 2015, 54, 991–996. [Google Scholar] [CrossRef]
- Kozou, H.; Azouz, H.G.; Abdou, R.M.; Shaltout, A. Evaluation and remediation of central auditory processing disorders in children with autism spectrum disorders. Int. J. Pediatr. Otorhinolaryngol. 2018, 104, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, D.J.C.; Amitay, S.; Moore, D. Early and rapid perceptual learning. Nat. Neurosci. 2004, 7, 1055–1056. [Google Scholar] [CrossRef] [PubMed]
- Green, C.S.; Banai, K.; Lu, Z.L.; Bevalier, D. Perceptual learning. In Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience, Sensation, Perception, and Attention, 4th ed.; Wixted, J.T., Serences, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; Volume 2, pp. 755–802. [Google Scholar]
- Ortiz, J.A.; Wright, B.A. Differential rates of consolidation of conceptual and stimulus learning following training on an auditory skill. Exp. Brain Res. 2010, 201, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Tzounopoulos, T.; Kraus, N. Learning to encode timing: Mechanisms of plasticity in the auditory brainstem. Neuron 2016, 62, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Ahissar, M.; Nahum, M.; Nelken, I.; Hochstein, S. Reverse hierarchies and sensory learning. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Pisoni, D. Long-term memory in speech perception: Some new findings on talker variability, speaking rate and perceptual learning. Speech Commun. 1993, 13, 109–125. [Google Scholar] [CrossRef]
- Alain, C.; Snyder, H.J.S.; He, Y.; Reinke, K.S. Changes in auditory cortex parallel rapid perceptual learning. Cereb. Cortex 2007, 17, 1074–1084. [Google Scholar] [CrossRef]
