Oxytocin Enhances Time-Dependent Responses in the Aggressive Zebrafish (Danio rerio)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chemicals
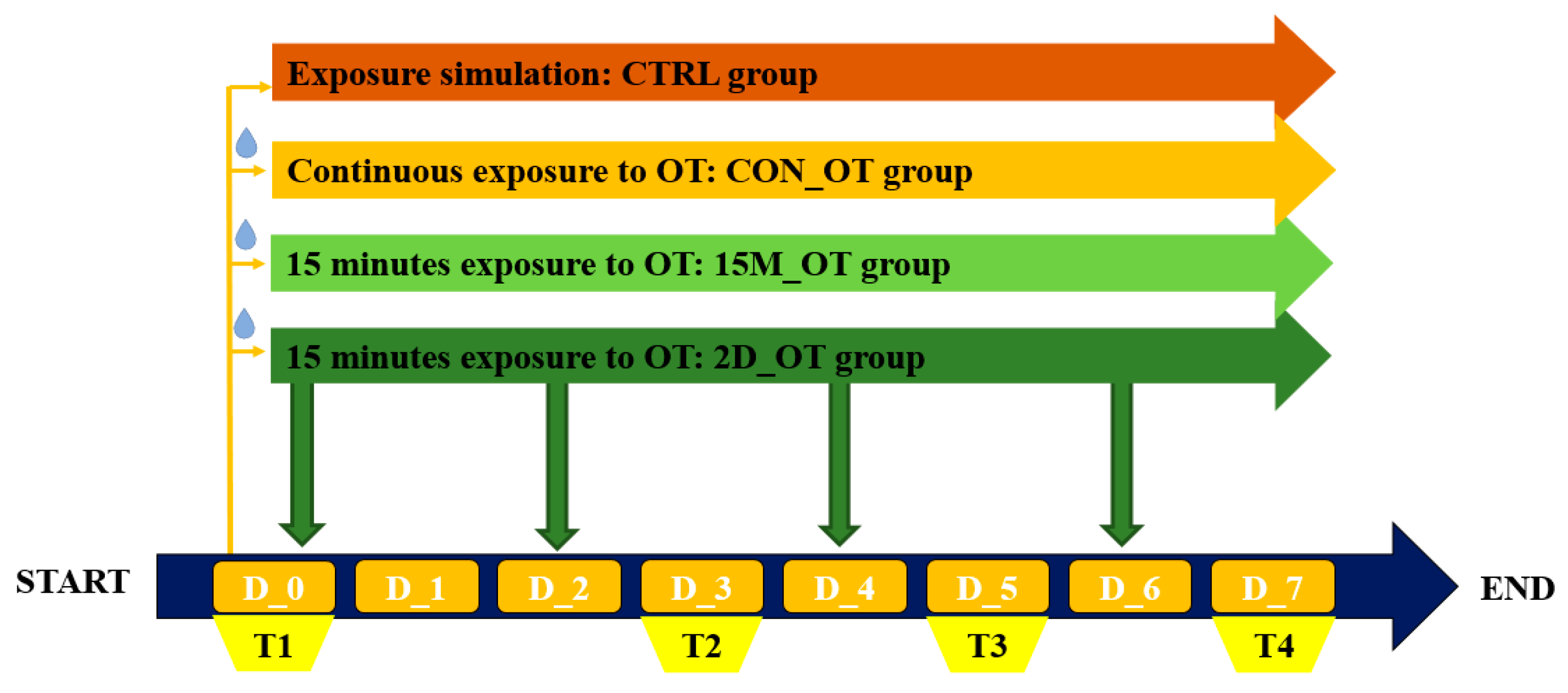
2.3. Behavioral Test Procedure
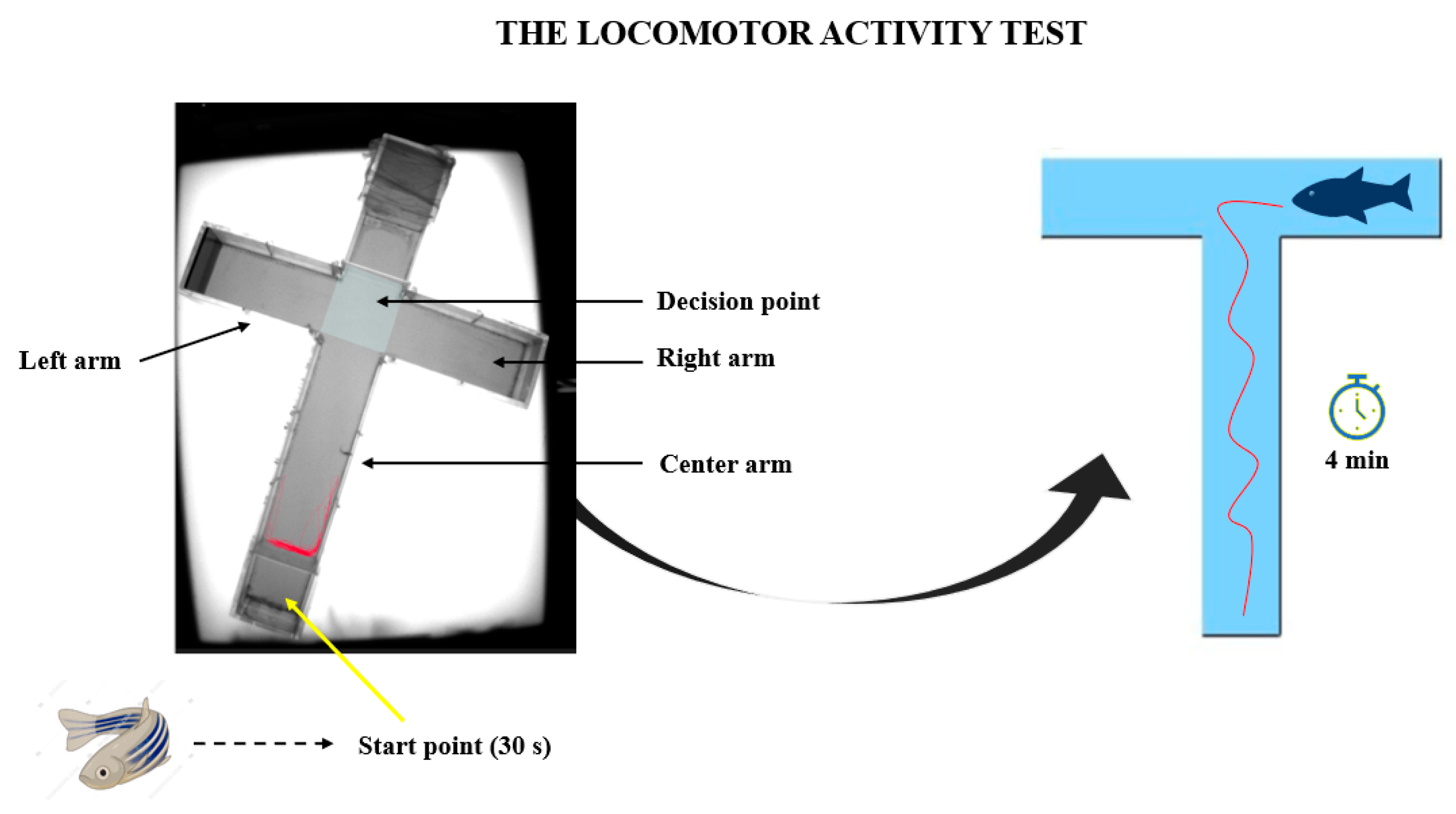
2.3.1. The Locomotor Activity Test
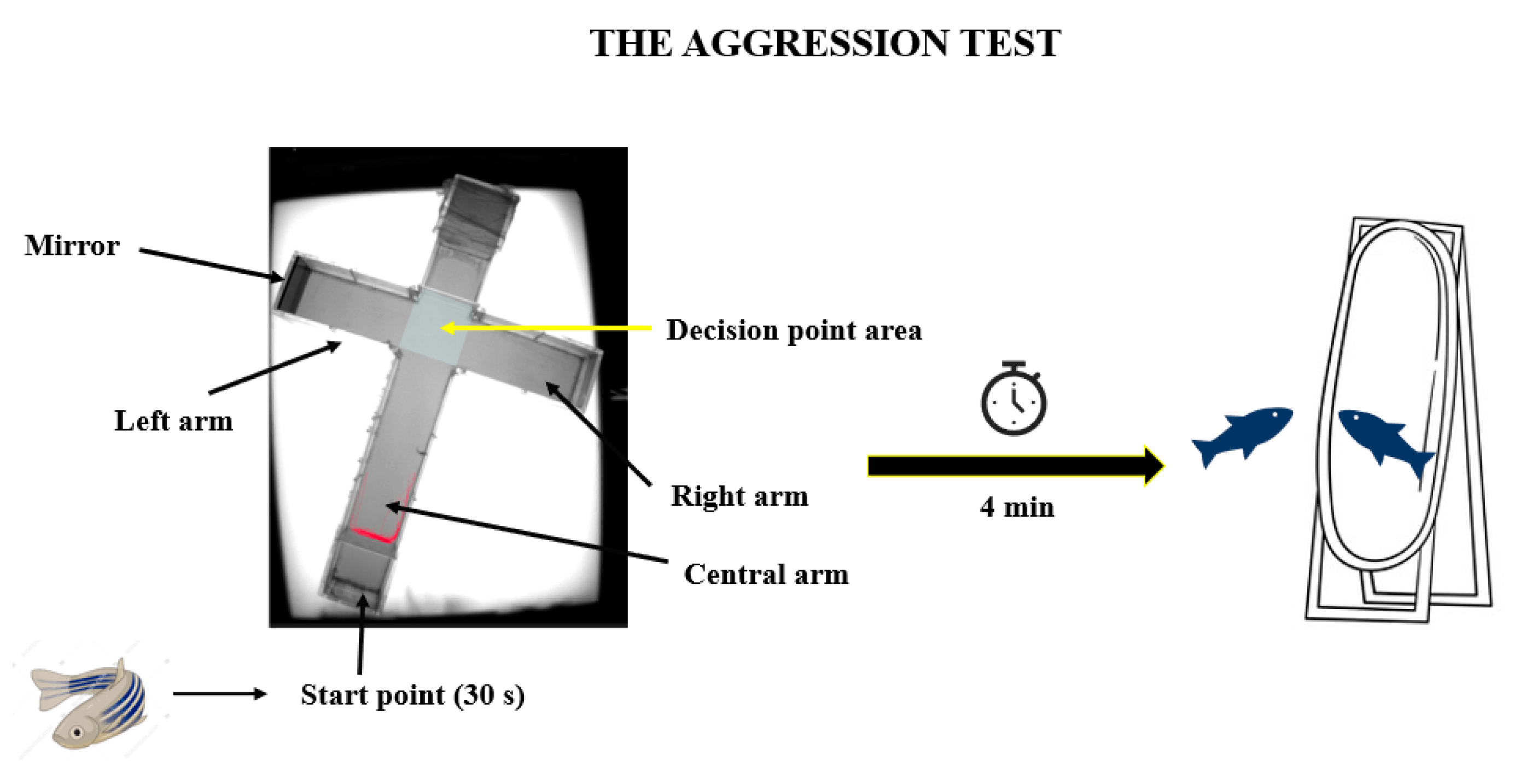
2.3.2. The Aggression Test
2.4. Quantification of Behavior and Statistical Analysis
3. Results
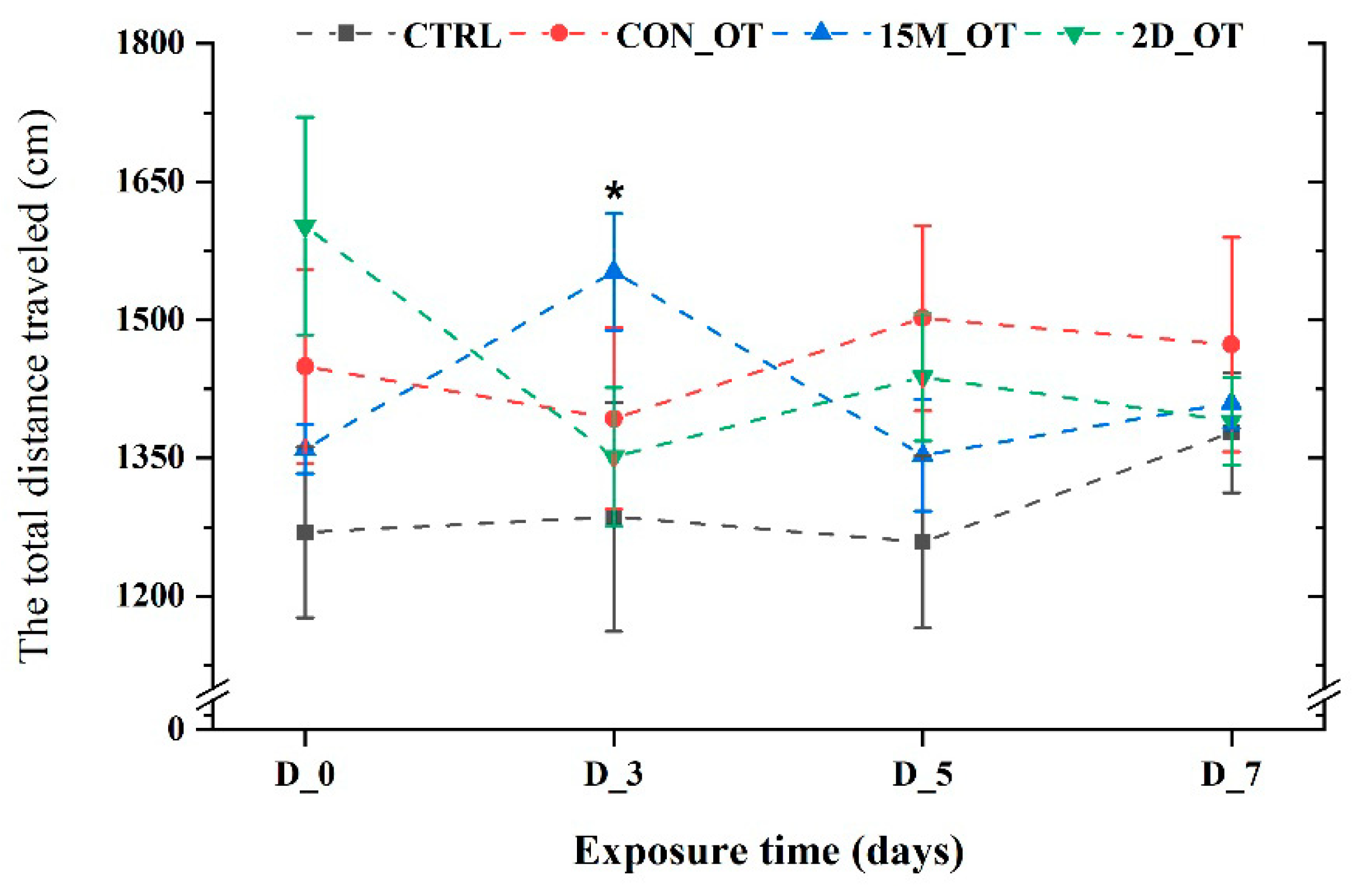
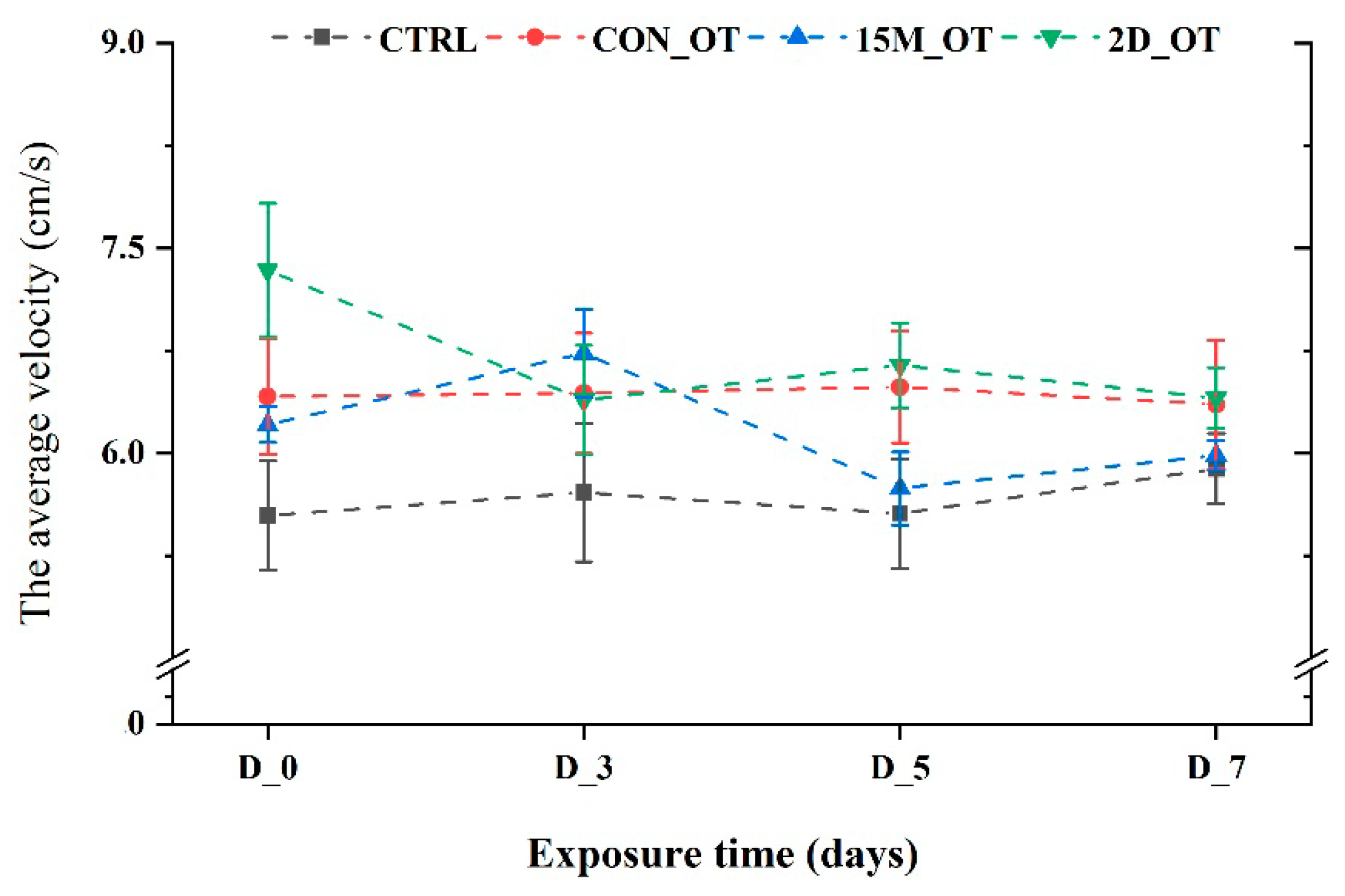
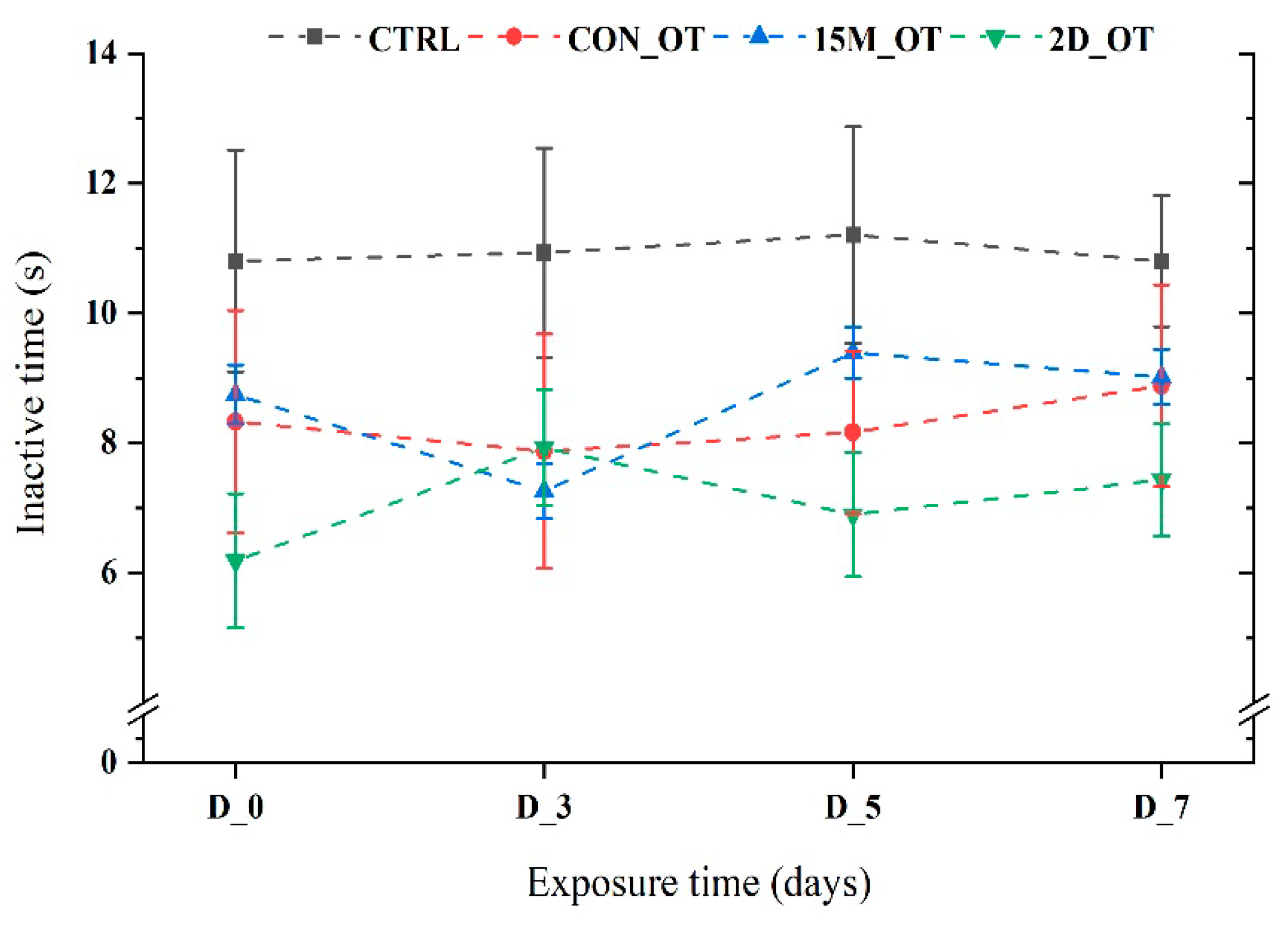
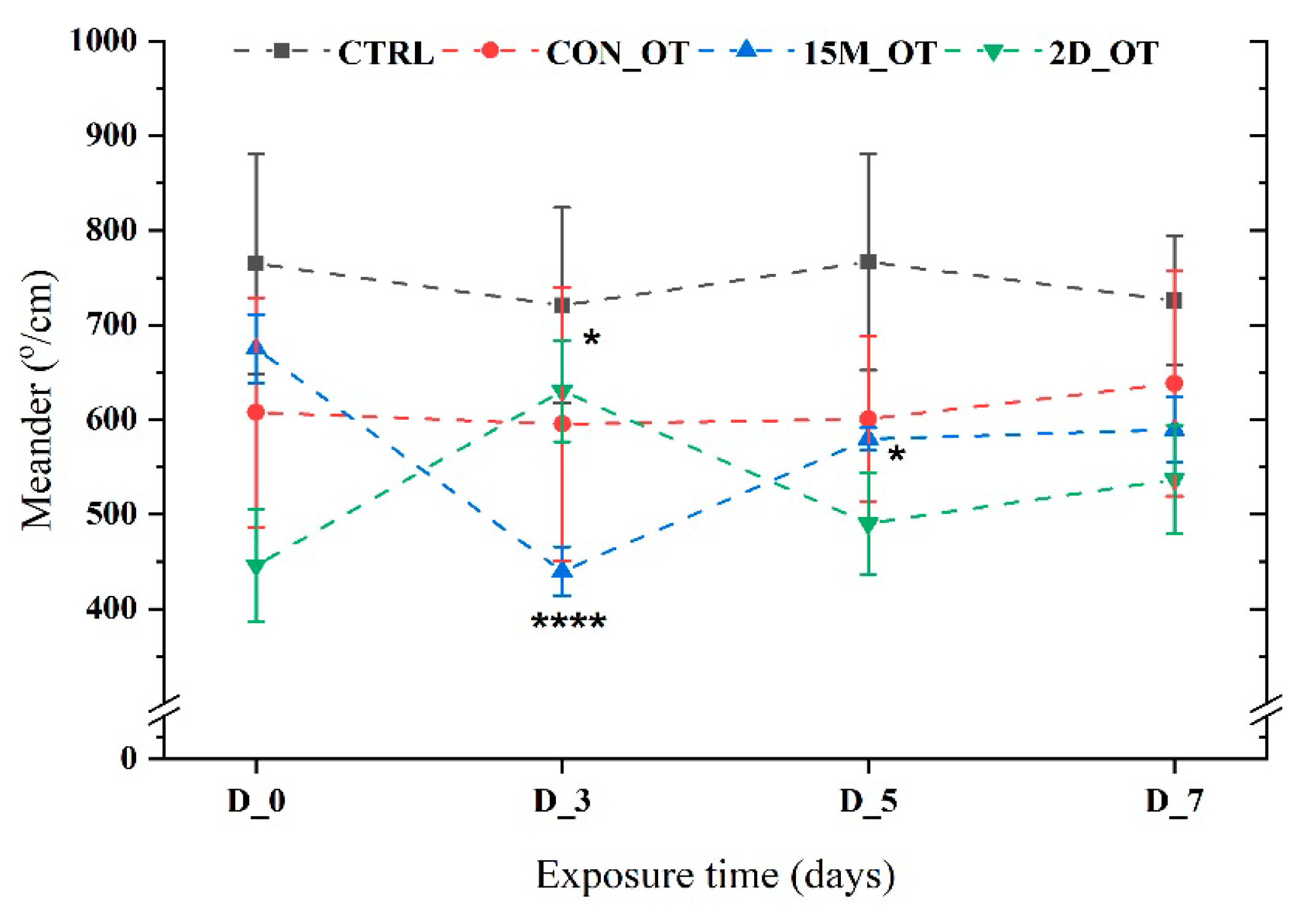
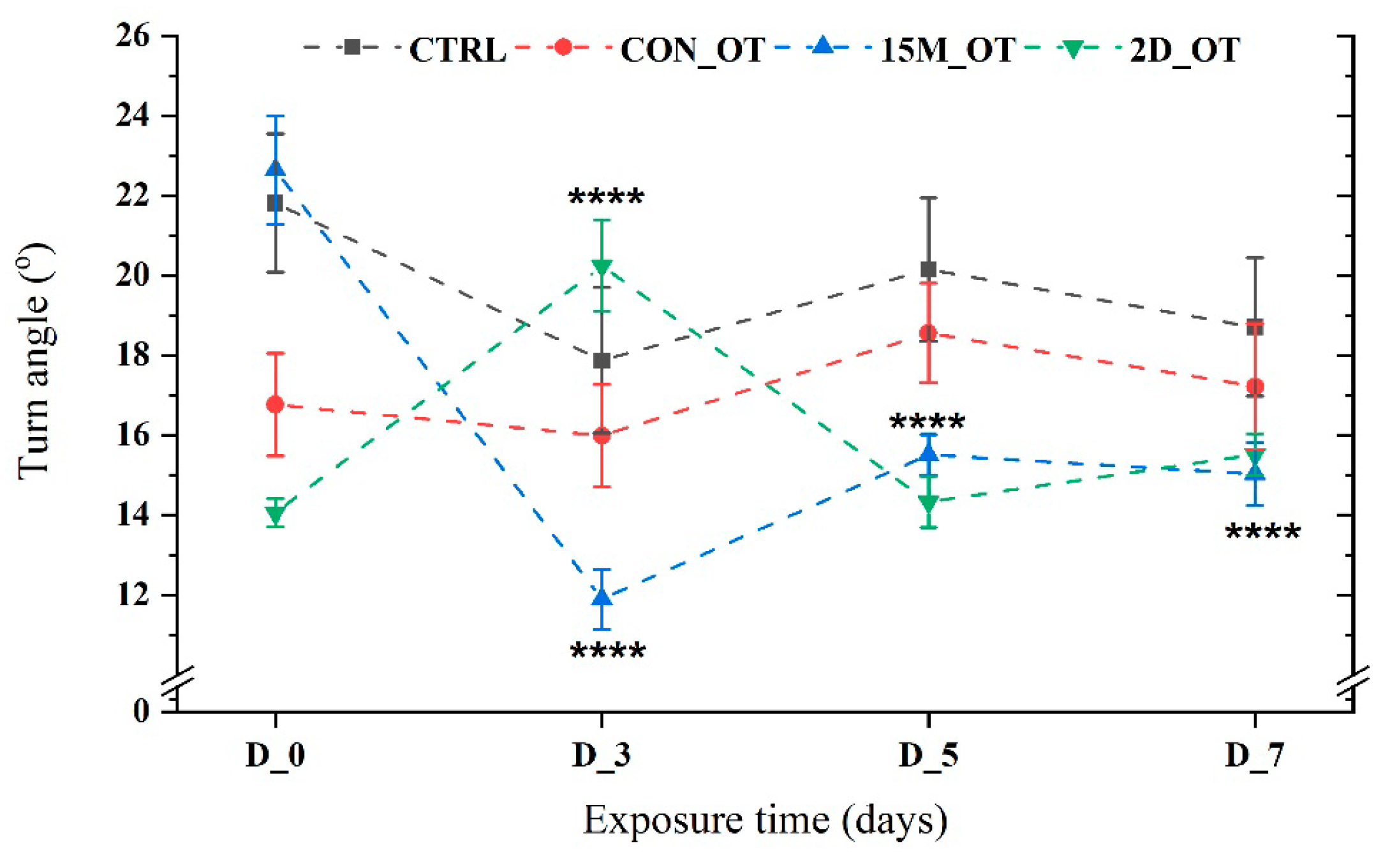
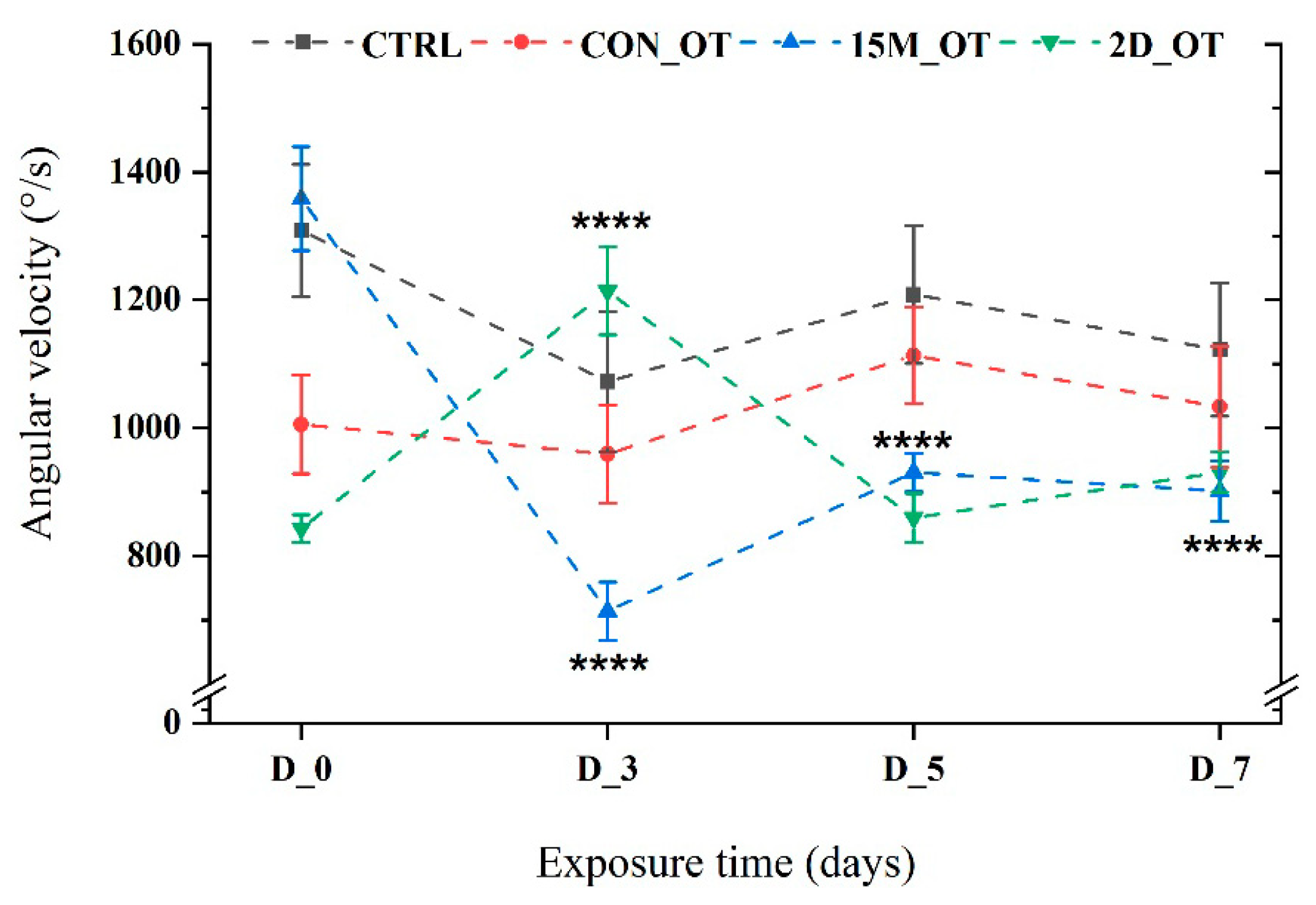
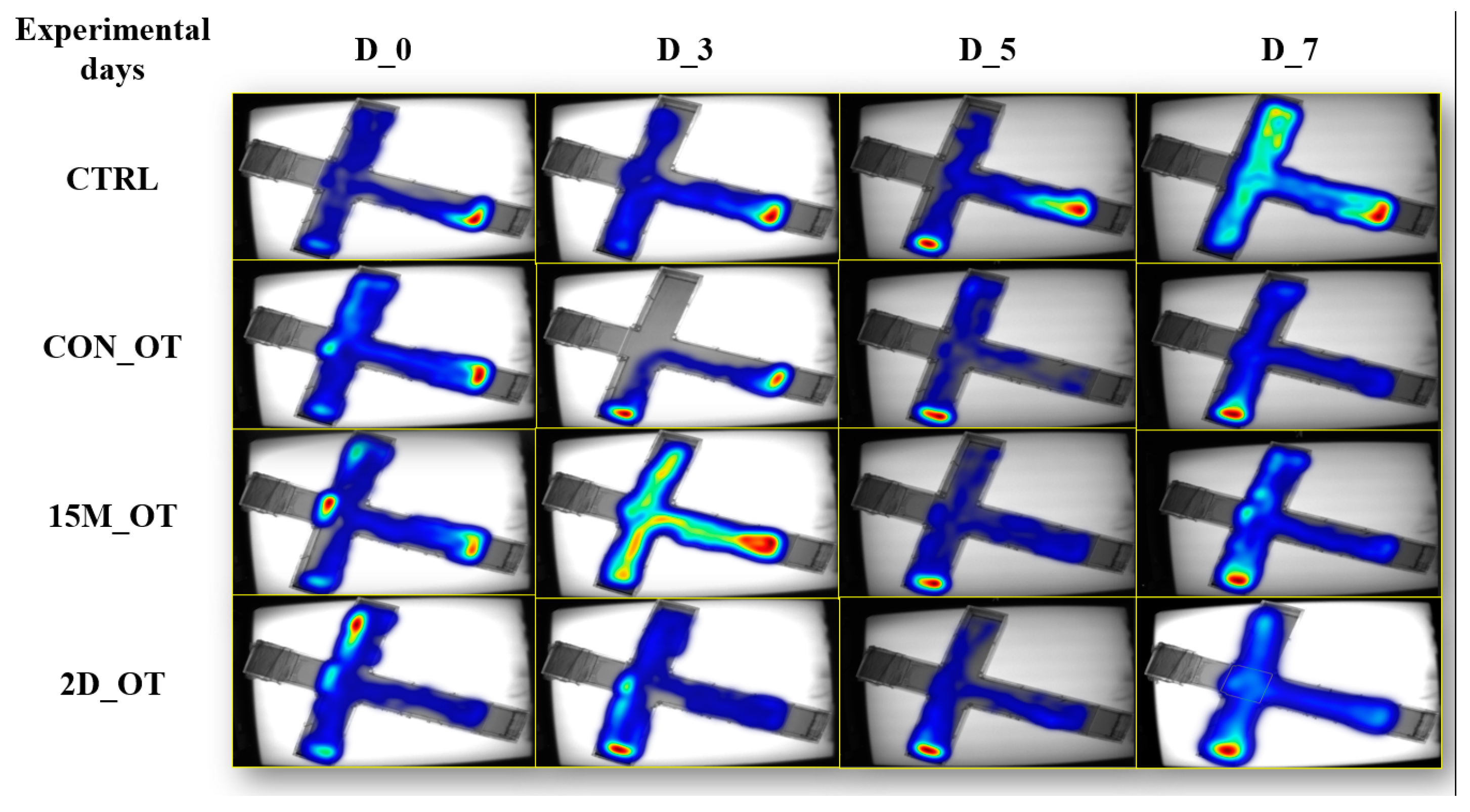
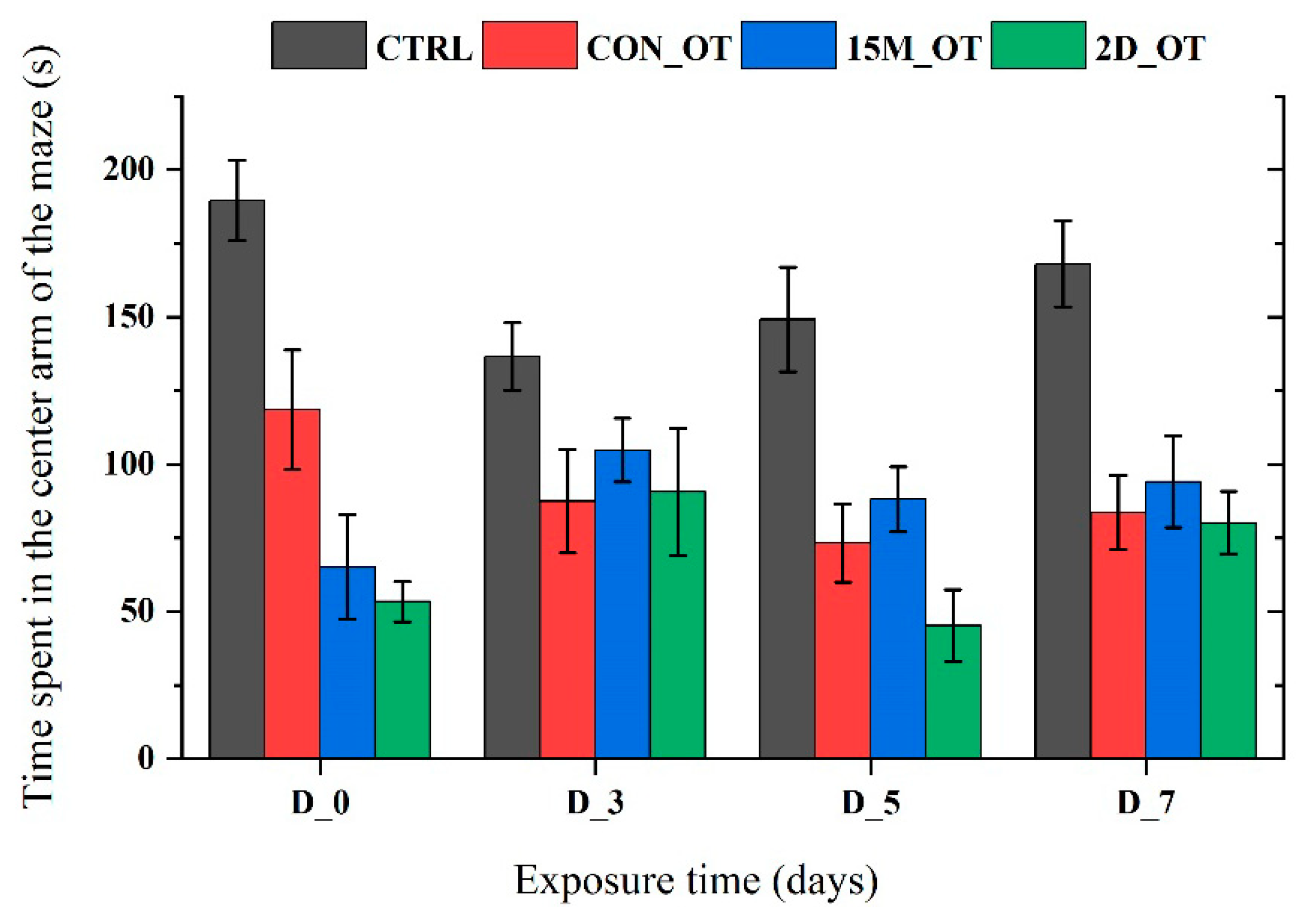
3.1. Oxytocin Has Time-Dependent Effects on Zebrafish Locomotion
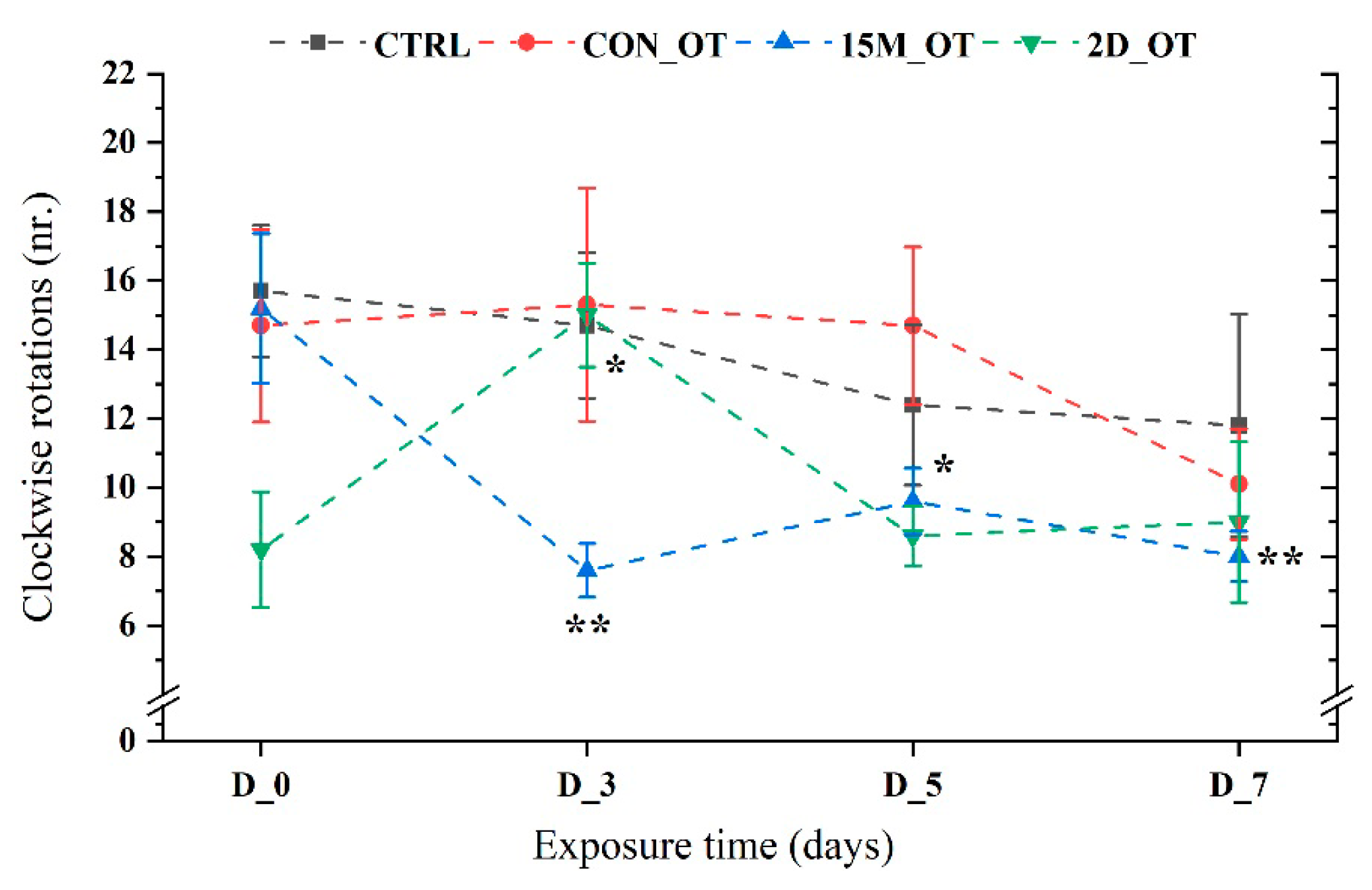
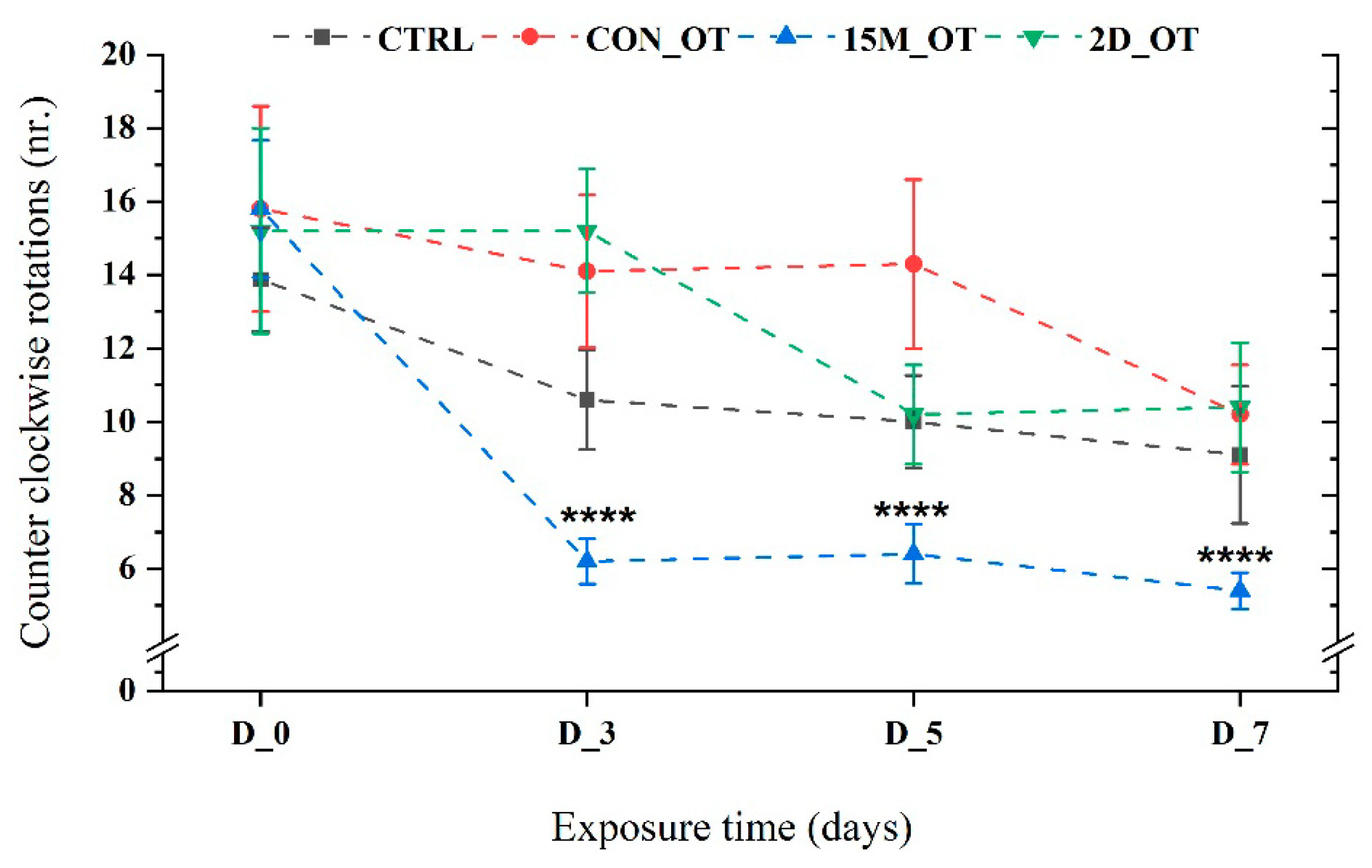
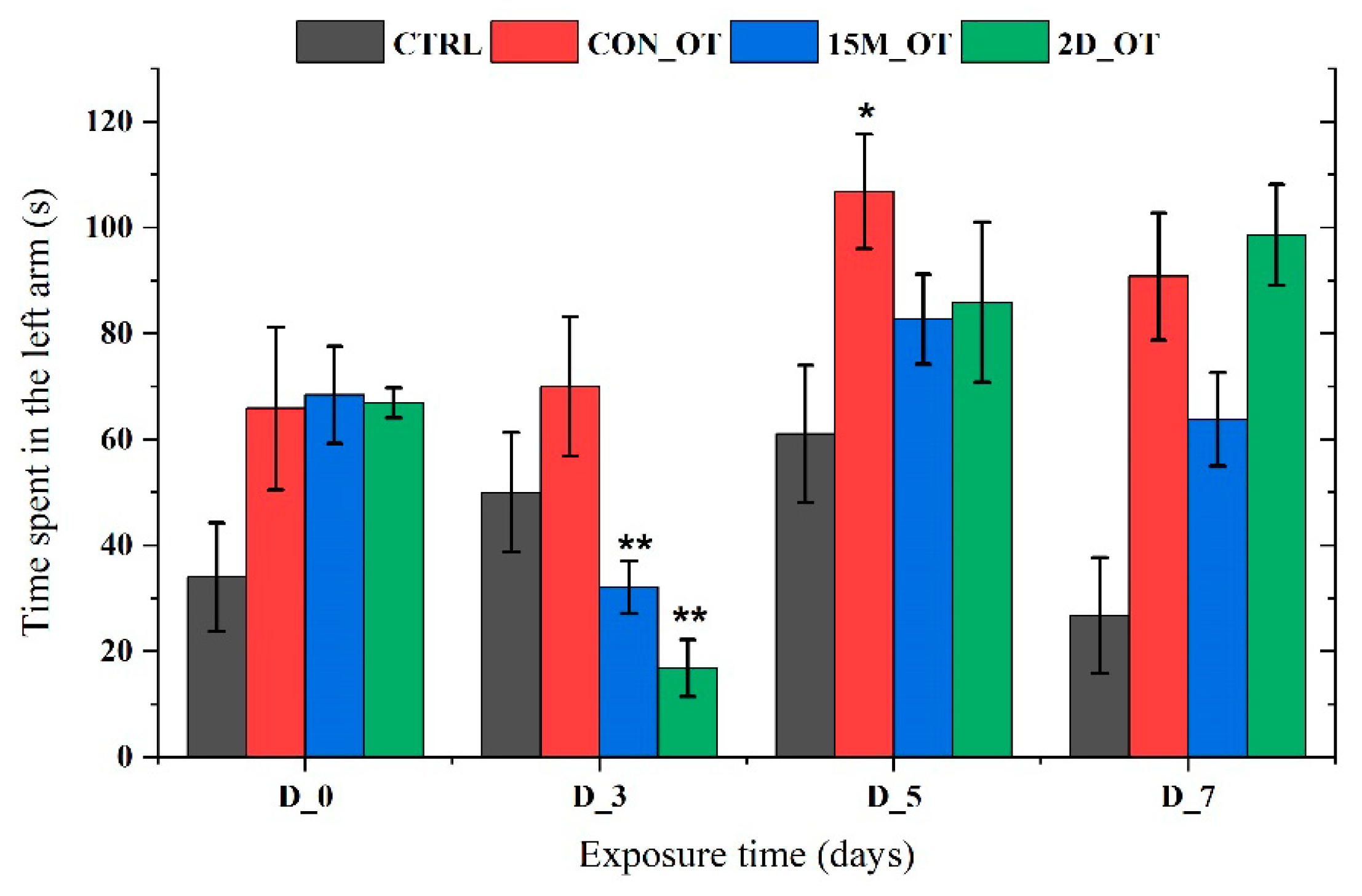
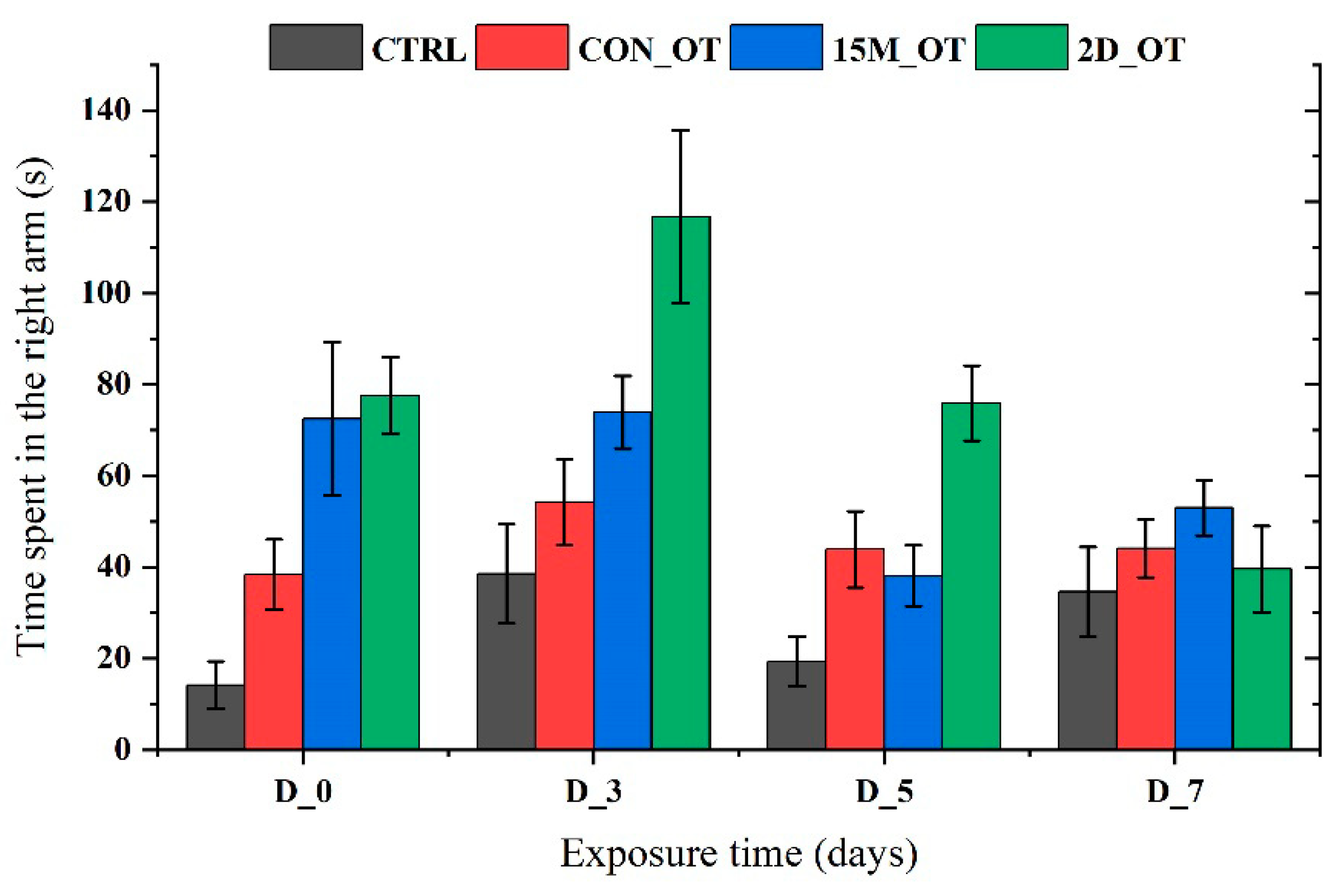
3.2. Oxytocin Triggers Aggressiveness in Zebrafish
4. Discussion
4.1. Limitations
4.2. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, C.-C.; Lin, L.-S.; Long, S.; Ke, X.-Y.; Fukunaga, K.; Lu, Y.-M.; Han, F. Signalling pathways in autism spectrum disorder: Mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 2022, 7, 229. [Google Scholar] [CrossRef]
- American Psychiatric Association, A.P.A. Diagnostic and Statiscal Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2013; pp. 50–51. [Google Scholar]
- Napolitano, A.; Schiavi, S.; La Rosa, P.; Rossi-Espagnet, M.C.; Petrillo, S.; Bottino, F.; Tagliente, E.; Longo, D.; Lupi, E.; Casula, L.; et al. Sex Differences in Autism Spectrum Disorder: Diagnostic, Neurobiological, and Behavioral Features. Front. Psychiatry 2022, 13, 889636. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Wu, C.; Wang, J.; Sun, M. Autism Spectrum Disorder: Neurodevelopmental Risk Factors, Biological Mechanism, and Precision Therapy. Int. J. Mol. Sci. 2023, 24, 1819. [Google Scholar] [CrossRef]
- Sealey, L.A.; Hughes, B.W.; Sriskanda, A.N.; Guest, J.R.; Gibson, A.D.; Johnson-Williams, L.; Pace, D.G.; Bagasra, O. Environmental factors in the development of autism spectrum disorders. Environ. Int. 2016, 88, 288–298. [Google Scholar] [CrossRef]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Noorimotlagh, Z.; Mirzaee, S.A.; Jaafarzadeh, N.; Martinez, S.S.; Rahim, F.; Kaffashian, M. An updated systematic review on the maternal exposure to environmental pesticides and involved mechanisms of autism spectrum disorder (ASD) progression risk in children. Rev. Environ. Health 2023, 38, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, M.T.; Golshani, P. The utility of rodent models of autism spectrum disorders. Curr. Opin. Neurol. 2015, 28, 103–109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, S.; Chun, H.-S.; Lee, J.; Park, H.-J.; Kim, K.-T.; Kim, C.-H.; Yoon, S.; Kim, W.-K. Plausibility of the zebrafish embryos/larvae as an alternative animal model for autism: A comparison study of transcriptome changes. PLoS ONE 2018, 13, e0203543. [Google Scholar] [CrossRef] [PubMed]
