Integrating Augmented Reality in Spine Surgery: Redefining Precision with New Technologies
Abstract
1. Introduction
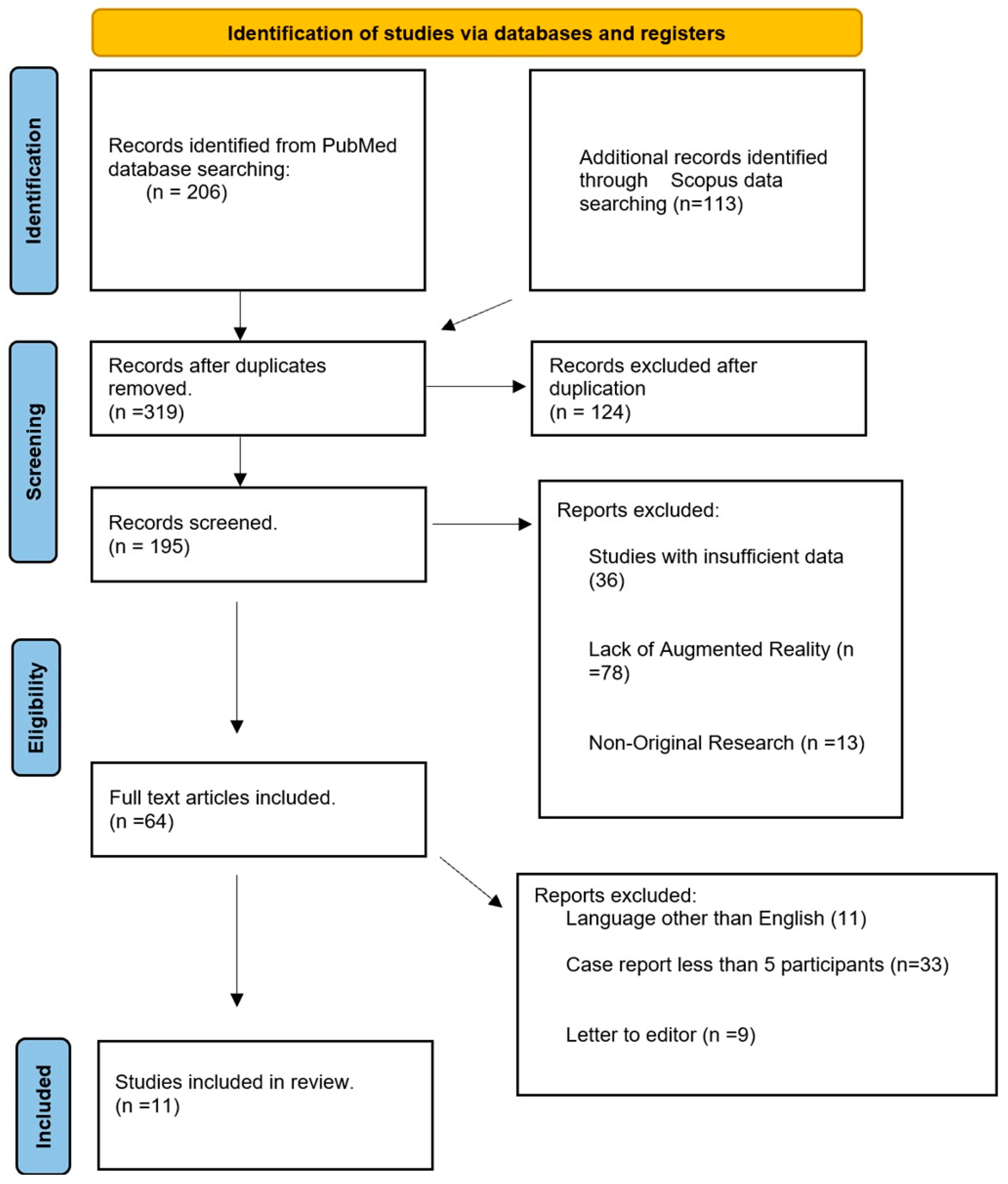
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Analysis
2.5. Quality Assessment
2.6. Risk of Bias thorough Assessment of Risk of Bias Was Conducted in Included Studies, Considering Several Potential Sources of Bias
- (1)
- Selection Bias: Selection bias was a concern due to the non-randomized selection of participants in case series and observational studies. Some studies had small sample sizes, which could limit the generalizability of the findings. The varying inclusion criteria across studies further contributed to the risk of selection bias.
- (2)
- Performance Bias: Performance bias may have been introduced by the variability in the experience levels of surgeons and the learning curve associated with augmented reality technologies. The novelty of these technologies might have influenced performance outcomes, with some studies potentially favoring those more familiar with the technology.
- (3)
- Detection Bias: Detection bias was considered due to the subjective nature of certain outcome assessments, such as user satisfaction and cognitive load. The use of non-standardized assessment tools across different studies may have further contributed to detection bias, impacting the reliability of the findings.
- (4)
- Attrition Bias: Attrition bias was a potential issue as some studies did not report on the long-term follow-up of participants or provided incomplete data. The lack of comprehensive reporting on participant outcomes raises concerns about the robustness of the findings.
- (5)
- Reporting Bias: Reporting bias was addressed by considering the possibility of selective publication of studies with positive outcomes. Negative or non-significant results might be underrepresented, skewing the overall assessment of augmented reality’s effectiveness in spine surgery.
