Independence Threat or Interdependence Threat? The Focusing Effect on Social or Physical Threat Modulates Brain Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Materials
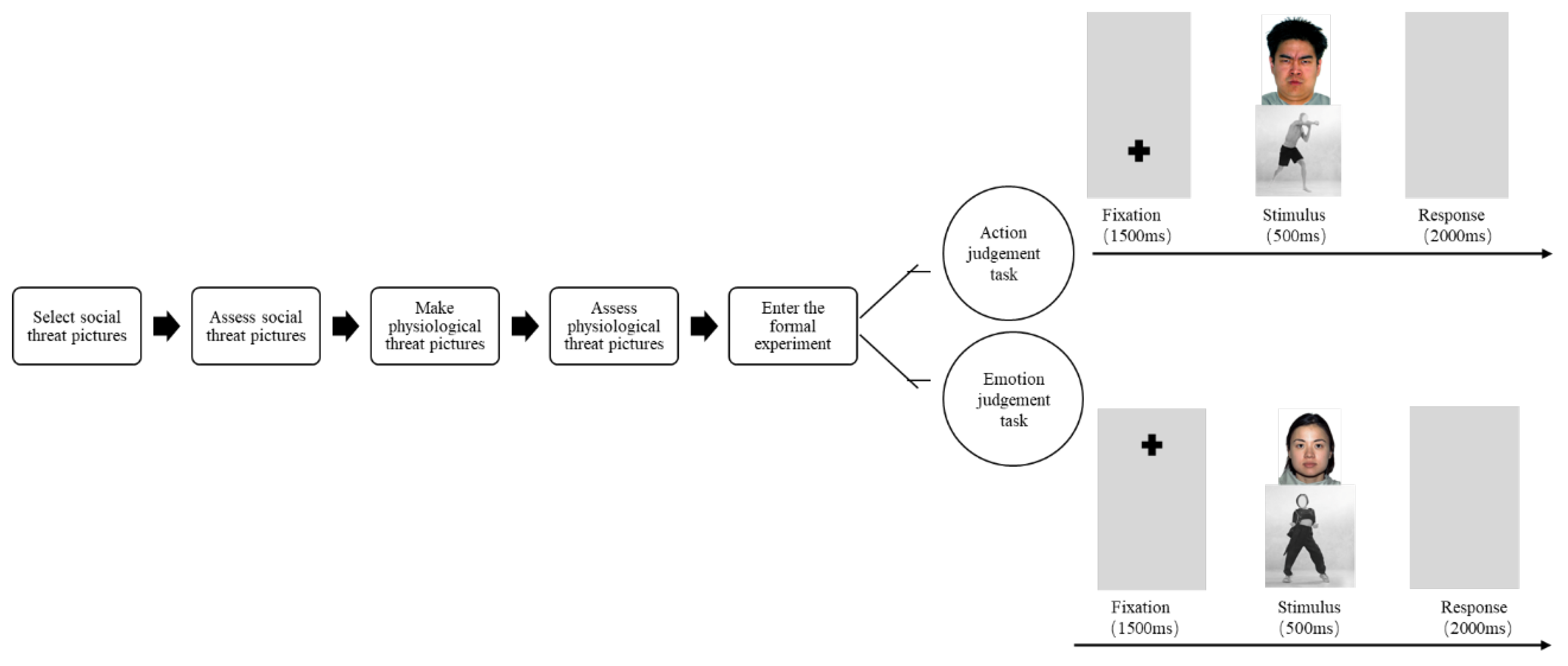
2.3. Procedure
2.4. Data Recording and Analysis
3. Results
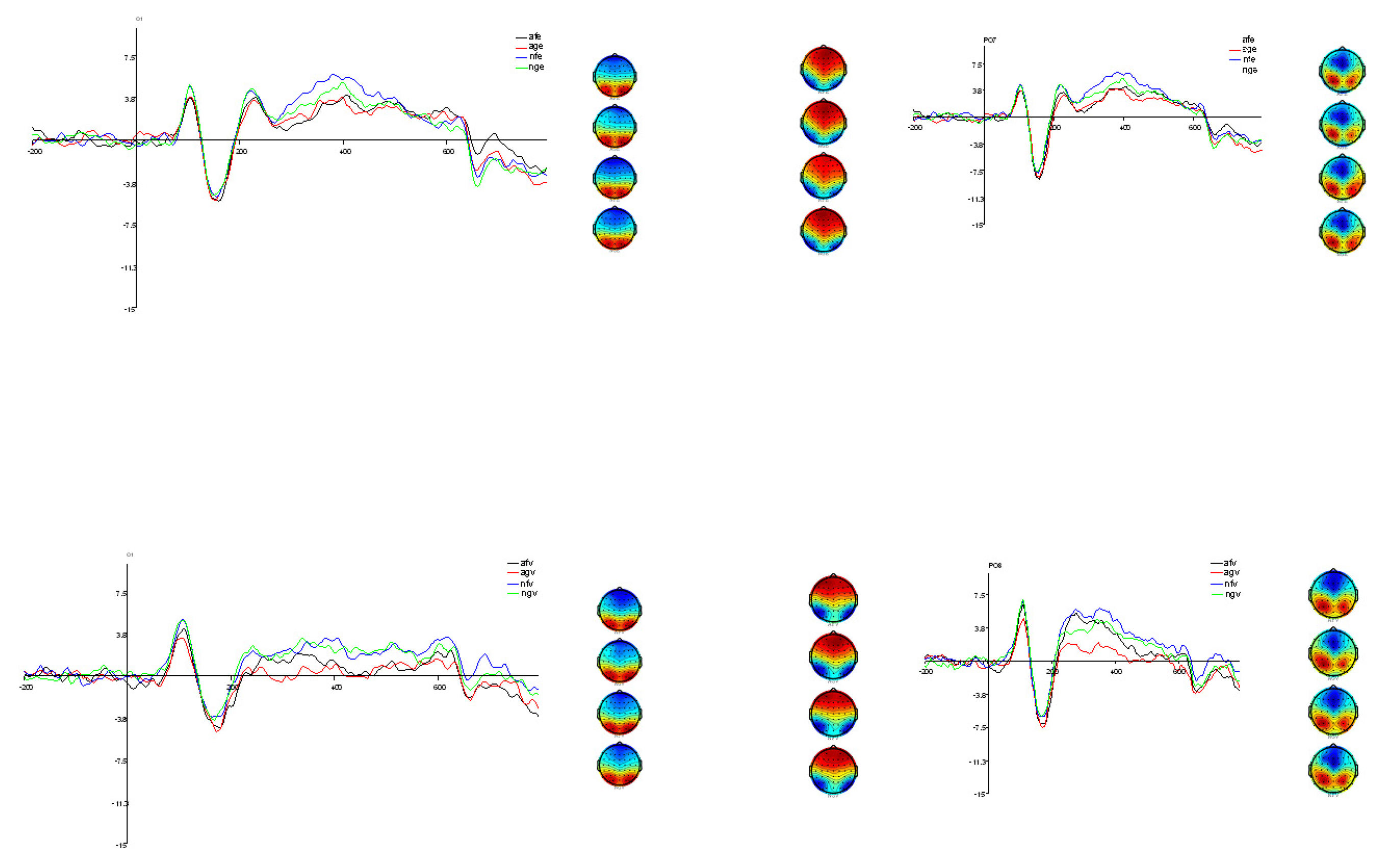
3.1. Electrophysiological Analysis
3.2. Questionnaire Results and Ratings
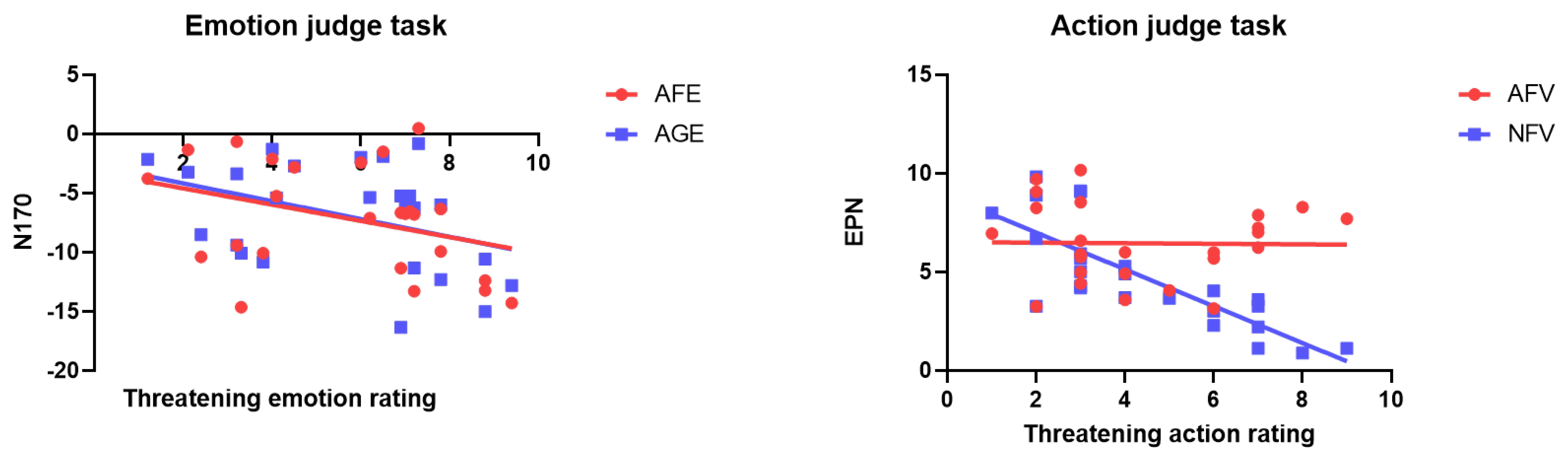
3.3. Correlation and Regression between Subjective Rating and Event-Related Potential Amplitude
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- LeDoux, J.E.; Pine, D.S. Using Neuroscience to help understand Fear and Anxiety: A Two-System Framework. Am. J. Psychiatry 2016, 173, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Bulley, A.; Henry, J.D.; Suddendorf, T. Thinking about threats: Memory and prospection in human threat management. Conscious. Cogn. 2017, 49, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Shapouri, S.; Martin, L.L. Snakes vs. Guns: A Systematic Review of Comparisons Between Phylogenetic and Ontogenetic Threats. Adapt. Hum. Behav. Physiol. 2021, 8, 131–155. [Google Scholar] [CrossRef]
- Dalmaso, M.; Castelli, L.; Galfano, G. Social modulators of gaze-mediated orienting of attention: A review. Psychon. Bull. Rev. 2020, 27, 833–855. [Google Scholar] [CrossRef] [PubMed]
- Azarian, B.; Esser, E.G.; Peterson, M.S. Watch out! Directional threat-related postures cue attention and the eyes. Cogn. Emot. 2015, 30, 561–569. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, C.; Martínez, R.M.; Fan, Y.T.; Liu, C.C.; Chen, C.Y.; Cheng, Y. An amygdala-centered hyper-connectivity signature of threatening face processing predicts anxiety in youths with autism spectrum conditions. Autism Res. 2021, 14, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Bértolo, C.; Moratti, S.; Toledano, R.; López-Sosa, F.; Martínez-Álvarez, R.; Mah, Y.H.; Vuilleumier, P.; Gil-Nágel, A.; Strange, B.A. A fast pathway for fear in human amygdala. Nat. Neurosci. 2016, 19, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Borhani, K.; Làdavas, E.; Maier, M.E.; Avenanti, A.; Bertini, C. Emotional and movement-related body postures modulate visual processing. Soc. Cogn. Affect. Neurosci. 2015, 10, 1092–1101. [Google Scholar] [CrossRef]
