The Effect of Upright Stance and Vision on a Cognitive Task in Elderly Subjects and Patients with Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Participant Characteristics
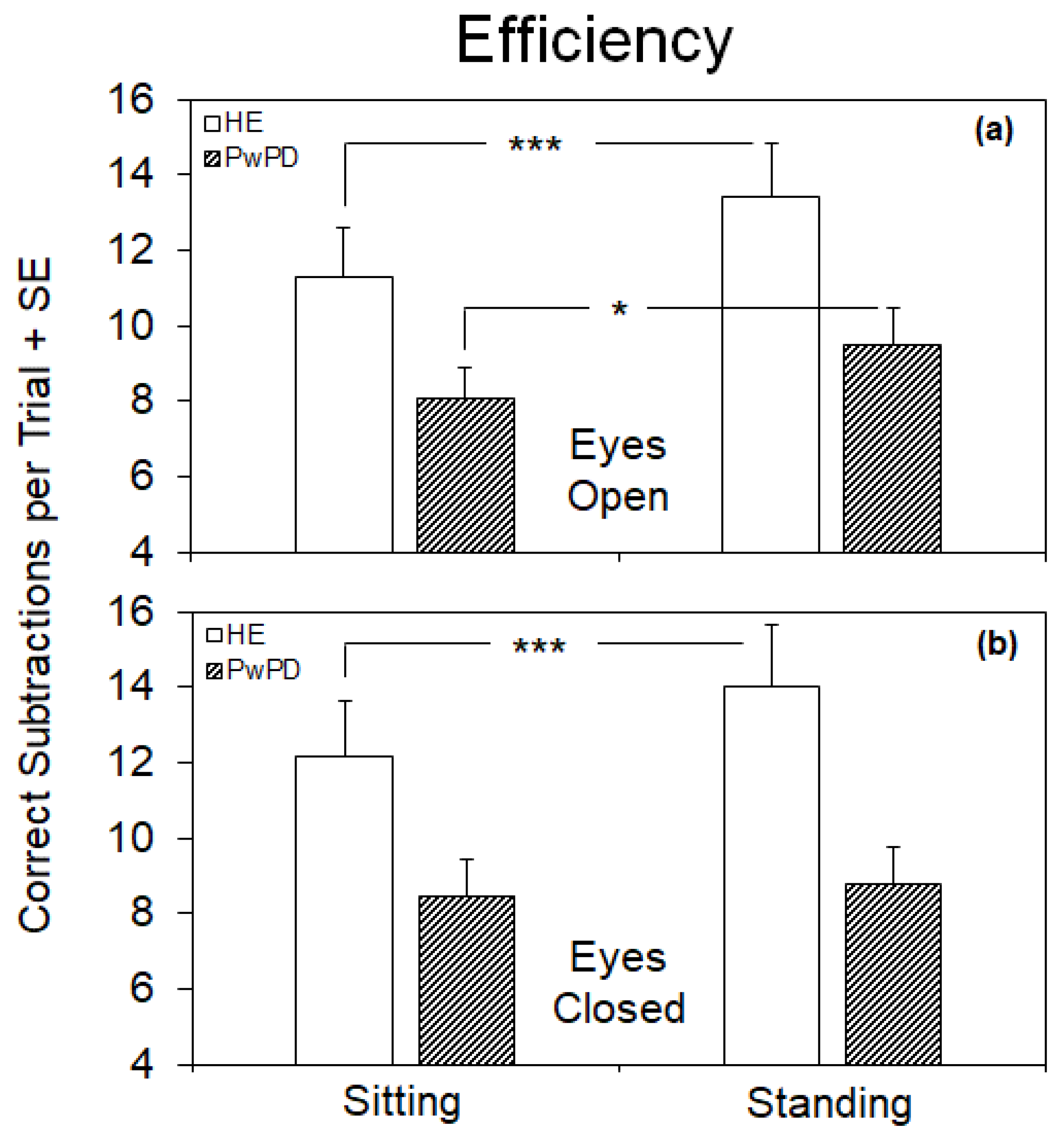
3.2. Efficiency of the Cognitive Performance under the Different Postural and Visual Conditions
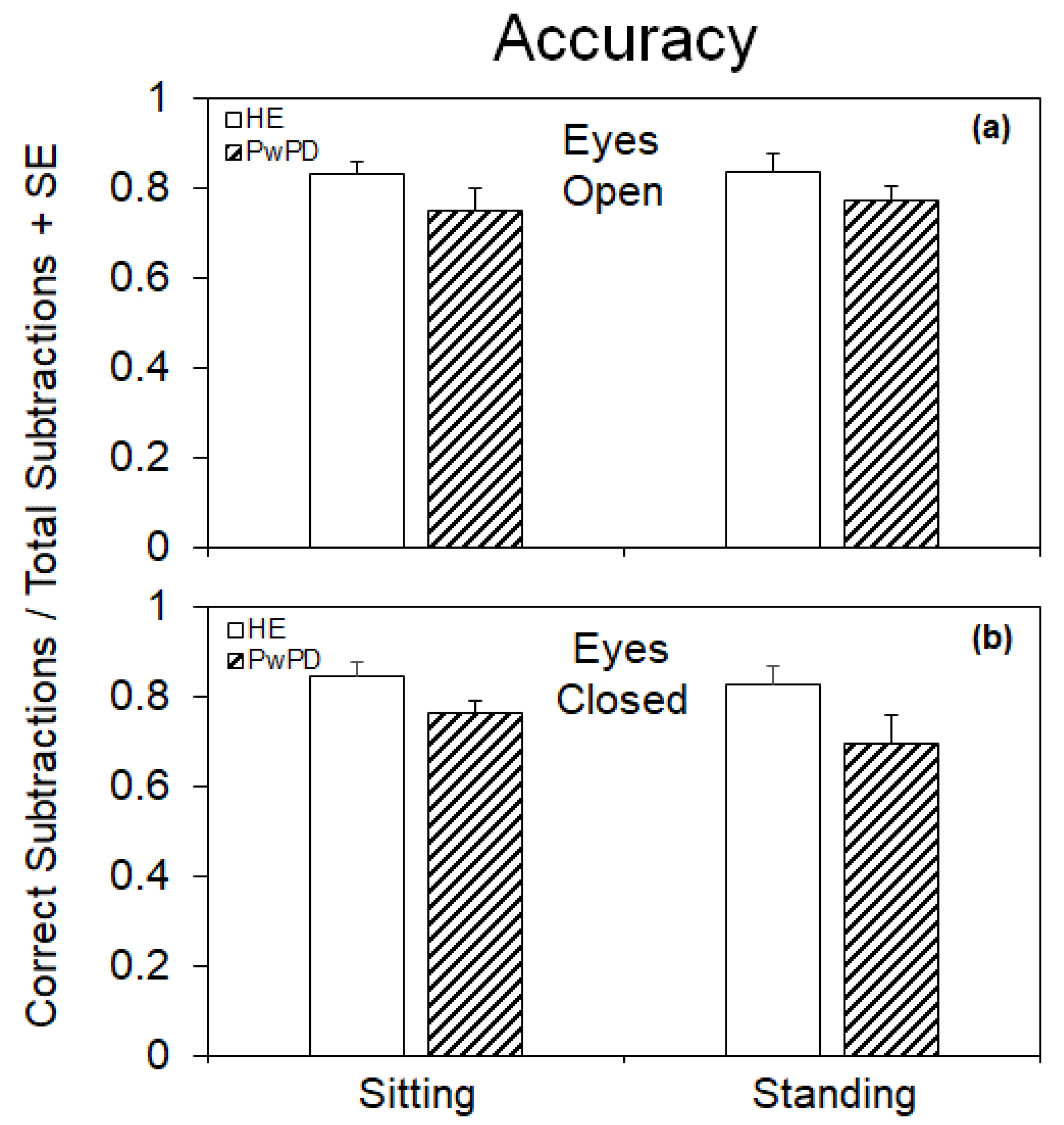
3.3. Accuracy of the Cognitive Performance under the Different Postural and Visual Conditions
3.4. Relationship between Cognitive Performance under the Different Postural and Visual Conditions and the Clinical Findings
4. Discussion
4.1. Differences between Efficiency and Accuracy of the Cognitive Task under the Different Postural and Visual Conditions in HE
4.2. Visual Deprivation Hampers the Improvement of the Cognitive Task While Standing in PwPD
4.3. Relationship between Clinical Variables and the Cognitive Task
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lajoie, Y.; Teasdale, N.; Bard, C.; Fleury, M. Attentional demands for static and dynamic equilibrium. Exp. Brain Res. 1993, 97, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Turcato, A.M. An overview of the physiology and pathophysiology of postural control. In Advanced Technologies for the Rehabilitation of Gait and Balance Disorders; Springer: Cham, Switzerland, 2018; pp. 3–28. [Google Scholar]
- Applegate, C.; Gandevia, S.; Burke, D. Changes in muscle and cutaneous cerebral potentials during standing. Exp. Brain Res. 1988, 71, 183–188. [Google Scholar] [CrossRef]
- Mouchnino, L.; Lhomond, O.; Morant, C.; Chavet, P. Plantar sole unweighting alters the sensory transmission to the cortical areas. Front. Hum. Neurosci. 2017, 11, 220. [Google Scholar] [CrossRef]
- Pellecchia, G.L. Postural sway increases with attentional demands of concurrent cognitive task. Gait Posture 2003, 18, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Bayot, M.; Dujardin, K.; Tard, C.; Defebvre, L.; Bonnet, C.T.; Allart, E.; Delval, A. The interaction between cognition and motor control: A theoretical framework for dual-task interference effects on posture, gait initiation, gait and turning. Neurophysiol. Clin. 2018, 48, 361–375. [Google Scholar] [CrossRef]
- Li, K.Z.; Bherer, L.; Mirelman, A.; Maidan, I.; Hausdorff, J.M. Cognitive involvement in balance, gait and dual-tasking in aging: A focused review from a neuroscience of aging perspective. Front. Neurol. 2018, 9, 913. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.L.; Perry, B.; Chow, J.W.; Wallace, C.; Stokic, D.S. Increased alertness, better than posture prioritization, explains dual-task performance in prosthesis users and controls under increasing postural and cognitive challenge. Exp. Brain Res. 2017, 235, 3527–3539. [Google Scholar] [CrossRef]
