Low-Cost 3D Models for Cervical Spine Tumor Removal Training for Neurosurgery Residents
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
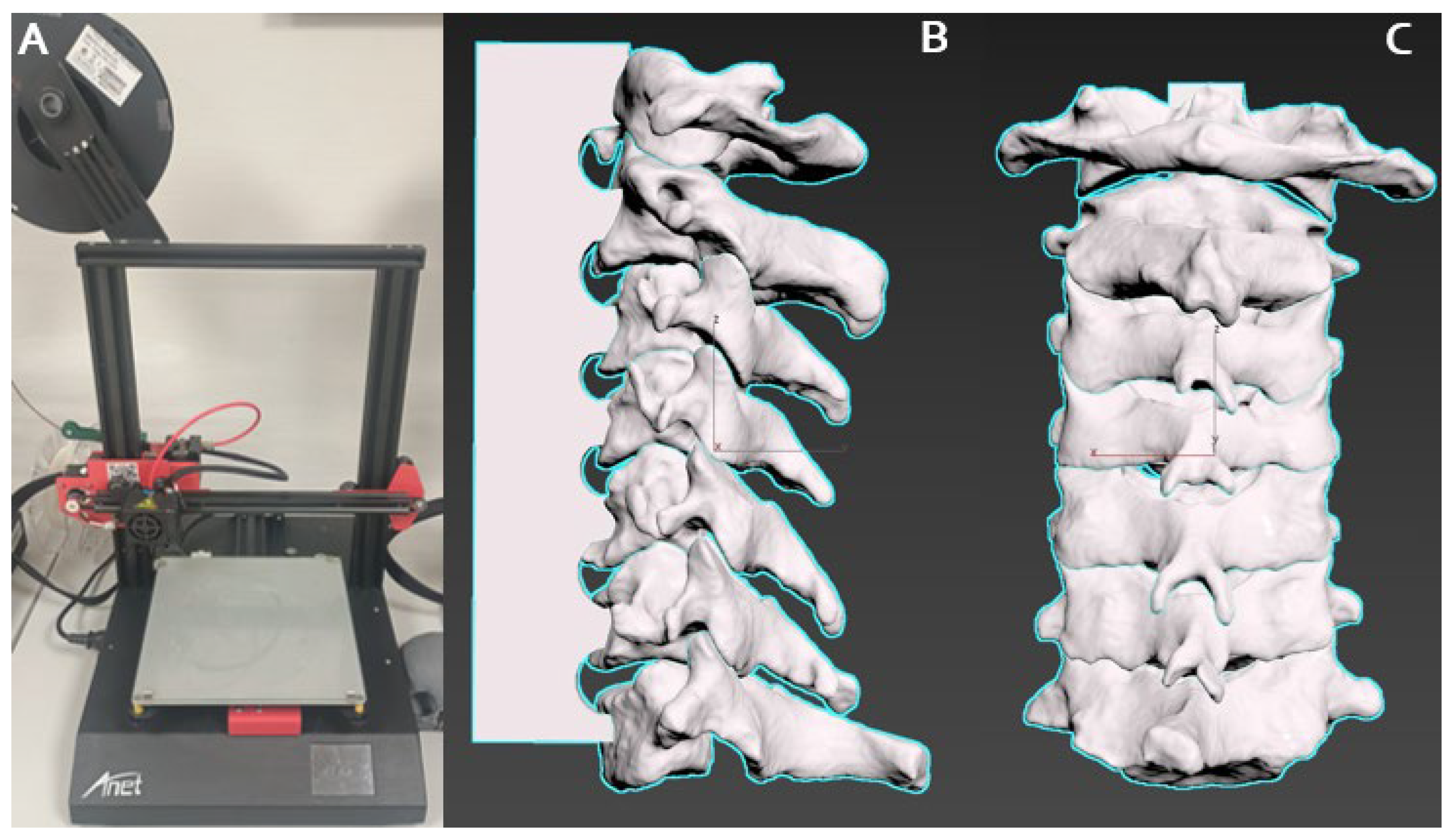
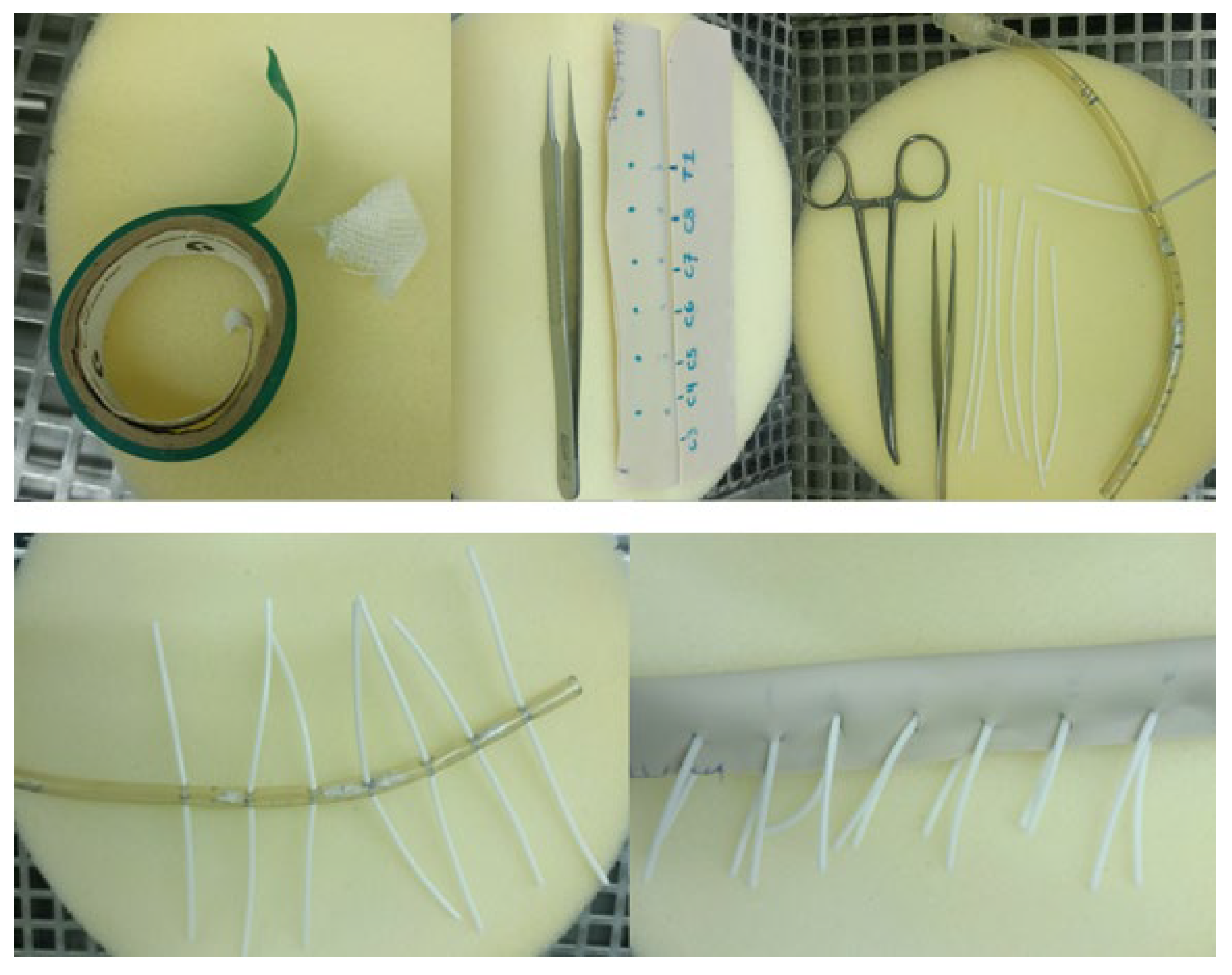
2.2. 3D Model Creation
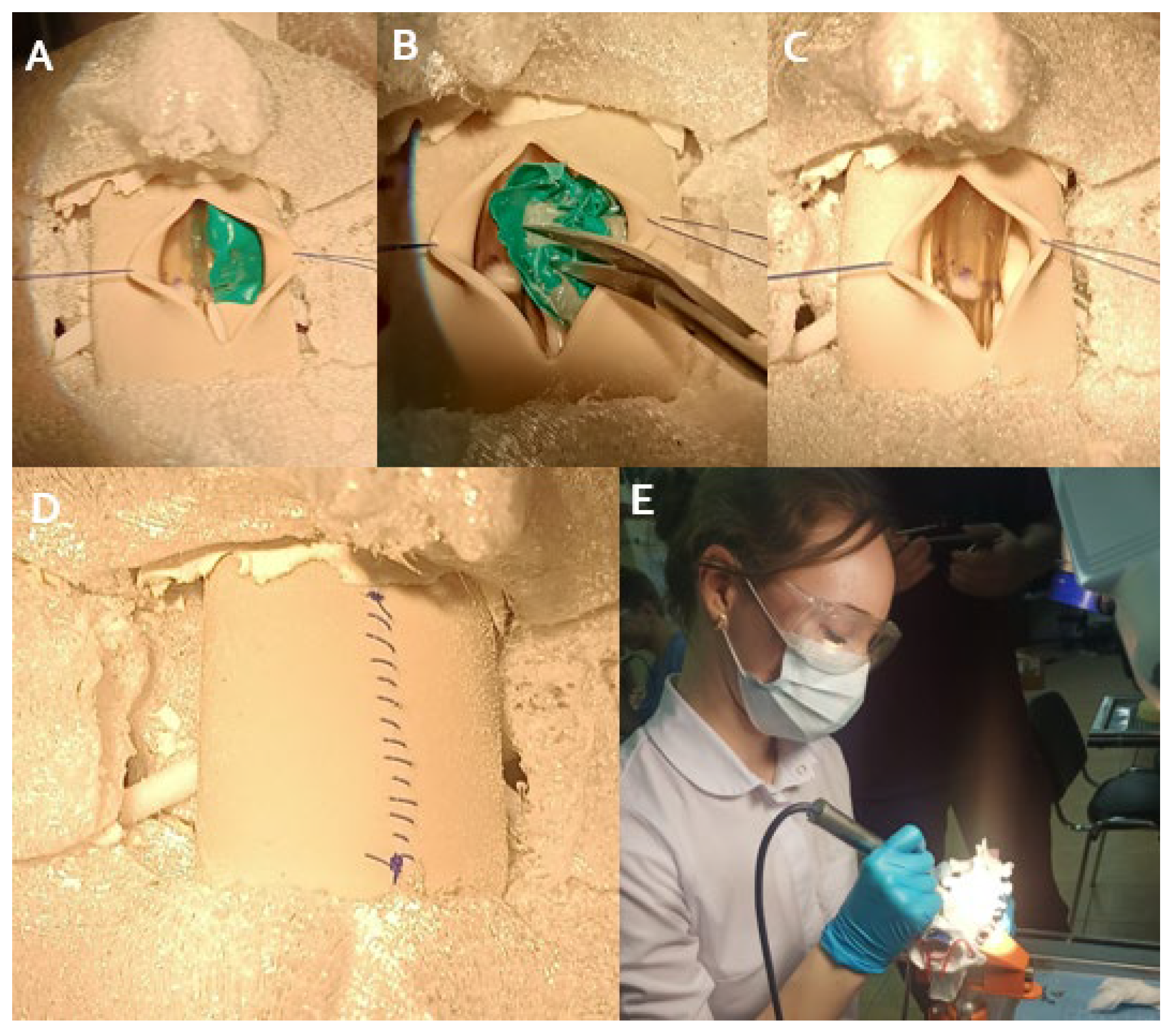
2.3. Simulation Training Protocol
2.4. Simulation Fidelity
2.5. Quality Control
2.5.1. Pre-Processing Verification, 3D Printer Calibration and Tumor Placement Standardization
2.5.2. Material Mixing, Curing, Anatomical Fidelity, and Dimensional Assessment
2.6. Ethical Material Selection, Waste Reduction, and Sustainable Procurement
2.7. Post-Training Evaluation and Statistical Analysis
3. Results
3.1. Participant Demographics
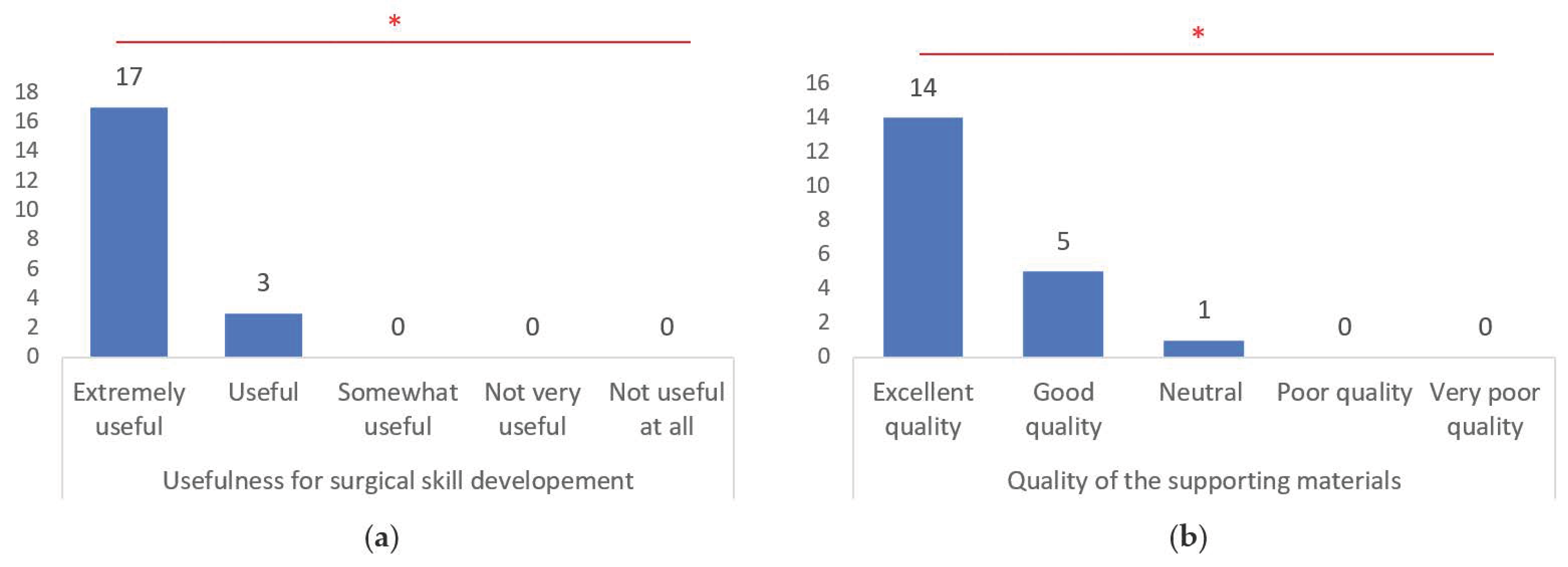
3.2. Realism of 3D Models, Accuracy of Pathologies Simulated, Tactile Feedback and Usefulness for Surgical Skill Development
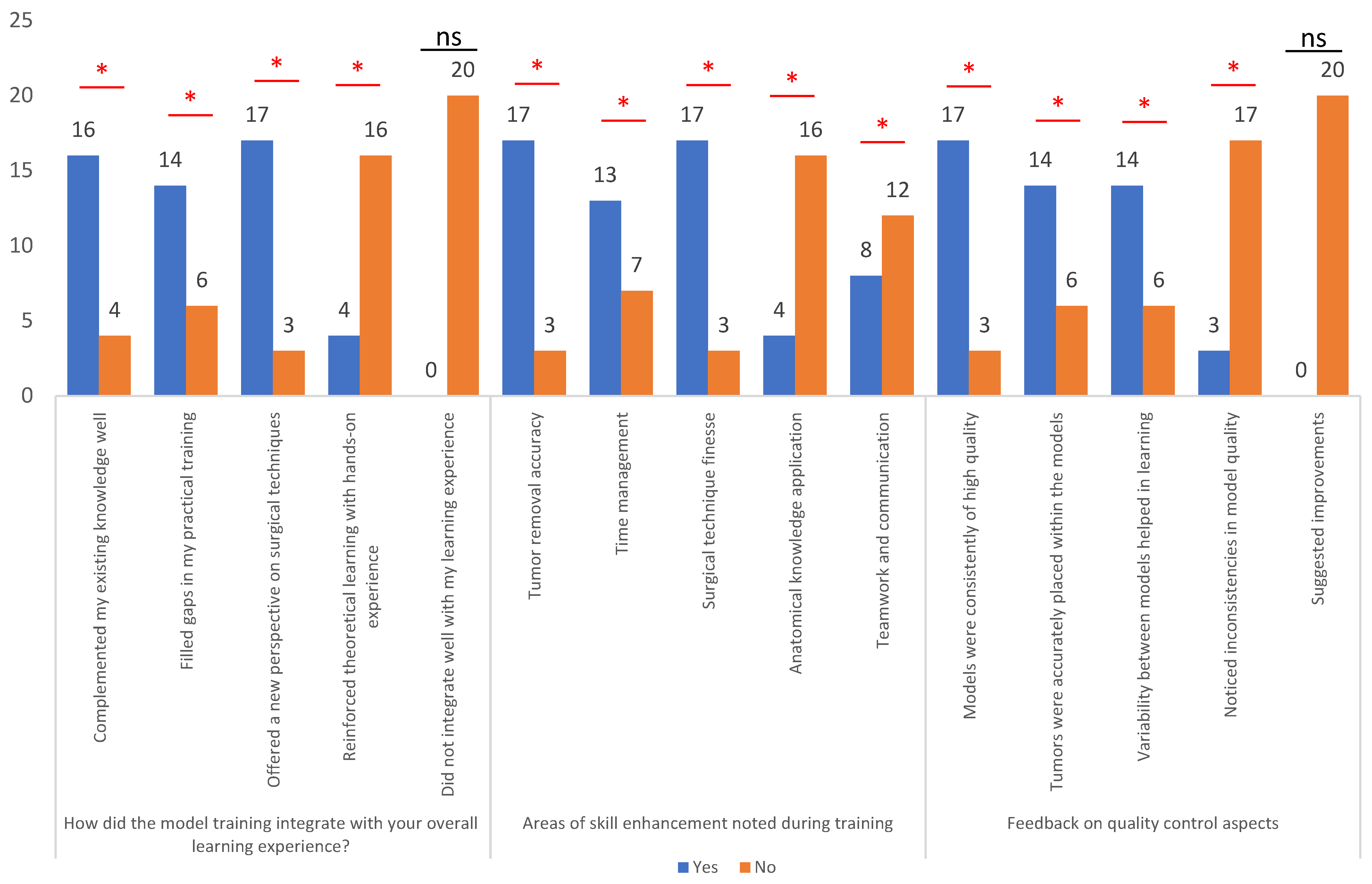
3.3. Integration with Overall Learning Experience and Skill Enhancement Areas
3.4. Post-Training Confidence and Willingness to Recommend It
4. Discussion
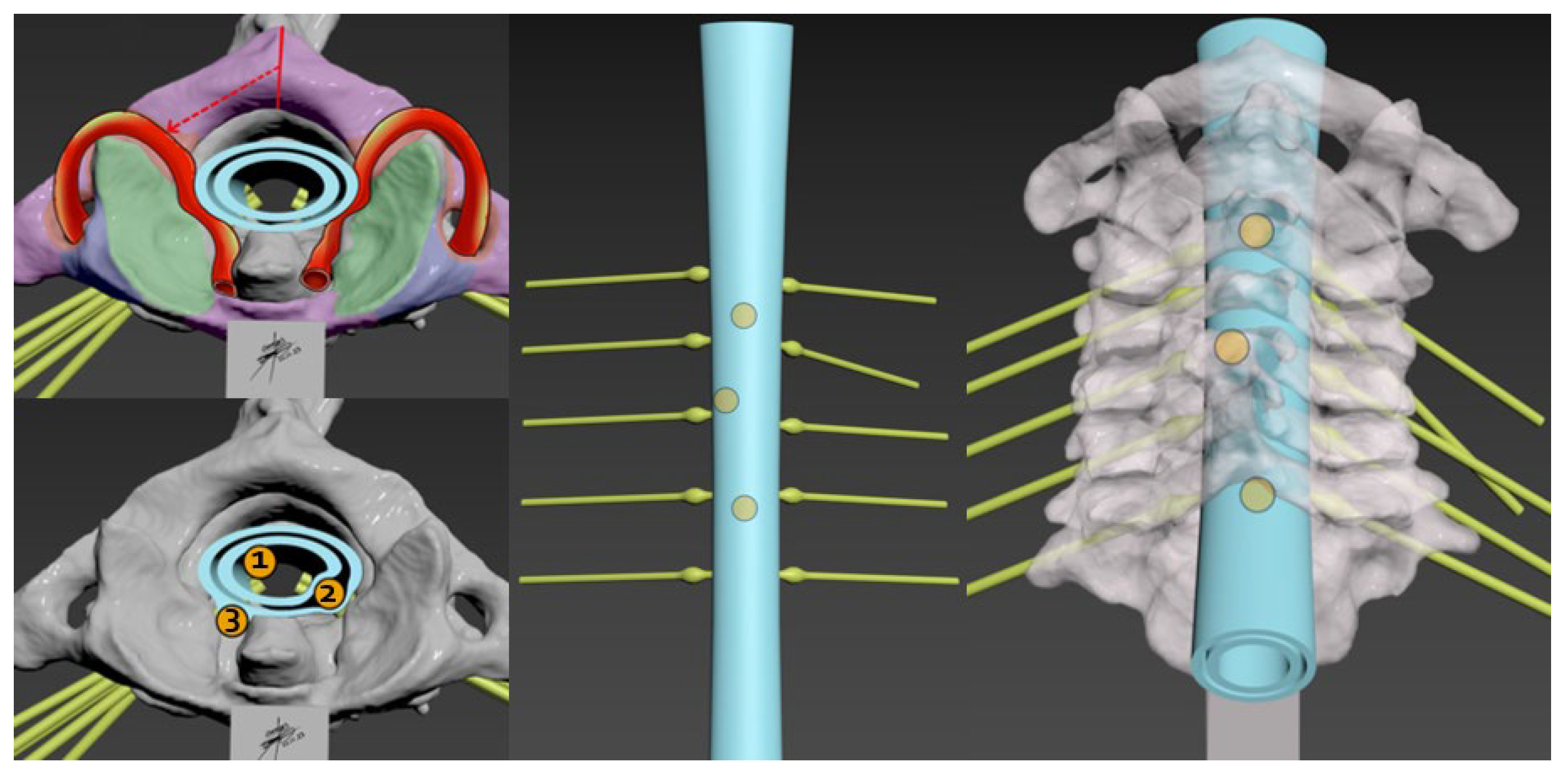
4.1. Model Components and Assembly
4.2. Complexities of Cervical Spine Tumors
4.3. Surgical Considerations
4.4. Role of 3D Models in Understanding Tumor Dynamics
4.5. Simulation Training Protocol, Simulation Fidelity and Quality Control