- Šimić, G.; Tkalčić, M.; Vukić, V.; Mulc, D.; Španić, E.; Šagud, M.; Olucha-Bordonau, F.E.; Vukšić, M.; Hof, P.R. Understanding Emotions: Origins and roles of the amygdala. Biomolecules 2021, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Berboth, S.; Morawetz, C. Amygdala-prefrontal connectivity during emotion regulation: A meta-analysis of psychophysiological interactions. Neuropsychologia 2021, 153, 107767. [Google Scholar] [CrossRef] [PubMed]
- Bedoya-Pérez, M.A.; Smith, K.L.; Kevin, R.C.; Luo, J.L.; Crowther, M.S.; McGregor, I.S. Parameters that affect fear responses in rodents and how to use them for management. Front. Ecol. Evol. 2019, 7, 136. [Google Scholar] [CrossRef]
- Kang, S.J.; Liu, S.; Ye, M.; Kim, D.-I.; Pao, G.M.; Copits, B.A.; Roberts, B.Z.; Lee, K.; Bruchas, M.R.; Han, S. A central alarm system that gates multi-sensory innate threat cues to the amygdala. Cell Rep. 2022, 40, 111222. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Keil, A. Sensing fear: Fast and precise threat evaluation in human sensory cortex. Trends Cogn. Sci. 2023, 27, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wilson, D.A. Threat Memory in the Sensory Cortex: Insights from Olfaction. Neurosciences 2023, 107385842211489. [Google Scholar] [CrossRef] [PubMed]
- Gantiva, C.; Sotaquirá, M.; Araujo, A.; Cuervo, P. Cortical processing of human and emoji faces: An ERP analysis. Behav. Inf. Technol. 2019, 39, 935–943. [Google Scholar] [CrossRef]
- Bublatzky, F.; Guerra, P.; Alpers, G.W. Watch out, he’s dangerous! Electrocortical indicators of selective visual attention to allegedly threatening persons. Cortex 2020, 131, 164–178. [Google Scholar] [CrossRef] [PubMed]