- Meshalkina, D.A.; N Kizlyk, M.; V Kysil, E.; Collier, A.D.; Echevarria, D.J.; Abreu, M.S.; Barcellos, L.J.G.; Song, C.; Warnick, J.E.; Kyzar, E.J.; et al. Zebrafish models of autism spectrum disorder. Exp. Neurol. 2018, 299, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Dougnon, G.; Matsui, H. Modelling Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD) Using Mice and Zebrafish. Int. J. Mol. Sci. 2022, 23, 7550. [Google Scholar] [CrossRef] [PubMed]
- Dooley, K.; Zon, L.I. Zebrafish: A model system for the study of human disease. Curr. Opin. Genet. Dev. 2000, 10, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Santoriello, C.; Zon, L.I. Hooked! Modeling human disease in zebrafish. J. Clin. Investig. 2012, 122, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.F. Mind the fish: Zebrafish as a model in cognitive social neuroscience. Front. Neural Circuits 2013, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-H.; Huang, P.; Dong, W.; Zhu, Z.-Y.; Liu, D. A brief history of zebrafish research--toward biomedicine. Yi Chuan Hered. 2013, 35, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Tayanloo-Beik, A.; Hamidpour, S.K.; Abedi, M.; Shojaei, H.; Tavirani, M.R.; Namazi, N.; Larijani, B.; Arjmand, B. Zebrafish Modeling of Autism Spectrum Disorders, Current Status and Future Prospective. Front. Psychiatry 2022, 13, 911770. [Google Scholar] [CrossRef] [PubMed]
- Dreosti, E.; Lopes, G.; Kampff, A.R.; Wilson, S.W. Development of social behavior in young zebrafish. Front. Neural Circuits 2015, 9, 39. [Google Scholar] [CrossRef]
- Nunes, A.R.; Ruhl, N.; Winberg, S.; Oliveira, R.F. Social Phenotypes in Zebrafish. In The Rights and Wrongs of Zebrafish: Behavioral Phenotyping of Zebrafish; Kalueff, A.V., Ed.; Springer: Cham, Switzerland, 2017; pp. 95–130. ISBN 978-3-319-33774-6. [Google Scholar]
- Saszik, S.M.; Smith, C.M. The impact of stress on social behavior in adult zebrafish (Danio rerio). Behav. Pharmacol. 2018, 29, 53–59. [Google Scholar] [CrossRef]
- Ricci, L.; Summers, C.H.; Larson, E.T.; O’Malley, D.; Melloni, R.H. Development of aggressive phenotypes in zebrafish: Interactions of age, experience and social status. Anim. Behav. 2013, 86, 245–252. [Google Scholar] [CrossRef]
