Gene Expression of GABAA Receptor Subunits and Association with Patient Survival in Glioma
Abstract
1. Introduction
2. Materials and Methods
2.1. Glioma Tumor and Patient Data
2.2. Statistics
3. Results
3.1. GABAA Receptor Genes Influencing OS in Patients with Glioma
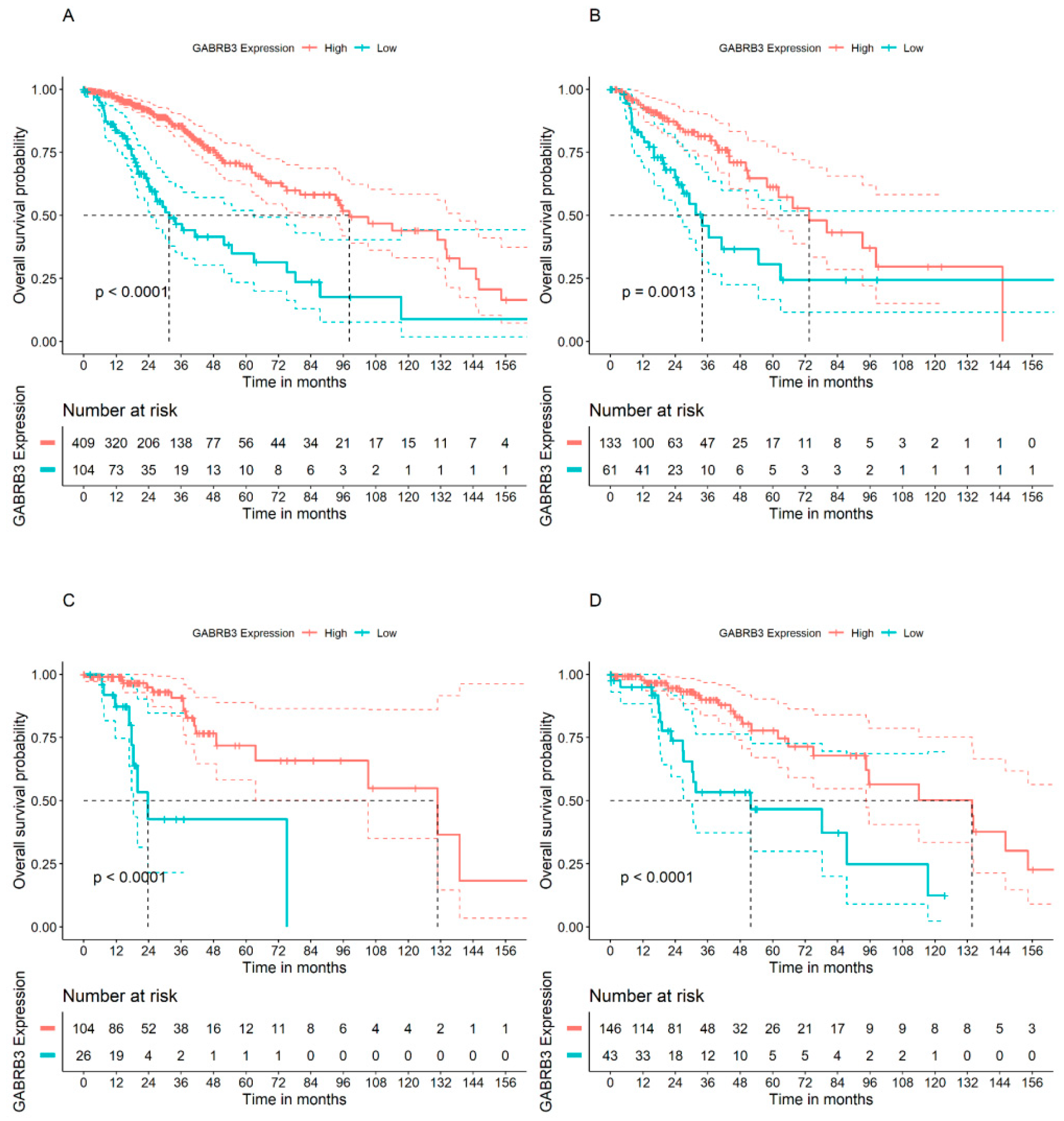
3.2. GABRA2 and GABRB3 Genes Display Opposite Patterns of Association with OS in Patients with GBM
3.3. GABAA Receptor Genes and OS in Patients with Lower Grade Glioma Types
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Toader, C.; Eva, L.; Costea, D.; Corlatescu, A.D.; Covache-Busuioc, R.A.; Bratu, B.G.; Glavan, L.A.; Costin, H.P.; Popa, A.A.; Ciurea, A.V. Low-grade gliomas: Histological subtypes, molecular mechanisms, and treatment strategies. Brain Sci. 2023, 13, 1700. [Google Scholar] [CrossRef]
- Alexander, B.M.; Cloughesy, T.F. Adult glioblastoma. J. Clin. Oncol. 2017, 35, 2402–2409. [Google Scholar] [CrossRef]
- Luskin, M.B. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 1993, 11, 173–189. [Google Scholar] [CrossRef]
- Lois, C.; Alvarez-Buylla, A. Long-distance neuronal migration in the adult mammalian brain. Science 1994, 264, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, F.; Caillé, I.; Lim, D.A.; García-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.E.; Kahng, J.Y.; Kim, S.H.; Park, J.S.; Yoon, S.J.; Um, J.Y.; Kim, W.K.; Lee, J.K.; Park, J.; et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature 2018, 560, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Altmann, C.; Keller, S.; Schmidt, M.H.H. The role of SVZ stem cells in glioblastoma. Cancers 2019, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.L.; Wang, C.F.; Qian, C.; Ji, Y.X.; Wang, Y.Z. Role and mechanism of neural stem cells of the subventricular zone in glioblastoma. World J. Stem Cells 2021, 13, 877–893. [Google Scholar] [CrossRef] [PubMed]
- Beiriger, J.; Habib, A.; Jovanovich, N.; Kodavali, C.V.; Edwards, L.; Amankulor, N.; Zinn, P.O. The subventricular zone in glioblastoma: Genesis, maintenance, and modeling. Front. Oncol. 2022, 12, 790976. [Google Scholar] [CrossRef] [PubMed]
- Loras, A.; Gonzalez-Bonet, L.G.; Gutierrez-Arroyo, J.L.; Martinez-Cadenas, C.; Marques-Torrejon, M.A. Neural stem cells as potential glioblastoma cells of origin. Life 2023, 13, 905. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, C.; Zong, S.; Piao, J.; Zhao, Y.; Chen, X. A review on surgical treatment options in gliomas. Front. Oncol. 2023, 13, 1088484. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Felistia, Y.; Wen, P.Y. Molecular profiling and targeted therapies in gliomas. Curr. Neurol. Neurosci. Rep. 2023, 23, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Bruno, F.; Nichelli, L.; Sanson, M.; Rudàm, R. Advances in molecular and imaging biomarkers in lower-grade gliomas. Expert Rev. Neurother. 2023, 23, 1217–1231. [Google Scholar] [CrossRef]