- De Sá, L.C.; Oliveira, M.E.D.E.S.; Ribeiro, F.; Rocha, T.L.; Bishop, K. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Gold, A.L.; Abend, R.; Britton, J.C.; Behrens, B.; Farber, M.J.; Ronkin, E.; Chen, G.; Leibenluft, E.; Pine, D.S. Age differences in the neural correlates of anxiety disorders: An FMRI study of response to learned threat. Am. J. Psychiatry 2020, 177, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Luck, C.C.; Patterson, R.R.; Lipp, O.V. “Prepared” fear or socio-cultural learning? Fear conditioned to guns, snakes, and spiders is eliminated by instructed extinction in a within-participant differential fear conditioning paradigm. Psychophysiology 2019, 57, e13516. [Google Scholar] [CrossRef]
- Tottenham, N.; Tanaka, J.W.; Leon, A.C.; McCarry, T.; Nurse, M.; Hare, T.A.; Marcus, D.J.; Westerlund, A.; Casey, B.J.; Nelson, C.A. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009, 168, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Gelder, B.; Van Den Stock, J. The Bodily Expressive Action Stimulus Test (BEAST). Construction and Validation of a Stimulus Basis for Measuring Perception of Whole Body Expression of Emotions. Front. Psychol. 2011, 2, 9824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Lu, J.M.; Chen, C.H.; Yan, Z.Y. The Sinicized Development, Reliability and Validity Analysis of the Emotional Contagion Scale. Psychol. Explor. 2017, 37, 241–246. [Google Scholar]
- Nelson, L.D.; Strickland, C.M.; Krueger, R.F.; Arbisi, P.A.; Patrick, C.J. Neurobehavioral traits as transdiagnostic predictors of clinical problems. Assessment 2015, 23, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Turano, M.T.; Lao, J.; Richoz, A.R.; De Lissa, P.; Degosciu, S.B.A.; Viggiano, M.P.; Caldara, R. Fear boosts the early neural coding of faces. Soc. Cogn. Affect. Neurosci. 2017, 12, 1959–1971. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, J.A.; Mercado, F.; Carretié, L. N170 sensitivity to facial expression: A meta-analysis. Neurosci. Biobehav. Rev. 2015, 55, 498–509. [Google Scholar] [CrossRef] [PubMed]
- De Gelder, B.; De Borst, A.W.; Watson, R. The perception of emotion in body expressions. WIREs Cogn. Sci. 2014, 6, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Dong, X.; Zhang, S. ERP evidence for emotional sensitivity in social anxiety. J. Affect. Disord. 2021, 279, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, P.; Luo, J.; Nan, W. Perception of threatening intention modulates brain processes to body actions: Evidence from Event-Related Potentials. Front. Psychol. 2018, 9, 352778. [Google Scholar] [CrossRef] [PubMed]