- Way, G.P.; Ruhl, N.; Snekser, J.L.; Kiesel, A.L.; McRobert, S.P. A comparison of methodologies to test aggression in zebrafish. Zebrafish 2015, 12, 144–151. [Google Scholar] [CrossRef]
- Zabegalov, K.N.; Kolesnikova, T.O.; Khatsko, S.L.; Volgin, A.D.; Yakovlev, O.A.; Amstislavskaya, T.G.; Friend, A.J.; Bao, W.; Alekseeva, P.A.; Lakstygal, A.M.; et al. Understanding zebrafish aggressive behavior. Behav. Process. 2019, 158, 200–210. [Google Scholar] [CrossRef]
- Fitzpatrick, S.E.; Srivorakiat, L.; Wink, L.K.; Pedapati, E.V.; Erickson, C.A. Aggression in autism spectrum disorder: Presentation and treatment options. Neuropsychiatr. Dis. Treat. 2016, 12, 1525–1538. [Google Scholar] [CrossRef] [PubMed]
- Geelhand, P.; Bernard, P.; Klein, O.; van Tiel, B.; Kissine, M. The role of gender in the perception of autism symptom severity and future behavioral development. Mol. Autism 2019, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Barrera, I.; Brothers, S.; Ring, R.; Wahlestedt, C. Oxytocin and social functioning. Dialogues Clin. Neurosci. 2017, 19, 193–201. [Google Scholar] [CrossRef]
- Kong, X.-J.; Liu, J.; Liu, K.; Koh, M.; Sherman, H.; Liu, S.; Tian, R.; Sukijthamapan, P.; Wang, J.; Fong, M.; et al. Probiotic and Oxytocin Combination Therapy in Patients with Autism Spectrum Disorder: A Randomized, Double-Blinded, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 1552. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, A.A.; Soliman, O.A.; Elbahnasi, A.I.; Alawy, A.Y.; Mansour, A.M.; Gowayed, M.A. Role of Oxytocin in Different Neuropsychiatric, Neurodegenerative, and Neurodevelopmental Disorders. Rev. Physiol. Biochem. Pharmacol. 2023, 186, 95–134. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J.; Hollander, E. Autism and Oxytocin: New Developments in Translational Approaches to Therapeutics. Neurotherapeutics 2010, 7, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, A.R.; Hwangbo, R.; Han, J.; Hong, M.; Bahn, G.H. Is Oxytocin Application for Autism Spectrum Disorder Evidence-Based? Exp. Neurobiol. 2015, 24, 312–324. [Google Scholar] [CrossRef]
- Miller, T.V.; Caldwell, H.K. Oxytocin during Development: Possible Organizational Effects on Behavior. Front. Endocrinol. 2015, 6, 76. [Google Scholar] [CrossRef]
- Walter, M.H.; Abele, H.; Plappert, C.F. The Role of Oxytocin and the Effect of Stress During Childbirth: Neurobiological Basics and Implications for Mother and Child. Front. Endocrinol. 2021, 12, 742236. [Google Scholar] [CrossRef]
- Xu, X.-J.; Shou, X.-J.; Li, J.; Jia, M.-X.; Zhang, J.-S.; Guo, Y.; Wei, Q.-Y.; Zhang, X.-T.; Han, S.-P.; Zhang, R.; et al. Mothers of autistic children: Lower plasma levels of oxytocin and Arg-vasopressin and a higher level of testosterone. PLoS ONE 2013, 8, e74849. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Jaeggi, A.V. Oxytocin levels tend to be lower in autistic children: A meta-analysis of 31 studies. Autism 2021, 25, 2152–2161. [Google Scholar] [CrossRef]