2.7. Ethical Consideration
3. Results
Study Selection
4. Discussion
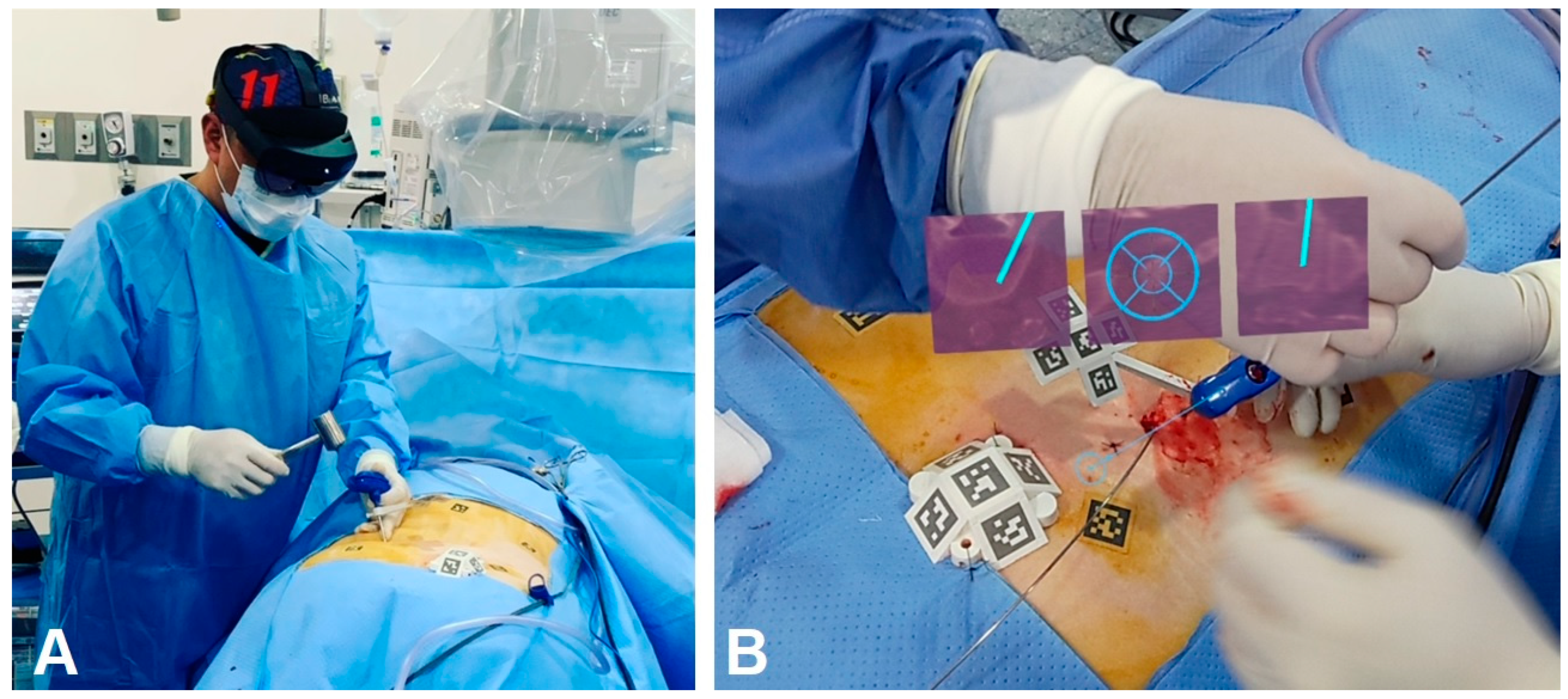
4.1. Applications of AR in Surgery
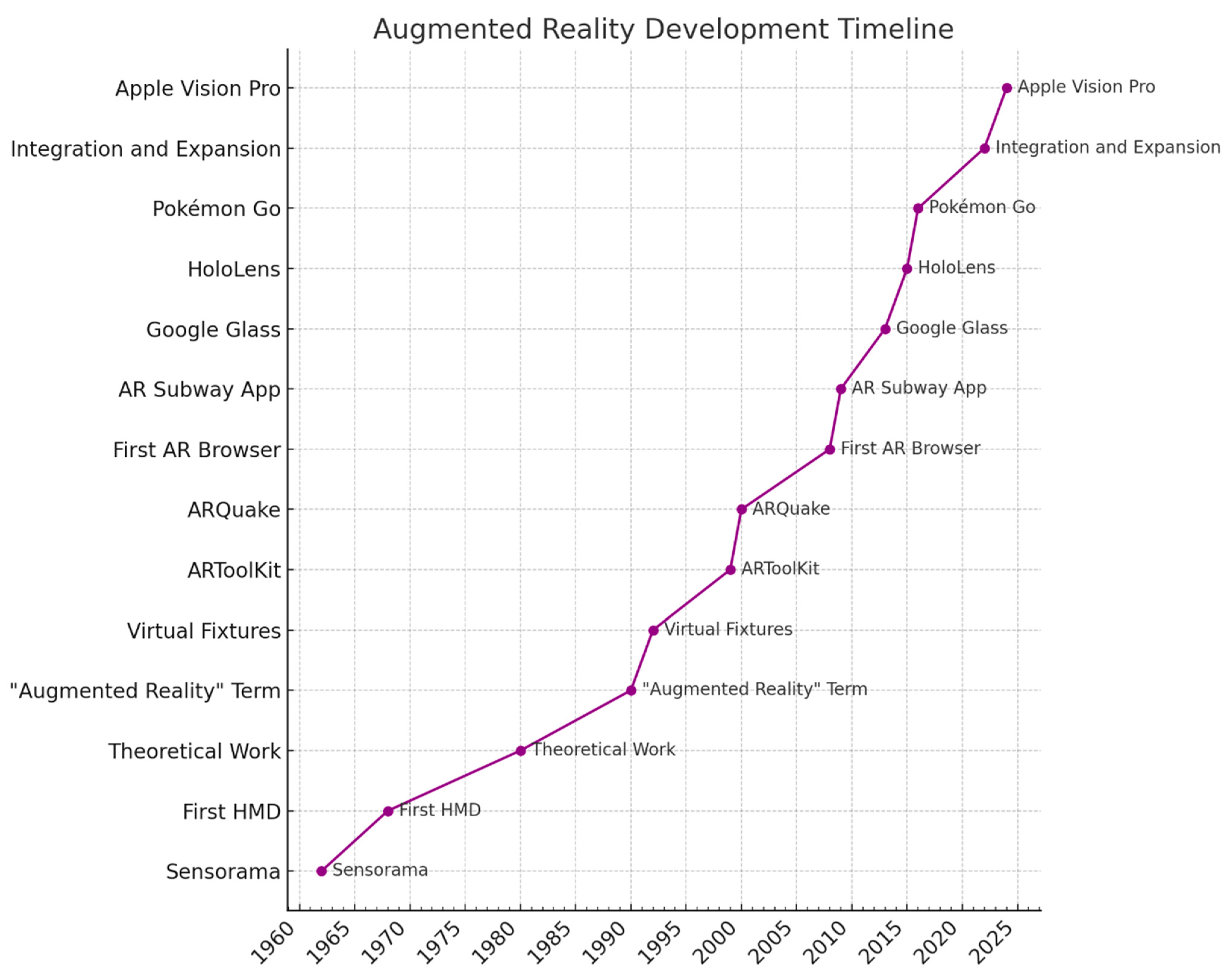
4.2. Technological Evolution and Impact
4.3. Research and Development
4.4. Learning Curve
4.5. Looking Ahead
4.6. Integration with AI and Machine Learning
4.7. Haptic Feedback Systems
4.8. Critical Analysis of Overall Findings
- (1)
- (2)
- Enhanced Surgical Education: The use of AR for surgical training and education was a prominent theme. Studies like those of Babichenko et al. [18] and Schonfeld et al. [25] highlighted AR’s role in reducing cognitive load and enhancing the learning experience for surgeons, which can lead to improved skill acquisition and performance.