- Jack, R.E.; Schyns, P.G. The human face as a dynamic tool for social communication. Curr. Biol. 2015, 25, R621–R634. [Google Scholar] [CrossRef] [PubMed]
- Abend, R.; Gold, A.L.; Britton, J.C.; Michalska, K.J.; Shechner, T.; Sachs, J.F.; Winkler, A.M.; Leibenluft, E.; Averbeck, B.B.; Pine, D.S. Anticipatory threat responding: Associations with anxiety, development, and brain structure. Biol. Psychiatry 2020, 87, 916–925. [Google Scholar] [CrossRef]
- Boeke, E.A.; Moscarello, J.M.; LeDoux, J.E.; Phelps, E.A.; Hartley, C.A. Active avoidance: Neural mechanisms and attenuation of Pavlovian conditioned responding. J. Neurosci. 2017, 37, 4808–4818. [Google Scholar] [CrossRef] [PubMed]
- Dhum, M.; Herwig, U.; Opialla, S.; Siegrist, M.; Brühl, A.B. Evolutionary and modern image content differentially influence the processing of emotional pictures. Front. Hum. Neurosci. 2017, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Framorando, D.; Moses, E.; Legrand, L.; Seeck, M.; Pegna, A.J. Rapid processing of fearful faces relies on the right amygdala: Evidence from individuals undergoing unilateral temporal lobectomy. Sci. Rep. 2021, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Lü, L.; Zhang, C.; Li, L. Mental imagery of face enhances face-sensitive event-related potentials to ambiguous visual stimuli. Biol. Psychol. 2017, 129, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Schupp, H.T.; Kirmse, U. Case-by-case: Emotional stimulus significance and the modulation of the EPN and LPP. Psychophysiology 2021, 58, e13766. [Google Scholar] [CrossRef]
- Adamczyk, A.K.; Wyczesany, M.; Roelofs, K.; Van Peer, J.M. Reappraisal is less effective than distraction in downregulation of neural responses to physical threats—Anevent-related potentialinvestigation. Psychophysiology 2023, 60, e14316. [Google Scholar] [CrossRef] [PubMed]