- Bustillo-Casero, P.; Villarrasa-Sapiña, I.; García-Massó, X. Effects of dual task difficulty in motor and cognitive performance: Differences between adults and adolescents. Hum. Mov. Sci. 2017, 55, 8–17. [Google Scholar] [CrossRef]
- Smith, K.C.; Davoli, C.C.; Knapp, W.H.; Abrams, R.A. Standing enhances cognitive control and alters visual search. Atten. Percept. Psychophys. 2019, 81, 2320–2329. [Google Scholar] [CrossRef]
- Rosenbaum, D.; Mama, Y.; Algom, D. Stand by your stroop: Standing up enhances selective attention and cognitive control. Psychol. Sci. 2017, 28, 1864–1867. [Google Scholar] [CrossRef]
- Kerchner, G.A.; Racine, C.A.; Hale, S.; Wilheim, R.; Laluz, V.; Miller, B.L.; Kramer, J.H. Cognitive processing speed in older adults: Relationship with white matter integrity. PLoS ONE 2012, 7, e50425. [Google Scholar] [CrossRef] [PubMed]
- Revonsuo, A.; Portin, R.; Koivikko, L.; Rinne, J.O.; Rinne, U.K. Slowing of information processing in Parkinson’s disease. Brain Cogn. 1993, 21, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Wang, G.; Liu, Q.; Wang, Y. Effect of cerebral small vessel disease on cognitive impairment in Parkinson’s disease: A systematic review and meta-analysis. Ann. Transl. Med. 2022, 10, 288. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.L.; Albin, R.L.; Kotagal, V.; Koeppe, R.A.; Scott, P.J.; Frey, K.A.; Bohnen, N.I. Thalamic cholinergic innervation and postural sensory integration function in Parkinson’s disease. Brain 2013, 136, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Brønnick, K.; Larsen, J.; Tysnes, O.; Alves, G. Cognitive impairment in incident, untreated Parkinson disease: The Norwegian ParkWest study. Neurology 2009, 72, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Fallon, S.J.; Gowell, M.; Maio, M.R.; Husain, M. Dopamine affects short-term memory corruption over time in Parkinson’s disease. Npj Park. Dis. 2019, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Brandão, P.R.P.; Munhoz, R.P.; Grippe, T.C.; Cardoso, F.E.C.; e Castro, B.M.d.A.; Titze-de-Almeida, R.; Tomaz, C.; Tavares, M.C.H. Cognitive impairment in Parkinson’s disease: A clinical and pathophysiological overview. J. Neurol. Sci. 2020, 419, 117177. [Google Scholar] [CrossRef]
- Nardone, A.; Schieppati, M. Balance in Parkinson’s disease under static and dynamic conditions. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21, 1515–1520. [Google Scholar] [CrossRef]
- Sciadas, R.; Dalton, C.; Nantel, J. Effort to reduce postural sway affects both cognitive and motor performances in individuals with Parkinson’s disease. Hum. Mov. Sci. 2016, 47, 135–140. [Google Scholar] [CrossRef]