- (3)
- Efficiency Gains: Several studies reported efficiency gains, such as reduced operative times and improved workflow. For example, DeSalvatore et al. [26] found that using AR technology decreased operative time and bleeding while increasing surgeon satisfaction.
- (4)
- Patient Outcomes and Satisfaction: The potential for AR to improve patient communication and satisfaction was noted, as it allows surgeons to better explain procedures and expected outcomes. This was particularly highlighted in studies like those by Rush et al. [27], which demonstrated the benefits of AR in preoperative planning and patient education.
4.9. Specific Challenges
- (1)
- Technological Barriers
- (2)
- Cost Issues
4.10. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yoo, J.S.; Patel, D.S.; Hrynewycz, N.M.; Brundage, T.S.; Singh, K. The utility of virtual reality and augmented reality in spine surgery. Ann. Transl. Med. 2019, 7 (Suppl. S5), S171. [Google Scholar] [CrossRef]
- Carl, B.; Bopp, M.; Saß, B.; Voellger, B.; Nimsky, C. Implementation of augmented reality support in spine surgery. Eur. Spine J. 2019, 28, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Burström, G.; Persson, O.; Edström, E.; Elmi-Terander, A. Augmented reality navigation in spine surgery: A systematic review. Acta Neurochir. 2021, 163, 843–852. [Google Scholar] [CrossRef]
- Wanivenhaus, F.; Neuhaus, C.; Liebmann, F.; Roner, S.; Spirig, J.M.; Farshad, M. Augmented reality-assisted rod bending in spinal surgery. Spine J. 2019, 19, 1687–1689. [Google Scholar] [CrossRef] [PubMed]
- Carl, B.; Bopp, M.; Saß, B.; Pojskic, M.; Gjorgjevski, M.; Voellger, B.; Nimsky, C. Reliable navigation registration in cranial and spine surgery based on intraoperative computed tomography. Neurosurg. Focus. 2019, 47, E11. [Google Scholar] [CrossRef] [PubMed]
- Bounajem, M.T.; Cameron, B.; Sorensen, K.; Parr, R.; Gibby, W.; Prashant, G.; Evans, J.J.; Karsy, M. Improved Accuracy and Lowered Learning Curve of Ventricular Targeting Using Augmented Reality-Phantom and Cadaveric Model Testing. Neurosurgery 2023, 92, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Rocks, T.; Samarasinghe, R.M.; Stephenson, G.; Smith, C. Augmented reality in medical education: Students’ experiences and learning outcomes. Med. Educ. Online 2021, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Elmi-Terander, A.; Burström, G.; Nachabe, R.; Skulason, H.; Pedersen, K.; Fagerlund, M.; Ståhl, F.; Charalampidis, A.; Söderman, M.; Holmin, S.; et al. Pedicle Screw Placement Using Augmented Reality Surgical Navigation with Intraoperative 3D Imaging: A First In-Human Prospective Cohort Study. Spine 2019, 44, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.A.; Theodore, N.; Ahmed, A.K.; Westbroek, E.M.; Mirovsky, Y.; Harel, R.; Orru’, E.; Khan, M.; Witham, T.; Sciubba, D.M. Augmented reality-assisted pedicle screw insertion: A cadaveric proof-of-concept study. J. Neurosurg. Spine 2019, 31, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Carl, B.; Bopp, M.; Saß, B.; Pojskic, M.; Nimsky, C. Augmented reality in intradural spinal tumor surgery. Acta Neurochir. 2019, 161, 2181–2193. [Google Scholar] [CrossRef]
- Liu, H.; Wu, J.; Tang, Y.; Li, H.; Wang, W.; Li, C.; Zhou, Y. Percutaneous placement of lumbar pedicle screws via intraoperative CT image-based augmented reality-guided technology. J. Neurosurg. Spine 2019, 32, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, F.; Roner, S.; von Atzigen, M.; Scaramuzza, D.; Sutter, R.; Snedeker, J.; Farshad, M.; Fürnstahl, P. Pedicle screw navigation using surface digitization on the Microsoft HoloLens. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Kimchi, G.; Orlev, A.; Hadanny, A.; Knoller, N.; Harel, R. Minimally Invasive Spine Surgery: The Learning Curve of a Single Surgeon. Glob. Spine J. 2020, 10, 1022–1026. [Google Scholar] [CrossRef]
- Edström, E.; Burström, G.; Omar, A.; Nachabe, R.; Söderman, M.; Persson, O.; Gerdhem, P.; Elmi-Terander, A. Augmented Reality Surgical Navigation in Spine Surgery to Minimize Staff Radiation Exposure. Spine 2020, 45, E45–E53. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, F.; Zhan, W.; Gan, M.; Sun, L. Optimization of virtual and real registration technology based on augmented reality in a surgical navigation system. Biomed. Eng. Online 2020, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Rahimpour, S.; Cutler, A.; Goodwin, C.R.; Lad, S.P.; Codd, P. Enhancing Reality: A Systematic Review of Augmented Reality in Neuronavigation and Education. World Neurosurg. 2020, 139, 186–195. [Google Scholar] [CrossRef]
- Matthews, J.H.; Shields, J.S. The Clinical Application of Augmented Reality in Orthopaedics: Where Do We Stand? Curr. Rev. Musculoskelet. Med. 2021, 14, 316–319. [Google Scholar] [CrossRef]
- Babichenko, D.; Andrews, E.G.; Canton, S.P.; Littleton, E.B.; Patel, R.; Labaze, D.; Mills, A. Evaluating Effect of Microsoft HoloLens on Extraneous Cognitive Load During Simulated Cervical Lateral Mass Screw Placement. In Proceedings of the Seventh International Congress on Information and Communication Technology, London, UK, 21–24 February 2022; Volume 3, pp. 191–201. [Google Scholar]
- Xin, B.; Chen, G.; Wang, Y.; Bai, G.; Gao, X.; Chu, J.; Xiao, J.; Liu, T. The efficacy of immersive virtual reality surgical simulator training for pedicle screw placement: A randomized double-blind controlled trial. Int. Orthop. 2020, 44, 927–934. [Google Scholar] [CrossRef]
- Buch, V.P.; Mensah-Brown, K.G.; Germi, J.W.; Park, B.J.; Madsen, P.J.; Borja, A.J.; Haldar, D.; Basenfelder, P.; Yoon, J.W.; Schuster, J.M.; et al. Development of an Intraoperative Pipeline for Holographic Mixed Reality Visualization During Spinal Fusion Surgery. Surg. Innov. 2021, 28, 427–437. [Google Scholar] [CrossRef]
- Pojskić, M.; Bopp, M.; Saß, B.; Nimsky, C. Single-center experience in resection of 120 cases of intradural spinal tumors. World Neurosurg. 2024, 18. [Google Scholar] [CrossRef]
- Charles, Y.P.; Al Ansari, R.; Collinet, A.; De Marini, P.; Schwartz, J.; Nachabe, R.; Schäfer, D.; Brendel, B.; Gangi, A.; Cazzato, R.L. Accuracy Assessment of Percutaneous Pedicle Screw Placement Using Cone Beam Computed Tomography with Metal Artifact Reduction. Sensors 2022, 22, 4615. [Google Scholar] [CrossRef] [PubMed]