- Van IJzendoorn, M.H.; Bakermans-Kranenburg, M.J. A sniff of trust: Meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology 2012, 37, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Veening, J.G.; Olivier, B. Intranasal administration of oxytocin: Behavioral and clinical effects, a review. Neurosci. Biobehav. Rev. 2013, 37, 1445–1465. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Kendrick, K.M. Effects of Intranasal Administration of Oxytocin and Vasopressin on Social Cognition and Potential Routes and Mechanisms of Action. Pharmaceutics 2022, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, E.; Soorya, L.; Brian, J.; Dupuis, A.; Mankad, D.; Smile, S.; Jacob, S. Intranasal oxytocin in the treatment of autism spectrum disorders: A review of literature and early safety and efficacy data in youth. Brain Res. 2014, 1580, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Karbasi, A.; Shafiezadegan Isfahani, S.; Maracy, M.R.; Sabzghabaee, A.M. Effect of intranasal oxytocin combination therapy with applied behavior analysis on social impairments in pediatric’s children with autism spectrum disorder. Middle East Curr. Psychiatry 2023, 30, 35. [Google Scholar] [CrossRef]
- Guastella, A.J.; Boulton, K.A.; Whitehouse, A.J.O.; Song, Y.J.; Thapa, R.; Gregory, S.G.; Pokorski, I.; Granich, J.; DeMayo, M.M.; Ambarchi, Z.; et al. The effect of oxytocin nasal spray on social interaction in young children with autism: A randomized clinical trial. Mol. Psychiatry 2023, 28, 834–842. [Google Scholar] [CrossRef]
- Maslahati, T.; Wingenfeld, K.; Hellmann-Regen, J.; Kraft, J.; Lyu, J.; Keinert, M.; Voß, A.; Cho, A.B.; Ripke, S.; Otte, C.; et al. Oxytocin vs. placebo effects on intrusive memory consolidation using a trauma film paradigm: A randomized, controlled experimental study in healthy women. Transl. Psychiatry 2023, 13, 42. [Google Scholar] [CrossRef]
- Crane, J.W.; Holmes, N.M.; Fam, J.; Westbrook, R.F.; Delaney, A.J. Oxytocin increases inhibitory synaptic transmission and blocks development of long-term potentiation in the lateral amygdala. J. Neurophysiol. 2019, 123, 587–599. [Google Scholar] [CrossRef]
- Triana-Del Rio, R.; Ranade, S.; Guardado, J.; LeDoux, J.; Klann, E.; Shrestha, P. The modulation of emotional and social behaviors by oxytocin signaling in limbic network. Front. Mol. Neurosci. 2022, 15, 1002846. [Google Scholar] [CrossRef] [PubMed]
- Sobota, R.; Mihara, T.; Forrest, A.; Featherstone, R.E.; Siegel, S.J. Oxytocin reduces amygdala activity, increases social interactions, and reduces anxiety-like behavior irrespective of NMDAR antagonism. Behav. Neurosci. 2015, 129, 389–398. [Google Scholar] [CrossRef]