- Morenilla, L.; Márquez, G.; Sánchez, J.A.; Bello, O.; López-Alonso, V.; Fernández-Lago, H.; Fernández-del-Olmo, M.Á. Postural stability and cognitive performance of subjects with Parkinson’s disease during a dual-task in an upright stance. Front. Psychol. 2020, 11, 1256. [Google Scholar] [CrossRef]
- Longhurst, J.K.; Rider, J.V.; Cummings, J.L.; John, S.E.; Poston, B.; Landers, M.R. Cognitive-motor dual-task interference in Alzheimer’s disease, Parkinson’s disease, and prodromal neurodegeneration: A scoping review. Gait Posture 2023, 105, 58–74. [Google Scholar] [CrossRef]
- Du, Y.H.; Ma, J.; Hu, J.Y.; Zhu, L.; Wang, L.Y.; Yang, R.Y.; Liang, L.C.; Jiang, M.; Cai, M.; Pu, J. Effects of dual-task training on gait and motor ability in patients with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2023, 37, 942–953. [Google Scholar] [CrossRef]
- Bonnet, C.T. Advantages and disadvantages of stiffness instructions when studying postural control. Gait Posture 2016, 46, 208–210. [Google Scholar] [CrossRef]
- Marchese, R.; Bove, M.; Abbruzzese, G. Effect of cognitive and motor tasks on postural stability in Parkinson’s disease: A posturographic study. Mov. Disord. 2003, 18, 652–658. [Google Scholar] [CrossRef]
- Measso, G.; Cavarzeran, F.; Zappalà, G.; Lebowitz, B.D.; Crook, T.H.; Pirozzolo, F.J.; Amaducci, L.A.; Massari, D.; Grigoletto, F. The mini-mental state examination: Normative study of an Italian random sample. Dev. Neuropsychol. 1993, 9, 77–85. [Google Scholar] [CrossRef]
- Pieper, N.T.; Grossi, C.M.; Chan, W.-Y.; Loke, Y.K.; Savva, G.M.; Haroulis, C.; Steel, N.; Fox, C.; Maidment, I.D.; Arthur, A.J. Anticholinergic drugs and incident dementia, mild cognitive impairment and cognitive decline: A meta-analysis. Age Ageing 2020, 49, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, L.G.; Vallée-Tourangeau, F. Numbers in action: Individual differences and interactivity in mental arithmetic. Cogn. Process 2018, 19, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Penati, R.; Schieppati, M.; Nardone, A. Cognitive performance during gait is worsened by overground but enhanced by treadmill walking. Gait Posture 2020, 76, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.; Kaski, D.; Schieppati, M.; Furman, M.; Arshad, Q.; Bronstein, A. Subjective stability perception is related to postural anxiety in older subjects. Gait Posture 2019, 68, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Grimbergen, Y.A.; van Dijk, J.G.; Munneke, M. The “posture second” strategy: A review of wrong priorities in Parkinson’s disease. J. Neurol. Sci. 2006, 248, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Ekman, U.; Rennie, L.; Peterson, D.S.; Leavy, B.; Franzén, E. Dual-Task Effects During a Motor-Cognitive Task in Parkinson’s Disease: Patterns of Prioritization and the Influence of Cognitive Status. Neurorehabilit. Neural Repair 2021, 35, 356–366. [Google Scholar] [CrossRef]