- Carcagno, S.; Plack, C.J. Short-term learning and memory: Training and perceptual learning. In Springer Handbook of Auditory Research Volume 61: The Frequency-Following Response: A Window into Human Communication; Kraus, N., Anderson, S., White-Schwoch, T., Fay, R.R., Popper, A.N., Eds.; ASA Press, Springer: Cham, Switzerland, 2017. [Google Scholar]
- Binder, J.R.; Rao, S.M.; Hammeke, T.A.; Yetkin, F.Z.; Jesmanowicz, A.; Bandettini, P.A.; Wong, E.C.; Estkowski, L.D.; Goldstein, M.D. Functional magnetic resonance imaging of human auditory cortex. Ann. Neurol. 1994, 35, 662–672. [Google Scholar] [CrossRef]
- Jäncke, L.; Wüstenberg, H.; Scheich, H.; Heinze, H.-J. Phonetic perception and the temporal cortex. Neuroimage 2002, 15, 733–746. [Google Scholar] [CrossRef]
- Broyd, S.J.; Demanuele, C.; Debener, S.; Helps, S.K.; James, C.J.; Sonuga-Barke, E.J.S. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci. Behav. Rev. 2009, 33, 279–296. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Ross, T.J.; Hoffmann, R.; Garavan, H.; Stein, E.A. Multiple neuronal networks mediate sustained attention. J. Cogn. Neurosci. 2003, 15, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Pastor, M.A.; Macaluso, E.; Day, B.L.; Frackowiak, R. The neural basis of temporal auditory discrimination. Neuroimage 2006, 30, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.X.; van der Heijden, M. Early binaural hearing: The comparison of temporal differences at the two ears. Ann. Rev. Neurosci. 2019, 42, 433–457. [Google Scholar] [CrossRef] [PubMed]
- Jäncke, L.; Buchanan, T.W.; Lutz, K.; Shah, N.J. Focused and non-focused attention in verbal and emotional dichotic listening: An FMRI study. Brain Lang. 2001, 78, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, T.; Rimol, L.M.; Ersland, L.; Hugdahl, K. Dichotic listening reveals functional specificity n prefrontal cortex: An fMRI study. Neuroimage 2003, 21, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Jäncke, L.; Specht, K.; Shah, J.N.; Hugdahl, K. Focused attention in a simple dichotic listening task: An fMRI experiment. Cogn. Bran Res. 2003, 16, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Hugdahl, K.; Westerhausen, R.; Alho, K.; Medvedev, S.; Laine, M.; Hämäläinen, H. Attention and cognitive control: Unfolding the dichotic listening story. Scand. J. Psychol. 2009, 50, 11–22. [Google Scholar] [CrossRef]
- Kompus, K.; Specht, K.; Ersland, L.; Juvodden, H.T.; van Wageningen, H.; Hugdahl, K.; Westernausen, R. A forced-attention dichotic listening fMRI study on 113 subjects. Brain Lang. 2012, 121, 240–247. [Google Scholar] [CrossRef]
- Westerhausen, R.; Hugdahl, K. Cognitive control of laterality. In The Two Halves of the Brain; Hugdahl, K., Westerhausen, Eds.; MIT Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Falkenberg, L.E.; Specht, K.; Westerhausen, R. Attention and cognitive control networks assessed in a dichotic listening fMRI study. Brain Cogn. 2011, 76, 276–285. [Google Scholar] [CrossRef]
- Piai, V.; Roelofs, A.; Acheson, D.J.; Takashima, A. Attention for speaking: Domain-general control from the anterior cingulate cortex in spoken word production. Front. Hum. Neurosci. 2013, 7, 832. [Google Scholar] [CrossRef]
- Emch, M.; von Bastian, C.C.; Koch, K. Neural correlates of verbal working memory: An fMRI meta-analysis. Front. Hum. Neurosci. 2019, 13, 180. [Google Scholar] [CrossRef]
- Naeser, M.A.; Martin, P.I.; Theoret, H.; Kobayashi, M.; Fregni, F.; Nicholas, M.; Tormos, J.M.; Steven, M.S.; Baker, E.H.; Pascual-Leone, A. TMS suppression of right pars triangularis, but not pars opercularis, improves naming in aphasia. Brain Lang. 2011, 119, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.T.; Buckner, R.L. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron 2002, 35, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Iacoboni, M. The role of premotor cortex in speech perception: Evidence from fMRI and rTMS. J. Physiol. 2008, 102, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Maess, B.; Herrmann, C.S.; Hahne, A.; Nakamura, A.; Friederici, A.D. Localizing the distributed language network responsible for the N400 measured by MEG during auditory sentence processing. Brain Res. 2006, 1096, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Moncrieff, D.; Jerger, J.; Wambacq, I.; Greenwald, R.; Black, J. ERP evidence of a dichotic left-ear deficit in some dyslexic children. J. Am. Acad. Audiol. 2004, 15, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Plakke, B.; Romanski, L.M. Auditory connections and functions of prefrontal cortex. Front. Neurosci. 2014, 8, 199. [Google Scholar] [CrossRef]
- Knight, R.T.; Staines, W.R.; Swick, D.; Chao, L.L. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol. 1999, 101, 159–178. [Google Scholar] [CrossRef]
- Scholz, J.; Klein, M.C.; Behrens TE, J.; Johansen-Berg, H. Training induces changes in white-matter architecture. Nat. Neurosci. 2009, 12, 1370–1371. [Google Scholar] [CrossRef]
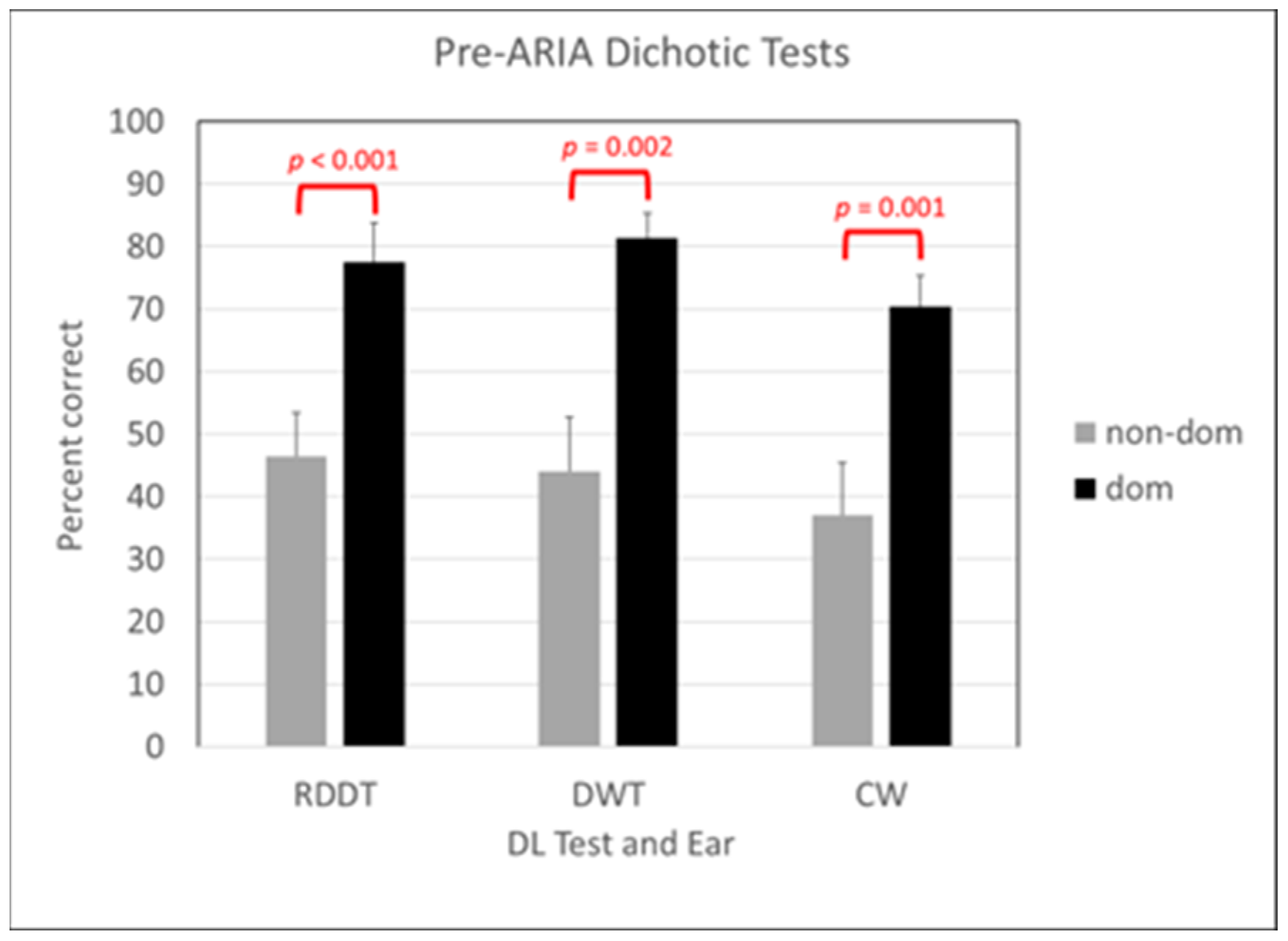

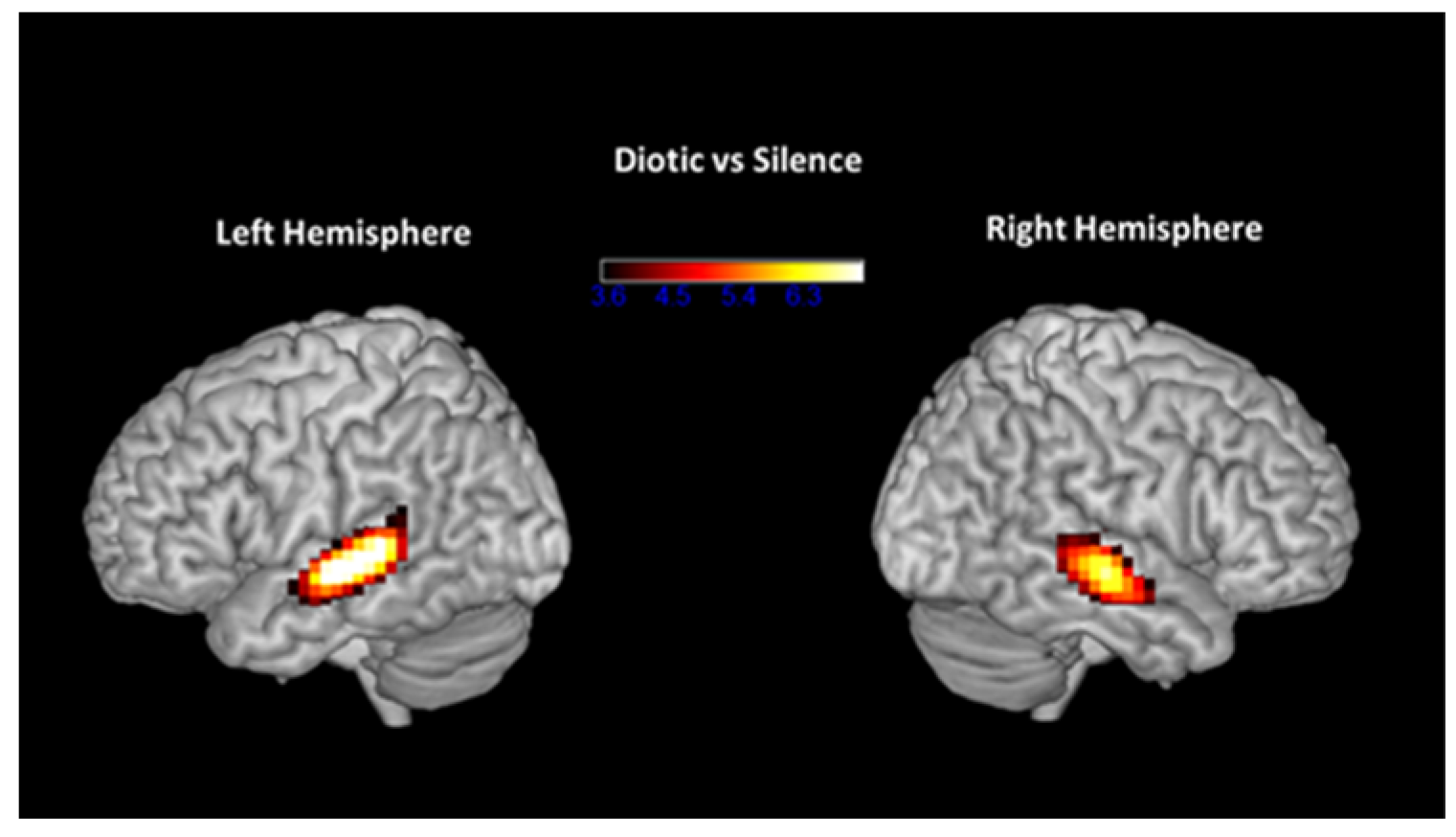

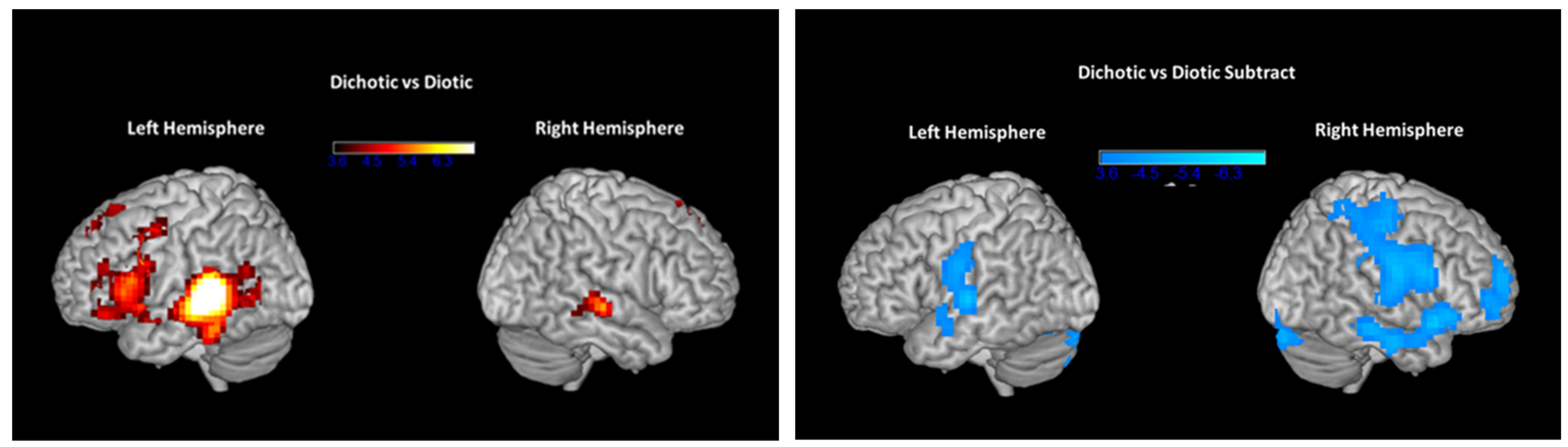
| Pre-ARIA | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RDDT | DWT | |||||||||||||
| Code | Age | non | dom | IA | non | dom | IA | WIN | FPT | ID | %ile | |||
| 2112 | 7 | 25 | 67 | 42 | 12 | 80 | 68 | 10 | 50 | 40 | 9.6 | 7 | AMB | 5th |
| 2114 | 9 | 61 | 97 | 36 | 88 | 92 | 4 | 73 | 93 | 20 | 8 | 70 | AMB | 10th |
| 2120 | 10 | 69 | 97 | 28 | 16 | 76 | 60 | 30 | 73 | 43 | 5.6 | 20 | AMB | 5th |
| 2126 | 7 | 27 | 67 | 40 | 28 | 68 | 40 | 30 | 70 | 40 | 9.6 | 0 | AMB | 5th |