- Xin, F.; Zhou, X.; Dong, D.; Zhao, Z.; Yang, X.; Wang, Q.; Gu, Y.; Kendrick, K.M.; Chen, A.; Becker, B. Oxytocin Differentially Modulates Amygdala Responses during Top-Down and Bottom-Up Aversive Anticipation. Adv. Sci. (Weinh. Baden-Wurtt. Ger.) 2020, 7, 2001077. [Google Scholar] [CrossRef]
- Tomizawa, K.; Iga, N.; Lu, Y.-F.; Moriwaki, A.; Matsushita, M.; Li, S.-T.; Miyamoto, O.; Itano, T.; Matsui, H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat. Neurosci. 2003, 6, 384–390. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-H.; Chung, C.; Kim, J.J.; Choi, S.-Y.; Han, J.-S. Oxytocin Protects Hippocampal Memory and Plasticity from Uncontrollable Stress. Sci. Rep. 2015, 5, 18540. [Google Scholar] [CrossRef] [PubMed]
- Talpo, F.; Spaiardi, P.; Castagno, A.N.; Maniezzi, C.; Raffin, F.; Terribile, G.; Sancini, G.; Pisani, A.; Biella, G.R. Neuromodulatory functions exerted by oxytocin on different populations of hippocampal neurons in rodents. Front. Cell. Neurosci. 2023, 17, 1082010. [Google Scholar] [CrossRef] [PubMed]
- Tabak, B.A.; Leng, G.; Szeto, A.; Parker, K.J.; Verbalis, J.G.; Ziegler, T.E.; Lee, M.R.; Neumann, I.D.; Mendez, A.J. Advances in human oxytocin measurement: Challenges and proposed solutions. Mol. Psychiatry 2023, 28, 127–140. [Google Scholar] [CrossRef]
- Quintana, D.S. Towards better hypothesis tests in oxytocin research: Evaluating the validity of auxiliary assumptions. Psychoneuroendocrinology 2022, 137, 105642. [Google Scholar] [CrossRef]
- MacLean, E.L.; Wilson, S.R.; Martin, W.L.; Davis, J.M.; Nazarloo, H.P.; Carter, C.S. Challenges for measuring oxytocin: The blind men and the elephant? Psychoneuroendocrinology 2019, 107, 225–231. [Google Scholar] [CrossRef]
- Bales, K.L. Oxytocin, Vasopressin, and Development of Social Behavior in Primates, 2nd ed.; Kaas, J., Krubitzer, L.A., Eds.; Academic Press: Oxford, UK, 2017; pp. 461–474. ISBN 978-0-12-804096-6. [Google Scholar]
- Braida, D.; Donzelli, A.; Martucci, R.; Capurro, V.; Busnelli, M.; Chini, B.; Sala, M. Neurohypophyseal hormones manipulation modulate social and anxiety-related behavior in zebrafish. Psychopharmacology 2012, 220, 319–330. [Google Scholar] [CrossRef]
- Landin, J.; Hovey, D.; Xu, B.; Lagman, D.; Zettergren, A.; Larhammar, D.; Kettunen, P.; Westberg, L. Oxytocin Receptors Regulate Social Preference in Zebrafish. Sci. Rep. 2020, 10, 5435. [Google Scholar] [CrossRef]
- Gemmer, A.; Mirkes, K.; Anneser, L.; Eilers, T.; Kibat, C.; Mathuru, A.; Ryu, S.; Schuman, E. Oxytocin receptors influence the development and maintenance of social behavior in zebrafish (Danio rerio). Sci. Rep. 2022, 12, 4322. [Google Scholar] [CrossRef] [PubMed]