- Wright, W.; Gurfinkel, V.; Nutt, J.; Horak, F.; Cordo, P. Axial hypertonicity in Parkinson’s disease: Direct measurements of trunk and hip torque. Exp. Neurol. 2007, 208, 38–46. [Google Scholar] [CrossRef]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa I sa D ouble-E dged S word for B alance and G ait in People with Parkinson’s D isease. Mov. Disord. 2015, 30, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Cools, R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci. Biobehav. Rev. 2006, 30, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Abou Khalil, G.; Doré-Mazars, K.; Legrand, A. Stand up to better pay attention, sit down to better subtract: A new perspective on the advantage of cognitive-motor interactions. Psychol. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Peelen, M.V.; Heslenfeld, D.J.; Theeuwes, J. Endogenous and exogenous attention shifts are mediated by the same large-scale neural network. Neuroimage 2004, 22, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Miyai, I.; Hatakenaka, M.; Kubota, K.; Sakoda, S. Role of the prefrontal cortex in human balance control. Neuroimage 2008, 43, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.S.; Rodnitzky, R.L.; Uc, E.Y. Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Rev. Neurosci. 2013, 24, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Lattari, E.; Costa, S.S.; Campos, C.; de Oliveira, A.J.; Machado, S.; Maranhao Neto, G.A. Can transcranial direct current stimulation on the dorsolateral prefrontal cortex improves balance and functional mobility in Parkinson’s disease? Neurosci. Lett. 2017, 636, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Tombu, M.; Jolicoeur, P. A central capacity sharing model of dual-task performance. J. Exp. Psychol. Hum. Percept. Perform. 2003, 29, 3–18. [Google Scholar] [CrossRef]
- Sapir, S. Multiple factors are involved in the dysarthria associated with Parkinson’s disease: A review with implications for clinical practice and research. J. Speech Lang. Hear. Res. 2014, 57, 1330–1343. [Google Scholar] [CrossRef]
- Schmid, M.; Nardone, A.; De Nunzio, A.M.; Schmid, M.; Schieppati, M. Equilibrium during static and dynamic tasks in blind subjects: No evidence of cross-modal plasticity. Brain 2007, 130, 2097–2107. [Google Scholar] [CrossRef]
- Sarabon, N.; Rosker, J.; Loefler, S.; Kern, H. The effect of vision elimination during quiet stance tasks with different feet positions. Gait Posture 2013, 38, 708–711. [Google Scholar] [CrossRef]