- Mancusi, R.; Monje, M. The neuroscience of cancer. Nature 2023, 618, 467–479. [Google Scholar] [CrossRef]
- Prillaman, M. How cancer hijacks the nervous system to grow and spread. Nature 2024, 626, 22–24. [Google Scholar] [CrossRef]
- Tochitani, S.; Furukawa, T.; Bando, R.; Kondo, S.; Ito, T.; Matsushima, Y.; Kojima, T.; Matsuzaki, H.; Fukuda, A. GABAA receptors and maternally derived taurine regulate the temporal specification of progenitors of excitatory glutamatergic neurons in the mouse developing cortex. Cereb. Cortex 2021, 31, 4554–4575. [Google Scholar] [CrossRef]
- Bao, H.; Asrican, B.; Li, W.; Gu, B.; Wen, Z.; Lim, S.A.; Haniff, I.; Ramakrishnan, C.; Deisseroth, K.; Philpot, B.; et al. Long-range GABAergic inputs regulate neural stem cell quiescence and control adult hippocampal neurogenesis. Cell Stem Cell 2017, 21, 604–617.e5. [Google Scholar] [CrossRef]
- Lattanzi, D.; Di Palma, M.; Cuppini, R.; Ambrogini, P. GABAergic input affects intracellular calcium levels in developing granule cells of adult rat hippocampus. Int. J. Mol. Sci. 2020, 21, 1715. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Guo, K.; Wu, B.; Wang, H. Overexpression of Shrm4 promotes proliferation and differentiation of neural stem cells through activation of GABA signaling pathway. Mol. Cell. Biochem. 2020, 463, 115–126. [Google Scholar] [CrossRef]
- Catavero, C.; Bao, H.; Song, J. Neural mechanisms underlying GABAergic regulation of adult hippocampal neurogenesis. Cell Tissue Res. 2018, 371, 33–46. [Google Scholar] [CrossRef]
- Song, D.; Chen, Y.; Chen, C.; Chen, L.; Cheng, O. GABAB receptor antagonist promotes hippocampal neurogenesis and facilitates cognitive function recovery following acute cerebral ischemia in mice. Stem Cell Res. Ther. 2021, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.N.; Oppenheimer, S.; Jeong, J.; Buyukdemirtas, B.; Naegele, J.R. Hippocampal transplants of fetal GABAergic progenitors regulate adult neurogenesis in mice with temporal lobe epilepsy. Neurobiol. Dis. 2022, 174, 105879. [Google Scholar] [CrossRef]
- Wang, D.D.; Krueger, D.D.; Bordey, A. GABA depolarizes neuronal progenitors of the postnatal subventricular zone via GABAA receptor activation. J. Physiol. 2003, 550 Pt 3, 785–800. [Google Scholar] [CrossRef]
- Young, S.Z.; Platel, J.C.; Nielsen, J.V.; Jensen, N.A.; Bordey, A. GABA(A) increases calcium in subventricular zone astrocyte-like cells through L- and T-type voltage-gated calcium channels. Front. Cell. Neurosci. 2010, 4, 8. [Google Scholar] [CrossRef]