4.6. Ethical Considerations, Sustainable Sourcing and Survey Findings
4.7. Integration in Medical Education and Practice
4.8. Enhanced Precision and Safety in Surgical Training
4.9. Bridging the Gap between Theoretical Knowledge and Clinical Application
4.10. Customization and Personalization of Training
4.11. Assessment, Feedback, Scalability and Accessibility
4.12. Future Directions
4.13. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Section 1 |
|---|
| Personal information |
| Specialty/area of residency |
| Section 2 |
| Overall experience |
| Realism of the 3D models |
| Variety and accuracy of pathologies simulated |
| Tactile feedback from the models |
| Usefulness for surgical skill development |
| Quality of the supporting materials (e.g., instructions, tutorials) |
| How did the model training integrate with your overall learning experience? |
| Areas of skill enhancement noted during training |
| Feedback on quality-control aspects |
| Post-training confidence level in handling real cases |
| Willingness to recommend this training to peers |
References
- Flippin, M.A. Cervical myelopathy. Orthopedics 2013, 36, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Bartleson, J.D. Cervical spondylotic myelopathy. Neurol. Clin. 2013, 31, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.C.; Riew, K.D.; Anderson, P.A.; Hilibrand, A.S.; Vaccaro, A.F. Cervical myelopathy. Current diagnostic and treatment strategies. Spine J. 2003, 3, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Witiw, C.D.; Fehlings, M.G. Degenerative cervical myelopathy. CMAJ 2017, 189, E116. [Google Scholar] [CrossRef] [PubMed]
- Laufer, I.; Rubin, D.G.; Lis, E.; Cox, B.W.; Stubblefield, M.D.; Yamada, Y.; Bilsky, M.H. The NOMS framework: Approach to the treatment of spinal metastatic tumors. Oncologist 2013, 18, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.; Tariq, A.; Aziz, H.F.; Shamim, M.S. Spinal Schwannomas; Classification, Management and Outcomes. J. Pak. Med. Assoc. 2023, 73, 2118–2120. [Google Scholar] [CrossRef] [PubMed]
- Celano, E.; Salehani, A.; Malcolm, J.G.; Reinertsen, E.; Hadjipanayis, C.G. Spinal cord ependymoma: A review of the literature and case series of ten patients. J. Neurooncol. 2016, 128, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Lizana, J.; Montemurro, N.; Aliaga, N.; Marani, W.; Tanikawa, R. From textbook to patient: A practical guide to train the end-to-side microvascular anastomosis. Br. J. Neurosurg. 2023, 37, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Abeysekera, N.; Whitmore, K.A.; Abeysekera, A.; Pang, G.; Laupland, K.B. Applications of 3D printing in critical care medicine: A scoping review. Anaesth. Intensive Care 2021, 49, 164–172. [Google Scholar] [CrossRef]