- Carl, B.; Bopp, M.; Saß, B.; Pojskic, M.; Voellger, B.; Nimsky, C. Spine Surgery Supported by Augmented Reality. Glob. Spine J. 2020, 10, 41s–55s. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, K.; Foster, C.H.; Rosner, M.K. Practical Use of Augmented Reality Modeling to Guide Revision Spine Surgery: An Illustrative Case of Hardware Failure and Overriding Spondyloptosis. Oper. Neurosurg. 2022, 23, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, E.; de Lotbiniere-Bassett, M.; Jansen, T.; Anthony, D.; Veeravagu, A. Vertebrae segmentation in reduced radiation CT imaging for augmented reality applications. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 775–783. [Google Scholar] [CrossRef] [PubMed]
- De Salvatore, S.; Vadalà, G.; Oggiano, L.; Russo, F.; Ambrosio, L.; Costici, P.F. Virtual reality in preoperative planning of adolescent idiopathic scoliosis surgery using google cardboard. Neurospine 2021, 18, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.J., 3rd; Shepard, N.; Nolte, M.; Siemionow, K.; Phillips, F. Augmented Reality in Spine Surgery: Current State of the Art. Int. J. Spine Surg. 2022, 16, S22–S27. [Google Scholar] [CrossRef] [PubMed]
- Edström, E.; Burström, G.; Nachabe, R.; Gerdhem, P.; Elmi Terander, A. A Novel Augmented-Reality-Based Surgical Navigation System for Spine Surgery in a Hybrid Operating Room: Design, Workflow, and Clinical Applications. Oper. Neurosurg. 2020, 18, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.; Mahapatra, S.; Weber-Levine, C.; Awosika, T.; Theodore, J.N.; Zakaria, H.M.; Liu, A.; Witham, T.F.; Theodore, N. Augmented reality in spine surgery: A narrative review. HSS J. 2021, 17, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Carl, B.; Bopp, M.; Saß, B.; Nimsky, C. Microscope-based augmented reality in degenerative spine surgery: Initial experience. World Neurosurg. 2019, 128, e541–e551. [Google Scholar] [CrossRef]
- Peuchot, B.; Tanguy, A.; Eude, M. Augmented reality in spinal surgery. Stud. Health Technol. Inform. 1997, 37, 441–444. [Google Scholar]
- Sakai, D.; Joyce, K.; Sugimoto, M.; Horikita, N.; Hiyama, A.; Sato, M.; Devitt, A.; Watanabe, M. Augmented, virtual and mixed reality in spinal surgery: A real-world experience. J. Orthop. Surg. 2020, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Theocharopoulos, N.; Perisinakis, K.; Damilakis, J.; Papadokostakis, G.; Hadjipavlou, A.; Gourtsoyiannis, N. Occupational exposure from common fluoroscopic projections used in orthopaedic surgery. J. Bone Jt. Surg. Ser. A 2003, 85, 1698–1703. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Wu, J.Y.; DiMaio, S.P.; Nassier, N.; Kazanzides, P. A review of augmented reality in robotic-assisted surgery. IEEE Trans. Med. Robot. Bionics 2020, 2, 1–16. [Google Scholar]
- A Brief History of Augmented Reality (+Future Trends & Impact)—G2. G2. 2019. Available online: https://www.g2.com/articles/history-of-augmented-reality (accessed on 1 March 2024).
- Marill, M.C. Hey Surgeon, Is That a Hololens on Your Head? Wired, Conde Nast. 2019. Available online: https://www.wired.com/story/hey-surgeon-is-that-a-hololens-on-your-head (accessed on 1 March 2024).
- John Hopkins Medicine. Johns Hopkins Performs Its First Augmented Reality Surgeries in Patients. 2021. Available online: https://www.hopkinsmedicine.org/news/articles/johns-hopkins-performs-its-first-augmented-reality-surgeries-in-patients (accessed on 1 March 2024).
- Molina, C.A.; Sciubba, D.M.; Greenberg, J.K.; Khan, M.; Witham, T. Clinical accuracy, technical precision, and workflow of the first in human use of an augmented-reality head-mounted display stereotactic navigation system for spine surgery. Oper Neurosurg. 2021, 20, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Alaraj, A.; Charbel, F.T.; Birk, D.; Tobin, M.; Luciano, C.; Banerjee, P.P.; Rizzi, S.; Sorenson, J.; Foley, K.; Slavin, K.; et al. Role of cranial and spinal virtual and augmented reality simulation using immersive touch modules in neurosurgical training. Neurosurgery 2013, 72 (Suppl. S1), 115–123. [Google Scholar] [CrossRef] [PubMed]
- Adida, S.; Legarreta, A.D.; Hudson, J.S.; McCarthy, D.; Andrews, E.; Shanahan, R.; Taori, S.; Lavadi, R.S.; Buell, T.J.; Hamilton, D.K.; et al. Machine Learning in Spine Surgery: A Narrative Review. Neurosurgery 2024, 94, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Lizana, J.; Montemurro, N.; Aliaga, N.; Marani, W.; Tanikawa, R. From textbook to patient: A practical guide to train the end-to-side microvascular anastomosis. Br J Neurosurg. 2023, 37, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Tigchelaar, S.S.; Medress, Z.A.; Quon, J.; Dang, P.; Barbery, D.; Bobrow, A.; Kin, C.; Louis, R.; Desai, A. Augmented Reality Neuronavigation for En Bloc Resection of Spinal Column Lesions. World Neurosurg. 2022, 167, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Yuk, F.J.; Maragkos, G.A.; Sato, K.; Steinberger, J. Current innovation in virtual and augmented reality in spine surgery. Ann. Transl. Med. 2021, 9, 94. [Google Scholar] [CrossRef]
- Luciano, C.J.; Banerjee, P.P.; Bellotte, B.; Oh, G.M.; Lemole MJr Charbel, F.T.; Roitberg, B. Learning retention of thoracic pedicle screw placement using a high-Resolution augmented reality simulator with haptic feedback. Oper. Neurosurg. 2011, 69, ons14–ons19. [Google Scholar] [CrossRef]
- Müller, F.; Roner, S.; Liebmann, F.; Spirig, J.M.; Fürnstahl, P.; Farshad, M. Augmented reality navigation for spinal pedicle screw instrumentation using intraoperative 3D imaging. Spine J. 2020, 20, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Narayanan, M.D.K.; Umana, G.E.; Montemurro, N.; Chaurasia, B.; Deora, H. Virtual Reality in Neurosurgery: Beyond Neurosurgical Planning. Int J Environ Res Public Health. 2022, 19, 1719. [Google Scholar] [CrossRef] [PubMed]
- Olexa, J.; Trang, A.; Cohen, J.; Kim, K.; Rakovec, M.; Saadon, J.; Sansur, C.; Woodworth, G.; Schwartzbauer, G.; Cherian, J.; et al. The Apple Vision Pro as a Neurosurgical Planning Tool: A Case Report. Cureus 2024, 16, e54205. [Google Scholar] [CrossRef] [PubMed]
- Apple Vision Pro Used in Spinal Surgery. Available online: https://wearable-technologies.com/news/apple-vision-pro-used-in-spinal-surgery (accessed on 1 March 2024).