- Young, S.Z.; Lafourcade, C.A.; Platel, J.C.; Lin, T.V.; Bordey, A. GABAergic striatal neurons project dendrites and axons into the postnatal subventricular zone leading to calcium activity. Front. Cell. Neurosci. 2014, 8, 10. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Puche, A.C. GABA modulation of SVZ-derived progenitor ventral cell migration. Dev. Neurobiol. 2015, 75, 791–804. [Google Scholar] [CrossRef]
- Gutiérrez-Castañeda, N.E.; González-Corona, J.; Griego, E.; Galván, E.J.; Ochoa-de la Paz, L.D. Taurine promotes differentiation and maturation of neural stem/progenitor cells from the subventricular zone via activation of GABAA receptors. Neurochem. Res. 2023, 48, 2206–2219. [Google Scholar] [CrossRef]
- Simon, J.; Wakimoto, H.; Fujita, N.; Lalande, M.; Barnard, E.A. Analysis of the set of GABA(A) receptor genes in the human genome. J. Biol. Chem. 2004, 279, 41422–41435. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Gawali, V.S.; Kallay, L.; Toukam, D.K.; Koehler, A.; Stambrook, P.; Krummel, D.P.; Sengupta, S. Therapeutically leveraging GABAA receptors in cancer. Exp. Biol. Med. 2021, 246, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Ghit, A.; Assal, D.; Al-Shami, A.S.; Hussein, D.E.E. GABAA receptors: Structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 2021, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.M.; Moody, O.A.; Schuler, E.; Coryell, J.; Alexander, J.J.; Jenkins, A.; Escayg, A. De novo variants in GABRA2 and GABRA5 alter receptor function and contribute to early-onset epilepsy. Brain 2018, 141, 2392–2405. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wei, Z.H.; Liu, C.; Li, G.Y.; Qiao, X.Z.; Gan, Y.J.; Zhang, C.C.; Deng, Y.C. Genetic variations in GABA metabolism and epilepsy. Seizure 2022, 101, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Joghataei, M.T.; Bakhtiarzadeh, F.; Dehghan, S.; Ketabforoush, A.H.M.E.; Golab, F.; Zarbakhsh, S.; Ahmadirad, N. The role of neurotransmitters in glioblastoma multiforme-associated seizures. Int. J. Dev. Neurosci. 2023, 83, 677–690. [Google Scholar] [CrossRef]
- Sengupta, S.; Weeraratne, S.D.; Sun, H.; Phallen, J.; Rallapalli, S.K.; Teider, N.; Kosaras, B.; Amani, V.; Pierre-Francois, J.; Tang, Y.; et al. α5-GABAA receptors negatively regulate MYC-amplified medulloblastoma growth. Acta Neuropathol. 2014, 127, 593–603. [Google Scholar] [CrossRef]
- Gravendeel, L.A.; Kouwenhoven, M.C.; Gevaert, O.; de Rooi, J.J.; Stubbs, A.P.; Duijm, J.E.; Daemen, A.; Bleeker, F.E.; Bralten, L.B.; Kloosterhof, N.K.; et al. Intrinsic gene expression profiles of gliomas are a better predictor of survival than histology. Cancer Res. 2009, 69, 9065–9072. [Google Scholar] [CrossRef]
- Rodrigues, E.M.; Giovanini, A.F.; Ribas, C.A.P.M.; Malafaia, O.; Roesler, R.; Isolan, G.R. The nervous system development regulator neuropilin-1 as a potential prognostic marker and therapeutic target in brain cancer. Cancers 2023, 15, 4922. [Google Scholar] [CrossRef]