- Uhl, J.F.; Sufianov, A.; Ruiz, C.; Iakimov, Y.; Mogorron, H.J.; Encarnacion Ramirez, M.; Prat, G.; Lorea, B.; Baldoncini, M.; Goncharov, E.; et al. The Use of 3D Printed Models for Surgical Simulation of Cranioplasty in Craniosynostosis as Training and Education. Brain Sci. 2023, 13, 894. [Google Scholar] [CrossRef]
- Bai, H.; Zhou, Z.; Liu, G.; Jiang, S.; Zhang, Y.; Zuo, X.; Xing, F.; Xu, L.; Wang, L.; Mu, X. 3D Printing Model of a Patient’s Specific Lumbar Vertebra. J. Vis. Exp. 2023, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Bohl, M.A.; Mooney, M.A.; Repp, G.J.; Nakaji, P.; Chang, S.W.; Turner, J.D.; Kakarla, U.K. The Barrow Biomimetic Spine: Fluoroscopic Analysis of a Synthetic Spine Model Made of Variable 3D-printed Materials and Print Parameters. Spine 2018, 43, E1368–E1375. [Google Scholar] [CrossRef]
- Ramirez, M.D.J.E.; Nurmukhametov, R.; Bernard, E.; Peralta, I.; Efe, I.E. A Low-Cost Three-Dimensional Printed Retractor for Transforaminal Lumbar Interbody Fusion. Cureus 2022, 14, e24185. [Google Scholar] [CrossRef]