- Madhavan, K.; Kolcun, J.P.G.; Chieng, L.O.; Wang, M.Y. Augmented-reality integrated robotics in neurosurgery: Are we there yet? Neurosurg. Focus. 2017, 42, E3. [Google Scholar] [CrossRef] [PubMed]
- Urakov, T.M.; Wang, M.Y.; Levi, A.D. Workflow Caveats in Augmented Reality-Assisted Pedicle Instrumentation: Cadaver Lab. World Neurosurg. 2019, 126, e1449–e1455. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.J.; McMillen, J. The surgical learning curve and accuracy of minimally invasive lumbar pedicle screw placement using CT based computer-assisted navigation plus continuous electromyography monitoring—A retrospective review of 627 screws in 150 patients. Int. J. Spine Surg. 2014, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Gasco, J.; Patel, A.; Ortega-Barnett, J.; Branch, D.; Desai, S.; Kuo, Y.F.; Luciano, C.; Rizzi, S.; Kania, P.; Matuyauskas, M.; et al. Virtual reality spine surgery simulation: An empirical study of its usefulness. Neurol. Res. 2014, 36, 968–997. [Google Scholar] [CrossRef]
- FDA. Clears Microsoft’s HoloLens for Pre-Operative Surgical Planning. Available online: https://www.fdanews.com/articles/188966-fda-clears-microsofts-hololens-for-pre-operative-surgical-planning (accessed on 10 January 2023).
- Montemurro, N.; Condino, S.; Carbone, M.; Cattari, N.; D’Amato, R.; Cutolo, F.; Ferrari, V. Brain Tumor and Augmented Reality: New Technologies for the Future. Int. J. Environ. Res. Public Health 2022, 19, 6347. [Google Scholar] [CrossRef] [PubMed]
- Encarnacion, M.J.; Castillo, R.E.B.; Matos, Y.; Bernard, E.; Elenis, B.; Oleinikov, B.; Nurmukhametov, R.; Castro, J.S.; Volovich, A.; Dosanov, M.; et al. EasyGO!-assisted microsurgical anterior cervical decompression: Technical report and literature review. Neurol. Neurochir. Pol. 2022, 56, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Condino, S.; Montemurro, N.; Cattari, N.; D’amato, R.; Thomale, U.; Ferrari, V.; Cutolo, F. Evaluation of a Wearable AR Platform for Guiding Complex Craniotomies in Neurosurgery. Ann. Biomed. Eng. 2021, 49, 2590–2605. [Google Scholar] [CrossRef]
- Nurmukhametov, R.; Dosanov, M.; Encarnacion, M.D.J.; Barrientos, R.; Matos, Y.; Alyokhin, A.I.; Baez, I.P.; Efe, I.E.; Restrepo, M.; Chavda, V.; et al. Transforaminal Fusion Using Physiologically Integrated Titanium Cages with a Novel Design in Patients with Degenerative Spinal Disorders: A Pilot Study. Surgeries 2022, 3, 175–184. [Google Scholar] [CrossRef]
- Torres, C.S.O.; Mora, A.E.; Campero, A.; Cherian, I.; Sufianov, A.; Sanchez, E.F.; Ramirez, M.E.; Pena, I.R.; Nurmukhametov, R.; Beltrán, M.A.; et al. Enhancing microsurgical skills in neurosurgery residents of low-income countries: A comprehensive guide. Surg. Neurol. Int. 2023, 14, 437. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Condino, S.; Cattari, N.; D’Amato, R.; Ferrari, V.; Cutolo, F. Augmented Reality-Assisted Craniotomy for Parasagittal and Convexity En Plaque Meningiomas and Custom-Made Cranio-Plasty: A Preliminary Laboratory Report. Int. J. Environ. Res. Public Health 2021, 18, 9955. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, G.M.; Sigismondi, S.; Nicoletta, M.; Condino, S.; Montemurro, N.; Vozzi, G.; Ferrari, V.; De Maria, C. Analysis of the Robotic-Based In Situ Bioprinting Workflow for the Regeneration of Damaged Tissues through a Case Study. Bioeng 2023, 10, 560. [Google Scholar] [CrossRef]
- Reyes Soto, G.; Ovalle Torres, C.; Perez Terrazas, J.; Partida, K.H.; Rosario, A.R.; Campero, A.; Baldoncini, M.; Ramirez, M.D.J.E.; Montemurro, N. Multiple Myeloma Treatment Challenges: A Case Report of Vertebral Artery Pseudoaneurysm Complicating Occipitocervical Arthrodesis and a Review of the Literature. Cureus 2023, 15, e49716. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Soto, G.; Corona De la Torre, A.; Honda Partida, K.G.; Nurmukhametov, R.; Encarnacion Ramirez, M.D.J.; Montemurro, N. Clivus-Cervical Stabilization through Transoral Approach in Patients with Craniocervical Tumor: Three Cases and Surgical Technical Note. Brain Sci. 2024, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.D.J.E.; Nurmukhametov, R.; Musa, G.; Castillo, R.E.B.; Encarnacion, V.L.A.; Sanchez, J.A.S.; Vazquez, C.A.; Efe, I.E. Three-Dimensional Plastic Modeling on Bone Frames for Cost-Effective Neuroanatomy Teaching. Cureus 2022, 14, e27472. [Google Scholar] [CrossRef]
- Meulstee, J.W.; Nijsink, J.; Schreurs, R.; Verhamme, L.M.; Xi, T.; Delye, H.H.K.; Borstlap, W.A.; Maal, T.J.J. Toward Holographic-Guided Surgery. Surg. Innov. 2019, 26, 86–94. [Google Scholar] [CrossRef]
- Encarnacion Ramirez, M.; Ramirez Pena, I.; Barrientos Castillo, R.E.; Sufianov, A.; Goncharov, E.; Soriano Sanchez, J.A.; Colome-Hidalgo, M.; Nurmukhametov, R.; Cerda Céspedes, J.R.; Montemurro, N. Development of a 3D Printed Brain Model with Vasculature for Neurosurgical Procedure Visualisation and Training. Biomedicines 2023, 11, 330. [Google Scholar] [CrossRef]
- Urlings, J.; de Jong, G.; Maal, T.; Henssen, D. Views on Augmented Reality, Virtual Reality, and 3D Printing in Modern Medicine and Education: A Qualitative Exploration of Expert Opinion. J Digit Imaging. 2023, 36, 1930–1939. [Google Scholar] [CrossRef]
- Ramirez, M.D.J.E.; Nurmukhametov, R.; Bernard, E.; Peralta, I.; Efe, I.E. A Low-Cost Three-Dimensional Printed Retractor for Transforaminal Lumbar Interbody Fusion. Cureus 2022, 14, e24185. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kumar, S.; Shallal, C.; Leo, K.T.; Girard, A.; Bai, Y.; Li, Y.; Jackson, E.M.; Cohen, A.R.; Yang, R. Caregiver Preferences for Three-Dimensional Printed or Augmented Reality Craniosynostosis Skull Models: A Cross-Sectional Survey. J. Craniofac. Surg. 2022, 33, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Haemmerli, J.; Davidovic, A.; Meling, T.R.; Chavaz, L.; Schaller, K.; Bijlenga, P. Evaluation of the precision of operative augmented reality compared to standard neuronavigation using a 3D-printed skull. Neurosurg. Focus. 2021, 50, E17. [Google Scholar] [CrossRef] [PubMed]