- Rahmati-Holasoo, H.; Salek Maghsoudi, A.; Akbarzade, M.; Gholami, M.; Shadboorestan, A.; Vakhshiteh, F.; Armandeh, M.; Hassani, S. Oxytocin protective effects on zebrafish larvae models of autism-like spectrum disorder. Iran. J. Basic Med. Sci. 2023, 26, 316–325. [Google Scholar] [CrossRef]
- Robb, A. Managing irritability and aggression in autism spectrum disorders in children and adolescents. Dev. Disabil. Res. Rev. 2010, 16, 258–264. [Google Scholar] [CrossRef]
- Hill, A.P.; Zuckerman, K.E.; Hagen, A.D.; Kriz, D.J.; Duvall, S.W.; van Santen, J.; Nigg, J.; Fair, D.; Fombonne, E. Aggressive behavior problems in children with autism spectrum disorders: Prevalence and correlates in a large clinical sample. Res. Autism Spectr. Disord. 2014, 8, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Svare, B.B. Maternal Aggression in Mammals BT-Parental Care in Mammals; Gubernick, D.J., Klopfer, P.H., Eds.; Springer: Boston, MA, USA, 1981; pp. 179–210. ISBN 978-1-4613-3150-6. [Google Scholar]
- Trainor, B.C.; Nelson, R.J. Chapter 22-Neuroendocrinology of Aggression; Fink, G., Pfaff, D.W., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 509–520. ISBN 978-0-12-375097-6. [Google Scholar]
- Williams Avram, S.K.; Cymerblit-Sabba, A. 3.14-Vasopressin: Roles in Modulating Social Behaviors. In Hormones, Brain and Behavior, 3rd ed.; Pfaff, D.W., Joëls, M., Eds.; Academic Press: Oxford, UK, 2017; pp. 279–304. ISBN 978-0-12-803608-2. [Google Scholar]
- Bosch, O.J.; Meddle, S.L.; Beiderbeck, D.I.; Douglas, A.J.; Neumann, I.D. Brain oxytocin correlates with maternal aggression: Link to anxiety. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 6807–6815. [Google Scholar] [CrossRef] [PubMed]
- Sabihi, S.; Dong, S.M.; Durosko, N.E.; Leuner, B. Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Front. Behav. Neurosci. 2014, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.-J.; Chang, C.-Y.; Ho, H.-P.; Chou, M.-Y. Oxytocin Signaling Acts as a Marker for Environmental Stressors in Zebrafish. Int. J. Mol. Sci. 2021, 22, 7459. [Google Scholar] [CrossRef]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.T.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G.; et al. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: An introduction. J. Vis. Exp. JoVE 2012, 69, e4196. [Google Scholar] [CrossRef]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2019, 54, 213–224. [Google Scholar] [CrossRef]
- The Commission Of The European Communities, Commission Recommendation Guidelines for the accommodation and care of animals used for experimental and other scientific purposes (notified under document number C(2007) 2525). Off. J. Eur. Union 2007, 89.