- Labrakakis, C.; Patt, S.; Hartmann, J.; Kettenmann, H. Functional GABA(A) receptors on human glioma cells. Eur. J. Neurosci. 1998, 10, 231–238. [Google Scholar] [CrossRef]
- Blanchart, A.; Fernando, R.; Häring, M.; Assaife-Lopes, N.; Romanov, R.A.; Andäng, M.; Harkany, T.; Ernfors, P. Endogenous GABAA receptor activity suppresses glioma growth. Oncogene 2017, 36, 777–786. [Google Scholar] [CrossRef]
- Babateen, O.; Jin, Z.; Bhandage, A.; Korol, S.V.; Westermark, B.; Forsberg Nilsson, K.; Uhrbom, L.; Smits, A.; Birnir, B. Etomidate, propofol and diazepam potentiate GABA-evoked GABAA currents in a cell line derived from human glioblastoma. Eur. J. Pharmacol. 2015, 748, 101–107. [Google Scholar] [CrossRef]
- Smits, A.; Jin, Z.; Elsir, T.; Pedder, H.; Nistér, M.; Alafuzoff, I.; Dimberg, A.; Edqvist, P.H.; Pontén, F.; Aronica, E.; et al. GABA-A channel subunit expression in human glioma correlates with tumor histology and clinical outcome. PLoS ONE 2012, 7, e37041. [Google Scholar] [CrossRef]
- Han, S.; Lium, Y.; Caim, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH mutation in glioma: Molecular mechanisms and potential therapeutic targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Dono, A.; Ballester, L.Y.; Primdahl, D.; Esquenazi, Y.; Bhatia, A. IDH-mutant low-grade glioma: Advances in molecular diagnosis, management, and future directions. Curr. Oncol. Rep. 2021, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Kayabolen, A.; Yilmaz, E.; Bagci-Onder, T. IDH mutations in glioma: Double-edged sword in clinical applications? Biomedicines 2021, 9, 799. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J. Targeting IDH-mutant glioma. Neurotherapeutics 2022, 19, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Sareen, H.; Ma, Y.; Becker, T.M.; Roberts, T.L.; de Souza, P.; Powter, B. Molecular biomarkers in glioblastoma: A systematic review and meta-analysis. Int. J. Mol. Sci. 2022, 23, 8835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.; Tang, Y.; Wang, C.; Chen, Y.; Shu, J.; Zhang, K. Systemic screening identifies GABRD, a subunit gene of GABAA receptor as a prognostic marker in adult IDH wild-type diffuse low-grade glioma. Biomed. Pharmacother. 2019, 118, 109215. [Google Scholar] [CrossRef] [PubMed]
- Kallay, L.; Keskin, H.; Ross, A.; Rupji, M.; Moody, O.A.; Wang, X.; Li, G.; Ahmed, T.; Rashid, F.; Stephen, M.R.; et al. Modulating native GABAA receptors in medulloblastoma with positive allosteric benzodiazepine-derivatives induces cell death. J. Neurooncol. 2019, 142, 411–422. [Google Scholar] [CrossRef]