- Boyaci, M.G.; Fidan, U.; Yuran, A.F.; Yildizhan, S.; Kaya, F.; Kimsesiz, O.; Ozdil, M.; Cengiz, A.; Aslan, A. Augmented Reality Supported Cervical Transpedicular Fixation on 3D-Printed Vertebrae Model: An Experimental Education Study. Turk. Neurosurg. 2020, 30, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Azad, T.D.; Warman, A.; Tracz, J.A.; Hughes, L.P.; Judy, B.F.; Witham, T.F. Augmented reality in spine surgery–past, present, and future. Spine J. 2024, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lohre, R.; Wang, J.C.; Lewandrowski, K.U.; Goel, D.P. Virtual reality in spinal endoscopy: A paradigm shift in education to support spine surgeons. J. Spine Surg. 2020, 6 (Suppl. S1), S208–S223. [Google Scholar] [CrossRef] [PubMed]
- Zawy Alsofy, S.; Nakamura, M.; Ewelt, C.; Kafchitsas, K.; Lewitz, M.; Schipmann, S.; Suero Molina, E.; Santacroce, A.; Stroop, R. Retrospective Comparison of Minimally Invasive and Open Monosegmental Lumbar Fusion, and Impact of Virtual Reality on Surgical Planning and Strategy. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2021, 82, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Pierzchajlo, N.; Stevenson, T.C.; Huynh, H.; Nguyen, J.; Boatright, S.; Arya, P.; Chakravarti, S.; Mehrki, Y.; Brown, N.J.; Gendreau, J.; et al. Augmented Reality in Minimally Invasive Spinal Surgery: A Narrative Review of Available Technology. World Neurosurg. 2023, 176, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Avrumova, F.; Lebl, D.R. Augmented reality for minimally invasive spinal surgery. Front. Surg. 2023, 9, 1086988. [Google Scholar] [CrossRef]
- Condino, S.; Cutolo, F.; Carbone, M.; Cercenelli, L.; Badiali, G.; Montemurro, N.; Ferrari, V. Registration Sanity Check for AR-guided Surgical Interventions: Experience from Head and Face Surgery. IEEE J Transl Eng Health Med. 2023, 12, 258–267. [Google Scholar] [CrossRef]
- Sufianov, A.; Ovalle, C.S.; Cruz, O.; Contreras, J.; Begagić, E.; Kannan, S.; Rosario Rosario, A.; Chmutin, G.; Askatovna, G.N.; Lafuente, J.; et al. Low-Cost 3D Models for Cervical Spine Tumor Removal Training for Neurosurgery Residents. Brain Sci. 2024, 14, 547. [Google Scholar] [CrossRef]
- Staartjes, V.E.; Stumpo, V.; Kernbach, J.M.; Klukowska, A.M.; Gadjradj, P.S.; Schröder, M.L.; Veeravagu, A.; Stienen, M.N.; van Niftrik, C.H.; Serra, C.; et al. Machine learning in neurosurgery: A global survey. Acta Neurochir. 2020, 162, 3081–3091. [Google Scholar] [CrossRef]
- Jumah, F.; Raju, B.; Nagaraj, A.; Shinde, R.; Lescott, C.; Sun, H.; Gupta, G.; Nanda, A. Uncharted Waters of Machine and Deep Learning for Surgical Phase Recognition in Neurosurgery. World Neurosurg. 2022, 160, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N. Telemedicine: Could it represent a new problem for spine surgeons to solve? Glob. Spine J. 2022, 12, 1306–1307. [Google Scholar] [CrossRef] [PubMed]
- Mah, E.T. Metaverse, AR, machine learning & AI in Orthopaedics? J. Orthop. Surg. 2023, 31, 10225536231165362. [Google Scholar]
- Gamba, I.A.D.; Hartery, A. The Virtual Reality Radiology Workstation: Current Technology and Future Applications. Can. Assoc. Radiol. J. 2024, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Maisto, M.; Pacchierotti, C.; Chinello, F.; Salvietti, G.; De Luca, A.; Prattichizzo, D. Evaluation of Wearable Haptic Systems for the Fingers in Augmented Reality Applications. IEEE Trans. Haptics 2017, 10, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, R.; Singh, D.; Tawk, C.; Sariyildiz, E. A 3D-Printed Soft Haptic Device with Built-in Force Sensing Delivering Bio-Mimicked Feedback. Biomimetics 2023, 8, 127. [Google Scholar] [CrossRef]
- Aggravi, M.; De Momi, E.; DiMeco, F.; Cardinale, F.; Casaceli, G.; Riva, M.; Ferrigno, G.; Prattichizzo, D. Hand-tool-tissue interaction forces in neurosurgery for haptic rendering. Med. Biol. Eng. Comput. 2016, 54, 1229–1241. [Google Scholar] [CrossRef]
- Sun, X.; Gu, S.; Jiang, L.; Wu, Y. A Low-cost Mobile System with Multi-AR Guidance for Brain Surgery Assistance. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; Volume 2021, pp. 2222–2225. [Google Scholar]
- Duryea, J.; Cheng, C.; Schaefer, L.F.; Smith, S.; Madore, B. Integration of accelerated MRI and post-processing software: A promising method for studies of knee osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1905–1909. [Google Scholar] [CrossRef]
- Canton, S.P.; Austin, C.N.; Steuer, F.; Dadi, S.; Sharma, N.; Kass, N.M.; Fogg, D.; Clayton, E.; Cunningham, O.; Scott, D.; et al. Feasibility and Usability of Augmented Reality Technology in the Orthopaedic Operating Room. Curr. Rev. Musculoskelet. Med. 2024, 17, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra-Becerra, J.E.; Acha Sánchez, J.L.; Castilla-Encinas, A.M.; Rios-Garcia, W.; Mendieta, C.D.; Quiroz-Marcelo, D.A.; Alhwaishel, K.; Aguilar-Zegarra, L.; Lopez-Gonzalez, M.A. Toward a Frontierless Collaboration in Neurosurgery: A Systematic Review of Remote Augmented and Virtual Reality Technologies. World Neurosurg. 2024, 187, 114–121. [Google Scholar] [CrossRef] [PubMed]


| Author(s) (Year) | Objective | AR Technology | Applications of AR in Spine Surgery | Methodology | Primary Findings | Sample Size | Primary Outcomes and Measures | Limitations |
|---|---|---|---|---|---|---|---|---|
| Babichenko et al. (2023) [18] | Systematically collect and examine the role of AR in spine surgery. aims to highlight the evolution of AR technology in this context, evaluate the existing body of research, and outline potential future directions for integrating AR into spine surgery. | Microsoft HoloLens | It highlights the strengths and weaknesses of existing investigations. Additionally, it presents insights into the potential for AR to enhance spine surgical education and speculates on future applications. | Case series | No significant differences presented in cognitive load when trials with the HoloLens 1 in comparison with the trials without the HoloLens 1. Cognitive load was measured with the Surgical Task Load Index (SURG-TLX) questionnaire and surgical performance metrics. | 22 Surgeons participated | The results from our literature review provide a centralized pool of in vivo metrics related to AR, virtual reality (VR), and mixed reality (MR) use in spine surgery. | Limited studies have been reported regarding the clinical results of AR, VR, and MR use in live surgery of the spine. |