- Baiano, C.; Barone, P.; Trojano, L.; Santangelo, G. Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: A meta-analysis. Mov. Disord. 2020, 35, 45–54. [Google Scholar] [CrossRef]
- Sawamoto, N.; Honda, M.; Hanakawa, T.; Fukuyama, H.; Shibasaki, H. Cognitive slowing in Parkinson’s disease: A behavioral evaluation independent of motor slowing. J. Neurosci. 2002, 22, 5198–5203. [Google Scholar] [CrossRef]
- Vette, A.H.; Masani, K.; Sin, V.; Popovic, M.R. Posturographic measures in healthy young adults during quiet sitting in comparison with quiet standing. Med. Eng. Phys. 2010, 32, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Nardone, A.; Schieppati, M. The role of instrumental assessment of balance in clinical decision making. Eur. J. Phys. Rehabil. Med. 2010, 46, 221–237. [Google Scholar] [PubMed]
- Schieppati, M.; Nardone, A. Free and supported stance in Parkinson’s disease: The effect of posture and ‘postural set’on leg muscle responses to perturbation, and its relation to the severity of the disease. Brain 1991, 114, 1227–1244. [Google Scholar] [CrossRef] [PubMed]
- Pieruccini-Faria, F.; Ehgoetz Martens, K.A.; Silveira, C.R.; Jones, J.A.; Almeida, Q.J. Interactions between cognitive and sensory load while planning and controlling complex gait adaptations in Parkinson’s disease. BMC Neurol. 2014, 14, 250. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G.; Wicki, A.; Baumann, C.R.; Straumann, D.; Palla, A. Impaired tilt perception in Parkinson’s disease: A central vestibular integration failure. PLoS ONE 2015, 10, e0124253. [Google Scholar] [CrossRef] [PubMed]
- Vaugoyeau, M.; Viel, S.; Assaiante, C.; Amblard, B.; Azulay, J.P. Impaired vertical postural control and proprioceptive integration deficits in Parkinson’s disease. Neuroscience 2007, 146, 852–863. [Google Scholar] [CrossRef]
- Azulay, J.P.; Mesure, S.; Amblard, B.; Pouget, J. Increased visual dependence in Parkinson’s disease. Percept. Mot. Ski. 2002, 95, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- García-López, H.; de Los Ángeles Castillo-Pintor, M.; Castro-Sánchez, A.M.; Lara-Palomo, I.C.; Obrero-Gaitán, E.; Cortés-Pérez, I. Efficacy of Dual-Task Training in Patients with Parkinson’s Disease: A Systematic Review with Meta-Analysis. Mov. Disord. Clin. Pract. 2023, 10, 1268–1284. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Folkerts, A.K.; Hammarström, I.; Kalbe, E.; Leavy, B. Effects of motor-cognitive training on dual-task performance in people with Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. 2023, 270, 2890–2907. [Google Scholar] [CrossRef] [PubMed]