| 2128 | 9 | 42 | 86 | 44 | 28 | 92 | 64 | 17 | 67 | 50 | 10.4 | 23 | AMB | 5th |
| 2179 | 8 | 50 | 83 | 33 | 64 | 72 | 8 | 47 | 63 | 16 | 12 | 40 | DD | 10th |
| 2181 | 13 | 86 | 97 | 14 | 72 | 96 | 24 | 4 | 87 | AMB | 10th | |||
| 2183 | 7 | 25 | 47 | 22 | 40 | 64 | 24 | 53 | 77 | 24 | 7.2 | 0 | DD | 5th |
| 2184 | 9 | 36 | 56 | 20 | 48 | 92 | 44 | 7.2 | 27 | MIX | 5th | |||
| Post-ARIA | ||||||||||||||
| RDDT | DWT | |||||||||||||
| non | dom | IA | non | dom | IA | WIN | FPT | |||||||
| 2112 | 7 | 72 | 83 | 11 | 72 | 84 | 12 | 4.4 | 0 | |||||
| 2114 | 10 | 56 | 61 | 5 | 60 | 60 | 0 | 5.2 | 27 | DD | 5th | |||
| 2120 | 10 | 89 | 100 | 11 | 88 | 92 | 4 | 6.0 | 67 | |||||
| 2126 | 7 | 81 | 97 | 16 | 76 | 100 | 24 | 4.4 | 40 | |||||
| 2128 | 9 | 58 | 69 | 11 | 64 | 88 | 24 | 5.6 | 13 | UND | 10th | |||
| 2179 | 8 | 83 | 100 | 7 | 96 | 100 | 4 | 2.4 | 73 | |||||
| 2181 | 14 | 100 | 100 | 0 | 84 | 88 | 4 | 5.8 | 100 | |||||
| 2183 | 7 | 42 | 44 | 2 | 32 | 80 | 48 | 4.4 | 20 | MIX | 5th | |||
| 2184 | 10 | 78 | 83 | 5 | 80 | 84 | 4 | 6.8 | 0 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moncrieff, D.; Schmithorst, V. Behavioral and Cortical Activation Changes in Children Following Auditory Training for Dichotic Deficits. Brain Sci. 2024, 14, 183. https://doi.org/10.3390/brainsci14020183
Moncrieff D, Schmithorst V. Behavioral and Cortical Activation Changes in Children Following Auditory Training for Dichotic Deficits. Brain Sciences. 2024; 14(2):183. https://doi.org/10.3390/brainsci14020183
Chicago/Turabian StyleMoncrieff, Deborah, and Vanessa Schmithorst. 2024. "Behavioral and Cortical Activation Changes in Children Following Auditory Training for Dichotic Deficits" Brain Sciences 14, no. 2: 183. https://doi.org/10.3390/brainsci14020183