- Byvaltsev, V.; Polkin, R.; Bereznyak, D.; Giers, M.B.; Hernandez, P.A.; Shepelev, V.; Aliyev, M. 3D-printed cranial models simulating operative field depth for microvascular training in neurosurgery. Surg. Neurol. Int. 2021, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Clifton, W.; Damon, A.; Soares, C.; Nottmeier, E.; Pichelmann, M. Investigation of a three-dimensional printed dynamic cervical spine model for anatomy and physiology education. Clin. Anat. 2021, 34, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Courvoisier, A.; Cebrian, A.; Simon, J.; Désauté, P.; Aubert, B.; Amabile, C.; Thiébaut, L. Virtual Scoliosis Surgery Using a 3D-Printed Model Based on Biplanar Radiographs. Bioengineering 2022, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.E.; Pena, I.R.; Castillo, R.E.B.; Sufianov, A.; Goncharov, E.; Sanchez, J.A.S.; Colome-Hidalgo, M.; Nurmukhametov, R.; Céspedes, J.R.C.; Montemurro, N. Development of a 3D Printed Brain Model with Vasculature for Neurosurgical Procedure Visualisation and Training. Biomedicines 2023, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.D.J.E.; Nurmukhametov, R.; Musa, G.; Castillo, R.E.B.; Encarnacion, V.L.A.; Sanchez, J.A.S.; Vazquez, C.A.; Efe, I.E. Three-Dimensional Plastic Modeling on Bone Frames for Cost-Effective Neuroanatomy Teaching. Cureus 2022, 14, e27472. [Google Scholar] [CrossRef]
- Horos. Available online: https://fr.freedownloadmanager.org/Mac-OS/Horos-GRATUIT.html (accessed on 1 September 2023).