- The European Parliament And The Council Of The European Union, Directive 63 The protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 33–79.
- Balmus, I.; Strungaru, Ș.-A.; Nicoara, M.; Plavan, G.; Cojocaru, S.; Simion, L. Preliminary Data Regarding the Effects of Oxytocin Administration on the Oxidative Stress Status of Zebrafish (Danio rerio). Rev. Chim. 2017, 68, 1640–1643. [Google Scholar] [CrossRef]
- Mottolese, R.; Redouté, J.; Costes, N.; Le Bars, D.; Sirigu, A. Switching brain serotonin with oxytocin. Proc. Natl. Acad. Sci. USA 2014, 111, 8637–8642. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.; Kim, E.J.; Jung, H.; Yoon, M. Relationship between oxytocin and serotonin and the fearfulness, dominance, and trainability of horses. J. Anim. Sci. Technol. 2021, 63, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Abozaid, A.; Gerlai, R. Behavioral Effects of Buspirone in Juvenile Zebrafish of Two Different Genetic Backgrounds. Toxics 2022, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Razali, K.; Mohd Nasir, M.H.; Othman, N.; Doolaanea, A.A.; Kumar, J.; Nabeel Ibrahim, W.; Mohamed, W.M.Y. Characterization of neurobehavioral pattern in a zebrafish 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced model: A 96-hour behavioral study. PLoS ONE 2022, 17, e0274844. [Google Scholar] [CrossRef] [PubMed]
- Destro Bisol, G.; Capocasa, M.; Anagnostou, P. When gender matters: New insights into the relationships between social systems and the genetic structure of human populations. Mol. Ecol. 2012, 21, 4917–4920. [Google Scholar] [CrossRef] [PubMed]
- Dadds, M.R.; MacDonald, E.; Cauchi, A.; Williams, K.; Levy, F.; Brennan, J. Nasal oxytocin for social deficits in childhood autism: A randomized controlled trial. J. Autism Dev. Disord. 2014, 44, 521–531. [Google Scholar] [CrossRef]
- Muscatelli, F.; Matarazzo, V.; Chini, B. Neonatal oxytocin gives the tempo of social and feeding behaviors. Front. Mol. Neurosci. 2022, 15, 1071719. [Google Scholar] [CrossRef]
- Bakos, J.; Lestanova, Z.; Strbak, V.; Havranek, T.; Bacova, Z. Neonatal manipulation of oxytocin prevents lipopolysaccharide-induced decrease in gene expression of growth factors in two developmental stages of the female rat. Neuropeptides 2014, 48, 281–286. [Google Scholar] [CrossRef]
- Bukatova, S.; Reichova, A.; Bacova, Z.; Bakos, J. Neonatal oxytocin treatment alters levels of precursor and mature BDNF forms and modifies the expression of neuronal markers in the male rat hippocampus. Neuropeptides 2023, 102, 102384. [Google Scholar] [CrossRef] [PubMed]