- De Borst, A.W.; De Gelder, B. Clear signals or mixed messages: Inter-individual emotion congruency modulates brain activity underlying affective body perception. Soc. Cogn. Affect. Neurosci. 2016, 11, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, B.W.; Pessoa, L. Discovering networks altered by potential threat (“anxiety”) using quadratic discriminant analysis. NeuroImage 2015, 116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rosén, J.; Kastrati, G.; Reppling, A.; Bergkvist, K.; Åhs, F. The effect of immersive virtual reality on proximal and conditioned threat. Sci. Rep. 2019, 9, 17407. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Ke, B.; Liu, Y.; Thigpen, N.; Keil, A.; Ding, M. Fear conditioning prompts sparser representations of conditioned threat in primary visual cortex. Soc. Cogn. Affect. Neurosci. 2020, 15, 950–964. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, J.; Park, K.; Kim, J.-Y.; Song, B.; Hong, I.; Kim, J.; Lee, S.; Choi, S. Sound tuning of amygdala plasticity in auditory fear conditioning. Sci. Rep. 2016, 6, 31069. [Google Scholar] [CrossRef] [PubMed]
- East, B.S.; Fleming, G.; Vervoordt, S.; Shah, P.; Sullivan, R.M.; Wilson, D.A. Basolateral amygdala to posterior piriform cortex connectivity ensures precision in learned odor threat. Sci. Rep. 2021, 11, 21746. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Angioletti, L. ERP as Biomarkers of Early Selective Attentional Bias, Reward Sensitivity, and Vulnerability to Excessive Internet Use. In Proceedings of the 2023 IEEE 23rd International Conference on Bioinformatics and Bioengineering (BIBE), Dayton, OH, USA, 4–6 December 2023. [Google Scholar] [CrossRef]
- De Gelder, B.; Solanas, M.P.; Seinfeld, S. The Aggressive body. Understanding aggression combining virtual reality, computational movement features, and neuroimaging. In Handbook of Anger, Aggression, and Violence; Springer: Cham, Switzerland, 2023; pp. 1–15. [Google Scholar] [CrossRef]
- Calvo, M.G.; Nummenmaa, L. Perceptual and affective mechanisms in facial expression recognition: An integrative review. Cogn. Emot. 2015, 30, 1081–1106. [Google Scholar] [CrossRef]
- Moses, E.; Nelson, N.L.; Taubert, J.; Pegna, A.J. Oxytocin differentially modulates the early neural responses to faces and non-social stimuli. Soc. Cogn. Affect. Neurosci. 2024, 19, nsae010. [Google Scholar] [CrossRef] [PubMed]