- Meshmixer 3.5.474. Available online: https://meshmixer.updatestar.com/fr (accessed on 1 September 2023).
- Cura 3.6.0. Available online: https://cura.updatestar.com/fr (accessed on 1 September 2023).
- Gardeck, A.M.; Pu, X.; Yang, Q.; Polly, D.W.; Jones, K.E. The effect of simulation training on resident proficiency in thoracolumbar pedicle screw placement using computer-assisted navigation. J. Neurosurg. Spine. 2020, 34, 127–134. [Google Scholar] [CrossRef]
- Goyal, D.; Kumar, H. In Vivo and 3D Imaging Technique(s) for Spatiotemporal Mapping of Pathological Events in Experimental Model(s) of Spinal Cord Injury. ACS Chem. Neurosci. 2023, 14, 809–819. [Google Scholar] [CrossRef]
- Reyes-Soto, G.; Corona De la Torre, A.; Honda Partida, K.G.; Nurmukhametov, R.; Encarnacion Ramirez, M.D.J.; Montemurro, N. Clivus-Cervical Stabilization through Transoral Approach in Patients with Craniocervical Tumor: Three Cases and Surgical Technical Note. Brain Sci. 2024, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Soto, G.R.; Torres, C.S.O.; Terrazas, J.P.; Partida, K.H.; Rosario, A.R.; Campero, A.; Baldoncini, M.; Ramirez, M.d.J.E.; Montemurro, N. Multiple Myeloma Treatment Challenges: A Case Report of Vertebral Artery Pseudoaneurysm Complicating Occipitocervical Arthrodesis and a Review of the Literature. Cureus 2023, 15, e49716. [Google Scholar] [CrossRef] [PubMed]
- Nurmukhametov, R.; Dosanov, M.; Encarnacion, M.D.J.; Barrientos, R.; Matos, Y.; Alyokhin, A.I.; Baez, I.P.; Efe, I.E.; Restrepo, M.; Chavda, V.; et al. Transforaminal Fusion Using Physiologically Integrated Titanium Cages with a Novel Design in Patients with Degenerative Spinal Disorders: A Pilot Study. Surgeries 2022, 3, 175–184. [Google Scholar] [CrossRef]
- Ramirez, M.D.J.E.; Musa, G.; Castillo, R.E.B.; Cherian, I.; Sufianov, A.; Lafuente, J.; Hernesniemi, J. Three-dimensional Cerebrovascular Bypass Training. A New Low-Cost Home-Made Model. Front. Med. Case Rep. 2021, 2, 1–10. [Google Scholar] [CrossRef]
- Guran, O.; Oflaz, H.; Gunal, İ. No Significant Effect of 3D Modelling on Surgical Planning In Spinal Deformities. Acta Ortop. Bras. 2022, 30, e248982. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gao, Y.; Ding, Y.; Wang, W.; Liu, L.; Zhao, A.; Yang, P. In vitro performance of 3D printed PCL-β-TCP degradable spinal fusion cage. J. Biomater. Appl. 2021, 35, 1304–1314. [Google Scholar] [CrossRef]
- Encarnacion, M.D.; Castillo, R.E.; Matos, Y.; Bernard, E.; Elenis, B.; Oleinikov, B.; Nurmukhametov, R.; Castro, J.S.; Volovich, A.; Dosanov, M.; et al. EasyGO!-assisted microsurgical anterior cervical decompression: Technical report and literature review. Neurol. Neurochir. Pol. 2022, 56, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Kabra, A.; Mehta, N.; Garg, B. 3D printing in spine care: A review of current applications. J. Clin. Orthop. Trauma. 2022, 35, 102044. [Google Scholar] [CrossRef] [PubMed]
- Keri, Z.; Sydor, D.; Ungi, T.; Holden, M.S.; McGraw, R.; Mousavi, P.; Borschneck, D.P.; Fichtinger, G.; Jaeger, M. Computerized training system for ultrasound-guided lumbar puncture on abnormal spine models: A randomized controlled trial. Can. J. Anaesth. 2015, 62, 777–784. [Google Scholar] [CrossRef]
- Odom, M.; Gomez, J.R.; Danelson, K.A.; Sarwal, A. Development of a Homemade Spinal Model for Simulation to Teach Ul-trasound Guidance for Lumbar Puncture. Neurocrit Care 2019, 31, 550–558. [Google Scholar] [CrossRef]
- Öztürk, A.M.; Süer, O.; Govsa, F.; Özer, M.A.; Akçalı, Ö. Patient-specific three-dimensional printing spine model for surgical planning in AO spine type-C fracture posterior long-segment fixation. Acta Orthop. Traumatol. Turc. 2022, 56, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Pearce, P.; Novak, J.; Wijesekera, A.; Loch-Wilkinson, T.; Redmond, M.; Winter, C.; Alexander, H.; Maclachlan, L. Properties and Implementation of 3-Dimensionally Printed Models in Spine Surgery: A Mixed-Methods Review with Meta-Analysis. World Neurosurg. 2023, 169, 57–72. [Google Scholar] [CrossRef]
- Ganapathy, M.K.; Reddy, V.; Tadi, P. Neuroanatomy, Spinal Cord Morphology; StatPearls Publishing: Treasure Island, FL, USA, 2022; Volume 24, p. 1. [Google Scholar]
- Torres, C.S.O.; Mora, A.E.; Campero, A.; Cherian, I.; Sufianov, A.; Sanchez, E.F.; Ramirez, M.E.; Pena, I.R.; Nurmukhametov, R.; Beltrán, M.A.; et al. Enhancing microsurgical skills in neurosurgery residents of low-income countries: A comprehensive guide. Surg. Neurol. Int. 2023, 14, 437. [Google Scholar] [CrossRef] [PubMed]