- Kiss, R.; Brueckner, D.; Muehlbauer, T. Effects of Single Compared to Dual Task Practice on Learning a Dynamic Balance Task in Young Adults. Front. Psychol. 2018, 9, 311. [Google Scholar] [CrossRef]
- Spanò, B.; De Tollis, M.; Taglieri, S.; Manzo, A.; Ricci, C.; Lombardi, M.G.; Polidori, L.; Griffini, I.A.; Aloisi, M.; Vinicola, V.; et al. The Effect of Dual-Task Motor-Cognitive Training in Adults with Neurological Diseases Who Are at Risk of Falling. Brain Sci. 2022, 12, 1207. [Google Scholar] [CrossRef]
| Characteristics | HE (n = 20) | PwPD (n = 20) | p Value |
|---|---|---|---|
| Sex | 9 M, 11 W | 10 M, 10 W | 0.75 # |
| Age (years) | 68.5 ± 8.8 | 72.3 ± 2.1 | 0.13 † |
| Disease duration (years) | n/a | 7.1 ± 4.5 | n/a |
| Hoehn-Yahr scale (score) | n/a | 2.5 ± 0.5 | n/a |
| UPDRS motor section (score) | n/a | 24.8 ± 11.5 | n/a |
| LEDD (mg) | n/a | 667.1 ± 328.8 | n/a |
| Mini-Mental State Examination (score) | 27.3 ± 1.8 | 27.1 ± 2.1 | 0.82 † |
| Conditions | F | dF | p-Value |
|---|---|---|---|
| Group (HE-PwPD) | 5.84 | 1.38 | 0.02 |
| Posture (Sitting-Standing) | 9.29 | 1.38 | 0.004 |
| Vision (EO-EC) | 0.98 | 1.38 | 0.33 |
| Group × Posture | 1.40 | 1.38 | 0.24 |
| Group × Vision | 2.59 | 1.38 | 0.12 |
| Posture × Vision | 3.95 | 1.38 | 0.05 |
| Group × Posture × Vision | 1.10 | 1.38 | 0.30 |
| Conditions | F | dF | p-Value |
|---|---|---|---|
| Group (HE-PwPD) | 3.13 | 1.38 | 0.08 |
| Posture (Sitting-Standing) | 0.51 | 1.38 | 0.48 |
| Vision (EO-EC) | 1.84 | 1.38 | 0.18 |
| Group × Posture | 0.15 | 1.38 | 0.70 |
| Group × Vision | 2.25 | 1.38 | 0.14 |
| Posture × Vision | 1.26 | 1.38 | 0.27 |
| Group × Posture × Vision | 0.04 | 1.38 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirando, M.; Penati, R.; Godi, M.; Giardini, M.; Nardone, A. The Effect of Upright Stance and Vision on a Cognitive Task in Elderly Subjects and Patients with Parkinson’s Disease. Brain Sci. 2024, 14, 305. https://doi.org/10.3390/brainsci14040305
Mirando M, Penati R, Godi M, Giardini M, Nardone A. The Effect of Upright Stance and Vision on a Cognitive Task in Elderly Subjects and Patients with Parkinson’s Disease. Brain Sciences. 2024; 14(4):305. https://doi.org/10.3390/brainsci14040305
Chicago/Turabian StyleMirando, Marta, Rachele Penati, Marco Godi, Marica Giardini, and Antonio Nardone. 2024. "The Effect of Upright Stance and Vision on a Cognitive Task in Elderly Subjects and Patients with Parkinson’s Disease" Brain Sciences 14, no. 4: 305. https://doi.org/10.3390/brainsci14040305
APA StyleMirando, M., Penati, R., Godi, M., Giardini, M., & Nardone, A. (2024). The Effect of Upright Stance and Vision on a Cognitive Task in Elderly Subjects and Patients with Parkinson’s Disease. Brain Sciences, 14(4), 305. https://doi.org/10.3390/brainsci14040305