- Northcott, P.A.; Korshunov, A.; Pfister, S.M.; Taylor, M.D. The clinical implications of medulloblastoma subgroups. Nat. Rev. Neurol. 2012, 8, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Northcott, P.A.; Korshunov, A.; Remke, M.; Cho, Y.J.; Clifford, S.C.; Eberhart, C.G.; Parsons, D.W.; Rutkowski, S.; Gajjar, A.; et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012, 123, 465–472. [Google Scholar] [CrossRef]
- Jonas, O.; Calligaris, D.; Methuku, K.R.; Poe, M.M.; Francois, J.P.; Tranghese, F.; Changelian, A.; Sieghart, W.; Ernst, M.; Krummel, D.A.; et al. First in vivo testing of compounds targeting group 3 medulloblastomas using an implantable microdevice as a new paradigm for drug development. J. Biomed. Nanotechnol. 2016, 12, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Weeraratne, S.D.; Cho, Y.J.; Pomeroy, S.L. Could α5-GABA-A receptor activation be used as a target for managing medulloblastomas? CNS Oncol. 2014, 3, 245–247. [Google Scholar] [CrossRef] [PubMed]

| Probe | Subunit | Gene | p Value | Adjusted p |
|---|---|---|---|---|
| 206678_at | GABAA receptor, alpha 1 | GABRA1 | 1.72 × 10−1 | 1 |
| 244118_at | GABAA receptor, alpha 1 | GABRA1 | 1.68 × 10−1 | 1 |
| 1554308_s_at | GABAA receptor, alpha 2 | GABRA2 | 1.00 × 10−3 | 3.60 × 10−2 |
| 207014_at | GABAA receptor, alpha 2 | GABRA2 | 1.29 × 10−2 | 4.64 × 10−1 |
| 216039_at | GABAA receptor, alpha 2 | GABRA2 | 1.24 × 10−3 | 4.45 × 10−2 |
| 207210_at | GABAA receptor, alpha 3 | GABRA3 | 1.05 × 10−7 | 3.78 × 10−6 |
| 208463_at | GABAA receptor, alpha 4 | GABRA4 | 2.99 × 10−1 | 1 |
| 233437_at | GABAA receptor, alpha 4 | GABRA4 | 1.83 × 10−1 | 1 |
| 206456_at | GABAA receptor, alpha 5 | GABRA5 | 7.45 × 10−2 | 1 |
| 215531_s_at | GABAA receptor, alpha 5 | GABRA5 | 2.69 × 10−1 | 1 |
| 217280_x_at | GABAA receptor, alpha 5 | GABRA5 | 1.16 × 10−1 | 1 |
| 207182_at | GABAA receptor, alpha 6 | GABRA6 | 1.42 × 10−2 | 5.10 × 10−1 |
| 1557256_a_at | GABAA receptor, beta 1 | GABRB1 | 0.01 | 0.48 |
| 207010_at | GABAA receptor, beta 1 | GABRB1 | 2.03 × 10−2 | 7.31 × 10−1 |
| 1557122_s_at | GABAA receptor, beta 2 | GABRB2 | 9.31 × 10−3 | 3.35 × 10−1 |
| 207352_s_at | GABAA receptor, beta 2 | GABRB2 | 3.49 × 10−1 | 1 |
| 242344_at | GABAA receptor, beta 2 | GABRB2 | 3.62 × 10−2 | 1 |
| 1569689_s_at | GABAA receptor, beta 3 | GABRB3 | 1.51 × 10−2 | 5.45 × 10−1 |
| 205850_s_at | GABAA receptor, beta 3 | GABRB3 | 2.43 × 10−13 | 8.74 × 10−12 |
| 227690_at | GABAA receptor, beta 3 | GABRB3 | 1.21 × 10−14 | 4.36 × 1013 |
| 227830_at | GABAA receptor, beta 3 | GABRB3 | 5.55 × 10−16 | 2.00 × 10−14 |
| 229724_at | GABAA receptor, beta 3 | GABRB3 | 0 | 0 |
| 208457_at | GABAA receptor, delta | GABRD | 2.04 × 10−2 | 7.35 × 10−1 |
| 230255_at | GABAA receptor, delta | GABRD | 1.34 × 10−1 | 1 |
| 1552943_at | GABAA receptor, gamma 1 | GABRG1 | 8.35 × 10−6 | 3.01 × 10−4 |
| 241805_at | GABAA receptor, gamma 1 | GABRG1 | 1.43 × 10−6 | 5.16 × 10−5 |