- Bogerts, B.; Schöne, M.; Breitschuh, S. Brain alterations potentially associated with aggression and terrorism. CNS Spectr. 2018, 23, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Choy, O.; Raine, A.; Hamilton, R.H. Stimulation of the Prefrontal Cortex Reduces Intentions to Commit Aggression: A Randomized, Double-Blind, Placebo-Controlled, Stratified, Parallel-Group Trial. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 6505–6512. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.I.; Zai, C.C.; Berall, L.; Abu, Z.; Din, F.; Nowrouzi, B.; Chen, S.; Beitchman, J.H. The role of genetic variants in genes regulating the oxytocin-vasopressin neurohumoral system in childhood-onset aggression. Psychiatr. Genet. 2014, 24, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, C.; Chang, H.; Yan, Q.; Wu, L.; Yuan, S.; Xiang, J.; Hao, W.; Yu, Y. Genetic variants in oxytocin receptor gene (OXTR) and childhood physical abuse collaborate to modify the risk of aggression in chinese adolescents. J. Affect. Disord. 2018, 229, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Alcorn, J.L., 3rd; Green, C.E.; Schmitz, J.; Lane, S.D. Effects of oxytocin on aggressive responding in healthy adult men. Behav. Pharmacol. 2015, 26, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.R.; Gliksberg, M.; Varela, S.A.M.; Teles, M.; Wircer, E.; Blechman, J.; Petri, G.; Levkowitz, G.; Oliveira, R.F. Developmental Effects of Oxytocin Neurons on Social Affiliation and Processing of Social Information. J. Neurosci. Off. J. Soc. Neurosci. 2021, 41, 8742–8760. [Google Scholar] [CrossRef]
- Ferguson, J.N.; Young, L.J.; Hearn, E.F.; Matzuk, M.M.; Insel, T.R.; Winslow, J.T. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000, 25, 284–288. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robea, M.-A.; Oprea, G.; Plavan, G.; Nicoara, M.N.; Mavroudis, I.; Burlui, V.; Ciobica, A. Oxytocin Enhances Time-Dependent Responses in the Aggressive Zebrafish (Danio rerio). Brain Sci. 2024, 14, 203. https://doi.org/10.3390/brainsci14030203
Robea M-A, Oprea G, Plavan G, Nicoara MN, Mavroudis I, Burlui V, Ciobica A. Oxytocin Enhances Time-Dependent Responses in the Aggressive Zebrafish (Danio rerio). Brain Sciences. 2024; 14(3):203. https://doi.org/10.3390/brainsci14030203
Chicago/Turabian StyleRobea, Madalina-Andreea, Georgiana Oprea, Gabriel Plavan, Mircea Nicusor Nicoara, Ioannis Mavroudis, Vasile Burlui, and Alin Ciobica. 2024. "Oxytocin Enhances Time-Dependent Responses in the Aggressive Zebrafish (Danio rerio)" Brain Sciences 14, no. 3: 203. https://doi.org/10.3390/brainsci14030203
APA StyleRobea, M.-A., Oprea, G., Plavan, G., Nicoara, M. N., Mavroudis, I., Burlui, V., & Ciobica, A. (2024). Oxytocin Enhances Time-Dependent Responses in the Aggressive Zebrafish (Danio rerio). Brain Sciences, 14(3), 203. https://doi.org/10.3390/brainsci14030203