| Xin et al., 2020 [19] | It shows the current evidence of the use of VR, AR, and MR simulators in minimally invasive spine surgery (MISS) and spinal endoscopic surgery, including study quality, level of evidence (LoE), and outcomes. | Phantom | Screw placement | RCT | To verify whether the pedicle screw placement (PSP) skills of young surgeons receiving immersive virtual reality surgical simulator (IVRSS) training could be improved effectively and whether the IVRSS-PSP training mode could produce real clinical value for clinical surgery. | 24 participants | The current scope of VR, AR, and MR surgical simulators in MISS was described. | A lack of clearly defined outcomes, absent statistical analyses, limited validity breadth including demonstration of transfer validity |
| Buch et al. (2021) [20] | Innovative solutions can be developed to enable the use of this technology during surgery. | HoloLens | 3D visualization, | Experiment | The pipeline uses intraoperatively acquired, low-resolution imaging to generate, deploy, and register holographic models onto patients on the operating table | 16 participants | A custom pipeline is described for the generation of intraoperative 3D holographic models during spine surgery. | More testing is required to confirm clinically adequate registration accuracy across multiple patients |
| Pojskić et al. (2024) [21] | Single center experience in resection of intradural spinal tumors either with or without using intraoperative CT (iCT)-based registration and microscope-based augmented reality (AR). Microscope-based AR was recently described for improved orientation in the operative field in spine surgery, using superimposed images of segmented structures of interest in a two- (2D) or three-dimensional (3D) mode | based registration and microscope-based augmented reality (AR) | resection of intradural spinal tumors | Case series | Its key advantage over robotics and navigated spine surgery is that the surgeon never has to take the focus from the patient | 112 patients | Operative time, extent of resection, clinical outcome and complication rate did not differ between the AR and non-AR group. However, use of AR improved orientation in the operative field by identification of important neurovascular structures. AR improved intraoperative orientation and increased surgeons comfort by enabling early identification of important anatomical structures, However, clinical and radiological outcomes did not differ, when AR was not used. | The cost of technology and justification of its use without differences in clinical and surgical outcomes and complication rates |
| Charles et al. (2022) [22] | To assess intra- and inter-observer reliability of pedicle screw placement and to compare the perception of baseline image quality (NoMAR) with optimized image quality (MAR). | Microsoft Holo Lens | Microscopic surgery, remote assistance | Case series | Assessment for accuracy of pedicle screw placement. | 24 patients | Intraoperative screw positioning can be reliably assessed on cone beam CT for AR surgical navigation when using optimized image quality. MAR and NoMAR images demonstrated good intra-observer and excellent inter-observer and intra-class correlation coefficients. | Various AR-enabled technologies are emerging without specific criteria for judging them. |
| Carl et al. (2020) [23] | To investigate how microscope-based augmented reality (AR) support can be utilized in various types of spine surgery. | Microscope-based AR support | The application of AR technology in various facets of spine surgery. The included studies illustrate the potential benefits and feasibility of utilizing AR in spine surgical procedures, highlighting its impact on patient outcomes and surgeon performance. | Case series | Anterior, lateral, posterior median, and posterior paramedian approaches for degenerative spine surgery as well as intradural and extradural tumor resections. | 42 patients (12 intra- and 8 extradural tumors, 7 other intradural lesions, 11 degenerative cases, 2 infections, and 2 deformities). AR was implemented using operating microscope head-up displays (HUDs) | AR smoothly supported various kinds of spine procedures and facilitated anatomical orientation in the surgical field. | It is difficult to exactly measure the additional benefit of the AR application in each individual procedure. Usability questionnaires might be a tool to document surgeon acceptance |
| Mozaffari et al. (2020) [24] | To investigate how microscope-based augmented reality (AR) support can be utilized in traumatic spine surgery and focal kyphosis. | Microscopic-AR, VisAR | Microscope-based AR can be applied successfully to various kinds of spinal procedures. AR improves anatomical orientation in the surgical field, supporting the surgeon, as well as offering a potential tool for education. | Clinical study | The entire process of intraoperative registration. Imaging was added only at about 5 min into the surgical procedure, and thereafter, AR was instantly available. We did not encounter any technical or surgical problems due to AR implementation. | 10 patients | A microscope-based AR environment was successfully implemented for spinal surgery. The application of Ict for registration imaging ensures high navigational accuracy. AR supports the surgeon in understanding the 3D anatomy, thereby facilitating surgery. | Among the limitations of our study is that it is difficult to exactly measure the additional benefit of the AR application in each individual procedure. Usability questionnaires might be a tool to document surgeon acceptance. |