- Ploch, C.C.; Mansi, C.S.S.A.; Jayamohan, J.; Kuhl, E. Using 3D Printing to Create Personalized Brain Models for Neurosurgical Training and Preoperative Planning. World Neurosurg. 2016, 90, 668–674. [Google Scholar] [CrossRef]
- Achey, R.L.; Karsy, M.; Azab, M.A.; Scoville, J.; Kundu, B.; Bowers, C.A.; Couldwell, W.T. Improved Surgical Safety via Intraoperative Navigation for Transnasal Transsphenoidal Resection of Pituitary Adenomas. J. Neurol. Surg. Part B Skull Base 2019, 80, 626–631. [Google Scholar] [CrossRef]
- Owusu, F.A.; Javed, H.; Saleem, A.; Singh, J.; Varrassi, G.; Raza, S.S.; Ram, R. Beyond the Scalpel: A Tapestry of Surgical Safety, Precision, and Patient Prosperity. Cureus 2023, 15, e50316. [Google Scholar]
- Shen, J.; Yuan, L.; Ge, R.; Shao, X.; Jiang, X. Improving medical student recruitment into neurosurgery through teaching reform. BMC Med. Educ. 2022, 22, 656. [Google Scholar] [CrossRef] [PubMed]
- Dho, Y.-S.; Lee, D.; Ha, T.; Ji, S.Y.; Kim, K.M.; Kang, H.; Kim, M.-S.; Kim, J.W.; Cho, W.-S.; Kim, Y.H.; et al. Clinical application of patient-specific 3D printing brain tumor model production system for neurosurgery. Sci. Rep. 2021, 11, 7005. [Google Scholar] [CrossRef] [PubMed]
- Paxton, N.C. Navigating the intersection of 3D printing, software regulation and quality control for point-of-care manufacturing of personalized anatomical models. 3D Print. Med. 2023, 9, 9. [Google Scholar]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R. 3D printing applications for healthcare research and development. Glob. Health J. 2022, 6, 217–226. [Google Scholar] [CrossRef]
- Petrone, S.; Cofano, F.; Nicolosi, F.; Spena, G.; Moschino, M.; Di Perna, G.; Lavorato, A.; Lanotte, M.M.; Garbossa, D. Virtual-Augmented Reality and Life-Like Neurosurgical Simulator for Training: First Evaluation of a Hands-On Experience for Residents. Front. Surg. 2022, 9, 862948. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Condino, S.; Carbone, M.; Cattari, N.; D’amato, R.; Cutolo, F.; Ferrari, V. Brain Tumor and Augmented Reality: New Technologies for the Future. Int. J. Environ. Res. Public Health 2022, 19, 6347. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Narayanan, M.K.; Umana, G.E.; Montemurro, N.; Chaurasia, B.; Deora, H. Virtual Reality in Neurosurgery: Be-yond Neurosurgical Planning. Int. J. Environ. Res. Public Health 2022, 19, 1719. [Google Scholar] [CrossRef] [PubMed]
- Si, W.-X.; Liao, X.-Y.; Qian, Y.-L.; Sun, H.-T.; Chen, X.-D.; Wang, Q.; Heng, P.A. Assessing performance of augmented reality-based neurosurgical training. Vis. Comput. Ind. Biomed. Art. 2019, 2, 66. [Google Scholar] [CrossRef] [PubMed]
- Mai, R.; Popov, V.; Mishina, E.; Osidak, E. 3D printing in pediatric neurosurgery: Experimental study of a novel approach using biodegradable materials. Childs Nerv. Syst. 2024, 40, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Aguado-Maestro, I.; Simón-Pérez, C.; García-Alonso, M.; Ailagas-De Las Heras, J.J.; Paredes-Herrero, E. Clinical Applications of “In-Hospital” 3D Printing in Hip Surgery: A Systematic Narrative Review. J. Clin. Med. 2024, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Alhaskawi, A.; Zhou, H.; Dong, Y.; Zou, X.; Ezzi, S.H.A.; Kota, V.G.; Abdulla, M.H.A.; Tu, T.; Alenikova, O.; Abdalbary, S.; et al. Advancements in 3D-printed artificial tendon. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35364. [Google Scholar] [CrossRef] [PubMed]
- González-López, P.; Kuptsov, A.; Gómez-Revuelta, C.; Fernández-Villa, J.; Abarca-Olivas, J.; Daniel, R.T.; Meling, T.R.; Nieto-Navarro, J. The Integration of 3D Virtual Reality and 3D Printing Technology as Innovative Approaches to Preoperative Planning in Neuro-Oncology. J. Pers. Med. 2024, 14, 187. [Google Scholar] [CrossRef]
- Sakaeyama, Y.; Sugo, N. Surgical Simulation Using a Three-Dimensional Printer. Neurol. Surg. 2024, 52, 254–262. (In Japanese) [Google Scholar] [CrossRef]
- Kopačin, V.; Zubčić, V.; Mumlek, I.; Mužević, D.; Rončević, A.; Lazar, A.M.; Pavić, A.K.; Koruga, A.S.; Krivdić, Z.; Martinović, I.; et al. Personalized 3D-printed cranial implants for complex cranioplasty using open-source software. Surg. Neurol. Int. 2024, 15, 39. [Google Scholar] [CrossRef]
- Bcharah, G.; Gupta, N.; Panico, N.; Winspear, S.; Bagley, A.; Turnow, M.; D’Amico, R.; Ukachukwu, A.K. Innovations in Spine Surgery: A Narrative Review of Current Integrative Technologies. World Neurosurg. 2024, 184, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Langenfeld, J.G.; Reinhart, B.C.; Lyden, E.I.; Campos, A.S.; Wadman, M.C.; Jamison, M.R.; Morin, S.A.; Barksdale, A.N. An evaluation of the usability and durability of 3D printed versus standard suture materials. Wound Repair. Regen. 2024, 32, 229–233. [Google Scholar] [CrossRef]