- Levy, I.; Schiller, D. Neural computations of threat. Trends Cogn. Sci. 2021, 25, 151–171. [Google Scholar] [CrossRef] [PubMed]
| Emotional Judgement Task | Action Judgement Task | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Condition | AFE | AGE | NFE | NGE | AFV | AGV | NFV | NGV | |
| P1 | M = 3.04, SD = 1.65 | M = 3.26, SD = 1.58 | M = 3.95, SD = 1.75 | M = 3.95, SD = 1.32 | M = 3.67, SD = 2.11 | M = 2.88, SD = 1.85 | M = 4.48, SD = 2.17 | M = 4.50, SD = 1.51 | P1 |
| N170 | M = −8.23, SD = 5.73 | M = −7.76, SD = 6.47 | M = −7.36, SD = 6.07 | M = −7.27, SD = 5.99 | M = −6.99, SD = 5.06 | M = −6.87, SD = 4.01 | M = −5.62, SD = 4.75 | M = −5.97, SD = 4.76 | N190 |
| EPN | M = 1.33, SD = 3.56 | M = −1.37, SD = 3.54 | M = 3.00, SD = 3.22 | M = −1.37, SD = 3.54 | M = 4.48, SD = 3.15 | M = 1.45, SD = 2.73 | M = 3.52, SD = 2.79 | M = 5.18, SD = 3.17 | EPN |
| ESQ | THT+ | Threat Perception | Emotion Judgement Task | Action Judgement Task | |||
|---|---|---|---|---|---|---|---|
| Anger Face | Fight Action | Anger Condition | Neutral Condition | Fighting Condition | Gymnastics Condition | ||
| M = 34.44, SD = 2.99 | M = 53.96, SD = 5.81 | M = 6.32, SD = 0.95 | M = 6.20, SD = 0.71 | M = 487.04, SD = 55.73 | M = 492.43, SD = 54.18 | M = 543.79, SD = 59.69 | M = 541.07, SD = 56.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Ma, L.; Wang, L.; Pang, W. Independence Threat or Interdependence Threat? The Focusing Effect on Social or Physical Threat Modulates Brain Activity. Brain Sci. 2024, 14, 368. https://doi.org/10.3390/brainsci14040368
Wang G, Ma L, Wang L, Pang W. Independence Threat or Interdependence Threat? The Focusing Effect on Social or Physical Threat Modulates Brain Activity. Brain Sciences. 2024; 14(4):368. https://doi.org/10.3390/brainsci14040368
Chicago/Turabian StyleWang, Guan, Lian Ma, Lili Wang, and Weiguo Pang. 2024. "Independence Threat or Interdependence Threat? The Focusing Effect on Social or Physical Threat Modulates Brain Activity" Brain Sciences 14, no. 4: 368. https://doi.org/10.3390/brainsci14040368