| Schonfeld et al. (2021) [25] | Analyzed several scenarios where we equipped OR personnel with augmented reality (AR) glasses, allowing a remote specialist to guide OR operations through voice and ad hoc visuals, superimposed to the field of view of the operator wearing them. | AR goggles, XVision | Remote assistance | Clinical study | Surgeons wearing the AR goggles reported positive feedback as for the ergonomics, wearability, and comfort during the procedure; being able to visualize a 3D reconstruction during surgery was perceived as a straightforward benefit, allowing surgeons to speed-up procedures, thus limiting post-operational complications. | 21 participants | By allowing surgeons to overlay digital medical content on actual surroundings, augmented reality surgery can be exploited easily in multiple scenarios by adapting commercially available or custom-made apps to several use cases. | Physical limitations, limited exploration of dose ranges, and denoising algorithm artifacts focus solely on AR auto-segmentation, potential impact on clinical workflow, and limited generalizability to diverse clinical environments and AR systems. |
| DeSalvatore et al. (2020) [26] | To assess a novel 3D model created using Google Cardboard for surgical planning for adolescent idiopathic scoliosis patients. | AR goggles, Google Cardboard | Intraoperative Navigation | Case series | The main findings were superior workflow and non-inferior accuracy when comparing AR to free-hand (FH) or conventional surgical navigation techniques. | 60 patients | Use of this VR-based technology led to decreased operative time and bleeding while increasing the surgeon’s satisfaction in a reproducible, cost-effective manner. | The current evidence base is limited and prospective studies on clinical outcomes and cost–benefit relationships are needed. |
| Rush et al. (2022) [27] | Augmented reality (AR) has the potential to dramatically improve the accuracy and reduce the time required for preoperative planning and performance of minimally invasive spine surgeries and procedures. | XVision | AR surgical navigation | Cadaveric study | This data set suggests that AR navigation, utilizing a VN, is an emerging, accurate, valuable additive method for surgical and procedural planning for percutaneous image-guided spinal procedures and has potential to be applied to a broad range of clinical and surgical applications. | 5 cadavers, 120 screws | application of AR navigation on a series of common percutaneous image-guided spine procedures | Physical limitations, limited exploration of dose ranges, denoising algorithm artifacts, focus solely on AR auto-segmentation, potential impact on clinical workflow, and limited generalizability to diverse clinical environments and AR systems. |
| Edström et al. (2020) [28] | To present a workflow for an ARSN system installed in a hybrid operating room. | augmented-reality-based surgical navigation (ARSN) system installed in a hybrid operating room | Pedicle screw placement. | Case series | Microscope AR displays offer the advantage of reducing attention shift and line-of-sight interruptions inherent in traditional instruments. | 20 cases | Navigated interventions were performed with a median total time of 379 min per procedure. The total procedure time was subdivided into surgical exposure (28%), cone beam computed tomography imaging and 3D segmentation (2%), software planning (6%), navigated surgery for screw placement (17%), and non-navigated instrumentation, wound closure (47%). | Limited clinical results, intraoperative CT scan required, registration markers unavailable for certain rigid locations. |
| Technology | Characteristics | Outcomes in Clinical Settings | Pricing |
|---|---|---|---|
| Augmedics XVision | Retina-projecting heads-up display, incorporating 3D anatomical precision and tool details, surgical navigation, integrated illumination, high-speed visual processing, cordless | Studies on cadavers: 94.6–98.9% precision according to the Gertzbein scale, and 96.7–99.1% according to the Heary–Gertzbein scale. Patient studies: 98.0–100% accuracy [38,43,44] | USD 200,000 (2020) |
| Microsoft HoloLens | Cordless headset with holographic lenses, quad visible light and dual IR cameras, integrated microphones and speakers, Bluetooth connectivity, Wi-Fi | Cadaver studies: Precision on par with top-tier tracking systems. Phantom studies: Reduced total time for rod bending and insertion by 20% [4,45,46] | USD 3500–USD 5200 (2023) |
| Applevision pro | Oculus-based headset with holographic lenses, precision measurement, adjustable opacity, sonic feedback, user-friendly menus and buttons | First case in the world registered using vision pro in ventricular catheter placement, good results not complications, first spine surgery case [47,48] | USD 3500 (2024) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Jesus Encarnacion Ramirez, M.; Chmutin, G.; Nurmukhametov, R.; Soto, G.R.; Kannan, S.; Piavchenko, G.; Nikolenko, V.; Efe, I.E.; Romero, A.R.; Mukengeshay, J.N.; et al. Integrating Augmented Reality in Spine Surgery: Redefining Precision with New Technologies. Brain Sci. 2024, 14, 645. https://doi.org/10.3390/brainsci14070645
De Jesus Encarnacion Ramirez M, Chmutin G, Nurmukhametov R, Soto GR, Kannan S, Piavchenko G, Nikolenko V, Efe IE, Romero AR, Mukengeshay JN, et al. Integrating Augmented Reality in Spine Surgery: Redefining Precision with New Technologies. Brain Sciences. 2024; 14(7):645. https://doi.org/10.3390/brainsci14070645
Chicago/Turabian StyleDe Jesus Encarnacion Ramirez, Manuel, Gennady Chmutin, Renat Nurmukhametov, Gervith Reyes Soto, Siddarth Kannan, Gennadi Piavchenko, Vladmir Nikolenko, Ibrahim E. Efe, Alberto Ramírez Romero, Jeff Ntalaja Mukengeshay, and et al. 2024. "Integrating Augmented Reality in Spine Surgery: Redefining Precision with New Technologies" Brain Sciences 14, no. 7: 645. https://doi.org/10.3390/brainsci14070645
APA StyleDe Jesus Encarnacion Ramirez, M., Chmutin, G., Nurmukhametov, R., Soto, G. R., Kannan, S., Piavchenko, G., Nikolenko, V., Efe, I. E., Romero, A. R., Mukengeshay, J. N., Simfukwe, K., Mpoyi Cherubin, T., Nicolosi, F., Sharif, S., Roa, J. C., & Montemurro, N. (2024). Integrating Augmented Reality in Spine Surgery: Redefining Precision with New Technologies. Brain Sciences, 14(7), 645. https://doi.org/10.3390/brainsci14070645