- Jeising, S.; Liu, S.; Blaszczyk, T.; Rapp, M.; Beez, T.; Cornelius, J.F.; Schwerter, M.; Sabel, M. Combined use of 3D printing and mixed reality technology for neurosurgical training: Getting ready for brain surgery. Neurosurg. Focus. 2024, 56, E12. [Google Scholar] [CrossRef] [PubMed]
- Ozgıray, E.; Husemoglu, B.; Cınar, C.; Bolat, E.; Akınturk, N.; Bıceroglu, H.; Kızmazoglu, C. The Effect of Preoperative Three Dimensional Modeling and Simulation on Outcome of Intracranial Aneursym Surgery. J. Korean Neurosurg. Soc. 2024, 67, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.W.; Kang, K.W.; Kim, W.K.; Yang, S.; Kang, B.J. Cervical spine reconstruction after total vertebrectomy using customized three-dimensional-printed implants in dogs. J. Vet. Sci. 2024, 25, e2. [Google Scholar] [CrossRef] [PubMed]
- Cingoz, I.D.; Gurkan, G.; Atar, M.; Uzunoglu, I.; Sahin, M.C.; Ozyoruk, S.; Tetik, H.; Kaya, I. Evaluation of Percutaneous Unilateral Kyphoplasty Results in Osteoporotic Vertebral Compression Fractures Using Individual 3D Printed Guide Template Support. Turk. Neurosurg. 2024, 34, 250–255. [Google Scholar] [CrossRef]
- Huang, A.Z.B.; Mobbs, R.J. Application of three-dimensional printed biomodels in endoscopic spinal surgery. J. Spine Surg. 2024, 10, 1. [Google Scholar] [CrossRef]
- Beri, A.; Pisulkar, S.G.; Iratwar, S.; Bansod, A.; Jain, R.; Shrivastava, A. Revolutionizing Neurosurgery: The Cutting-Edge Era of Digitally Fabricated Cranial Stents. Cureus 2024, 16, e53482. [Google Scholar] [CrossRef] [PubMed]
- Caruso, J.P.; Adenwalla, A.; Venishetty, N.; Tamimi, M.A.; Bagley, C.A.; Aoun, S.G. 3D-Printed Spine Models for Planning Staged Minimally Invasive Transverse Process Resections for Bertolotti Syndrome: Technical Note. J. Orthop. Case Rep. 2024, 14, 88–91. [Google Scholar] [CrossRef]
- Kumar, R.P.; Elsayed, G.A.; Hafez, D.M.; Agarwal, N. Advances in Anterolateral Approaches to the Lumbar Spine: A Focus on Technological Developments. Neurosurg. Clin. N. Am. 2024, 35, 199–205. [Google Scholar] [CrossRef]
- Winkler, D.; Kropla, F.; Busse, M.; Jung, S.; Scholz, S.; Güresir, E.; Gericke, M.; Vychopen, M.; Wach, J.; Grunert, R. Mixed reality for spine surgery: A step into the future with a human cadaveric accuracy study. Neurosurg. Focus. 2024, 56, E10. [Google Scholar] [CrossRef] [PubMed]
- Ghenbot, Y.; Ahmad, H.S.; Chauhan, D.; Wathen, C.; Arena, J.; Turlip, R.; Parr, R.; Gibby, W.; Yoon, J.W. Effects of Augmented Reality on Thoracolumbar Pedicle Screw Instrumentation Across Different Levels of Surgical Experience. World Neurosurg. 2024, 182, e284–e291. [Google Scholar] [CrossRef] [PubMed]
- Bardeesi, A.; Tabarestani, T.Q.; Bergin, S.M.; Huang, C.C.; Shaffrey, C.I.; Wiggins, W.F.; Abd-El-Barr, M.M. Using Augmented Reality Technology to Optimize Transfacet Lumbar Interbody Fusion: A Case Report. J. Clin. Med. 2024, 13, 1513. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sufianov, A.; Ovalle, C.S.; Cruz, O.; Contreras, J.; Begagić, E.; Kannan, S.; Rosario Rosario, A.; Chmutin, G.; Askatovna, G.N.; Lafuente, J.; et al. Low-Cost 3D Models for Cervical Spine Tumor Removal Training for Neurosurgery Residents. Brain Sci. 2024, 14, 547. https://doi.org/10.3390/brainsci14060547
Sufianov A, Ovalle CS, Cruz O, Contreras J, Begagić E, Kannan S, Rosario Rosario A, Chmutin G, Askatovna GN, Lafuente J, et al. Low-Cost 3D Models for Cervical Spine Tumor Removal Training for Neurosurgery Residents. Brain Sciences. 2024; 14(6):547. https://doi.org/10.3390/brainsci14060547
Chicago/Turabian StyleSufianov, Albert, Carlos Salvador Ovalle, Omar Cruz, Javier Contreras, Emir Begagić, Siddarth Kannan, Andreina Rosario Rosario, Gennady Chmutin, Garifullina Nargiza Askatovna, Jesus Lafuente, and et al. 2024. "Low-Cost 3D Models for Cervical Spine Tumor Removal Training for Neurosurgery Residents" Brain Sciences 14, no. 6: 547. https://doi.org/10.3390/brainsci14060547
APA StyleSufianov, A., Ovalle, C. S., Cruz, O., Contreras, J., Begagić, E., Kannan, S., Rosario Rosario, A., Chmutin, G., Askatovna, G. N., Lafuente, J., Sanchez, J. S., Nurmukhametov, R., Soto García, M. E., Peev, N., Pojskić, M., Reyes-Soto, G., Bozkurt, I., & Encarnación Ramírez, M. D. J. (2024). Low-Cost 3D Models for Cervical Spine Tumor Removal Training for Neurosurgery Residents. Brain Sciences, 14(6), 547. https://doi.org/10.3390/brainsci14060547