| 1568612_at | GABAA receptor, gamma 2 | GABRG2 | 1.63 × 10−6 | 5.88 × 10−5 |
| 206849_at | GABAA receptor, gamma 2 | GABRG2 | 7.95 × 10−8 | 2.86 × 10−6 |
| 1555517_at | GABAA receptor, gamma 3 | GABRG3 | 1.44 × 10−2 | 5.18 × 10−1 |
| 216895_at | GABAA receptor, gamma 3 | GABRG3 | 1.65 × 10−1 | 1 |
| 205044_at | GABAA receptor, pi | GABRP | 2.78 × 10−1 | 1 |
| 220886_at | GABAA receptor, theta | GABRQ | 3.44 × 10−1 | 1 |
| 238123_at | GABAA receptor, theta | GABRQ | 4.06 × 10−1 | 1 |
| 206525_at | GABAA receptor, rho 1 | GABRR1 | 4.44 × 10−3 | 1.60 × 10−1 |
| 208217_at | GABAA receptor, rho 2 | GABRR2 | 4.71 × 10−2 | 1 |
| 234410_at | GABAA receptor, rho 3 | GABRR3 | 1.34 × 10−2 | 4.84 × 10−1 |
| 206678_at | GABAA receptor, alpha 1 | GABRA1 | 1.72 × 10−1 | 1 |
| Probe | Subunit | Gene | p Value | Adjusted p |
|---|---|---|---|---|
| 1554308_s_at | GABAA receptor, alpha 2 | GABRA2 | 5.34 × 10−3 | 2.67 × 10−2 |
| 207210_at | GABAA receptor, alpha 3 | GABRA3 | 2.02 × 10−2 | 1.01 × 10−1 |
| 229724_at | GABAA receptor, beta 3 | GABRB3 | 4.39 × 10−3 | 2.19 × 10−2 |
| 206849_at | GABAA receptor, gamma 1 | GABRG1 | 1.57 × 10−1 | 7.83 × 10−1 |
| 241805_at | GABAA receptor, gamma 2 | GABRG2 | 8.95 × 10−2 | 4.48 × 10−1 |
| Subunit | Gene | p Value | Adjusted p |
|---|---|---|---|
| GABAA receptor, alpha 2 | GABRA2 | 5.37 × 10−2 | 2.69 × 10−1 |
| GABAA receptor, alpha 3 | GABRA3 | 6.25 × 10−14 | 3.13 × 10−13 |
| GABAA receptor, beta 3 | GABRB3 | 1.63 × 10−11 | 8.13 × 10−11 |
| GABAA receptor, gamma 1 | GABRG1 | 4.13 × 10−7 | 2.07 × 10−6 |
| GABAA receptor, gamma 2 | GABRG2 | 1.96 × 10−5 | 9.78 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badalotti, R.; Dalmolin, M.; Malafaia, O.; Ribas Filho, J.M.; Roesler, R.; Fernandes, M.A.C.; Isolan, G.R. Gene Expression of GABAA Receptor Subunits and Association with Patient Survival in Glioma. Brain Sci. 2024, 14, 275. https://doi.org/10.3390/brainsci14030275
Badalotti R, Dalmolin M, Malafaia O, Ribas Filho JM, Roesler R, Fernandes MAC, Isolan GR. Gene Expression of GABAA Receptor Subunits and Association with Patient Survival in Glioma. Brain Sciences. 2024; 14(3):275. https://doi.org/10.3390/brainsci14030275
Chicago/Turabian StyleBadalotti, Rafael, Matheus Dalmolin, Osvaldo Malafaia, Jurandir M. Ribas Filho, Rafael Roesler, Marcelo A. C. Fernandes, and Gustavo R. Isolan. 2024. "Gene Expression of GABAA Receptor Subunits and Association with Patient Survival in Glioma" Brain Sciences 14, no. 3: 275. https://doi.org/10.3390/brainsci14030275
APA StyleBadalotti, R., Dalmolin, M., Malafaia, O., Ribas Filho, J. M., Roesler, R., Fernandes, M. A. C., & Isolan, G. R. (2024). Gene Expression of GABAA Receptor Subunits and Association with Patient Survival in Glioma. Brain Sciences, 14(3), 275. https://doi.org/10.3390/brainsci14030275







