Short-Term Therapeutic Effect of Repetitive Transcranial Magnetic Stimulations of Sleep Disorders in Parkinson’s Disease: A Randomized Clinical Trial (Pilot Study)
Abstract
:1. Introduction
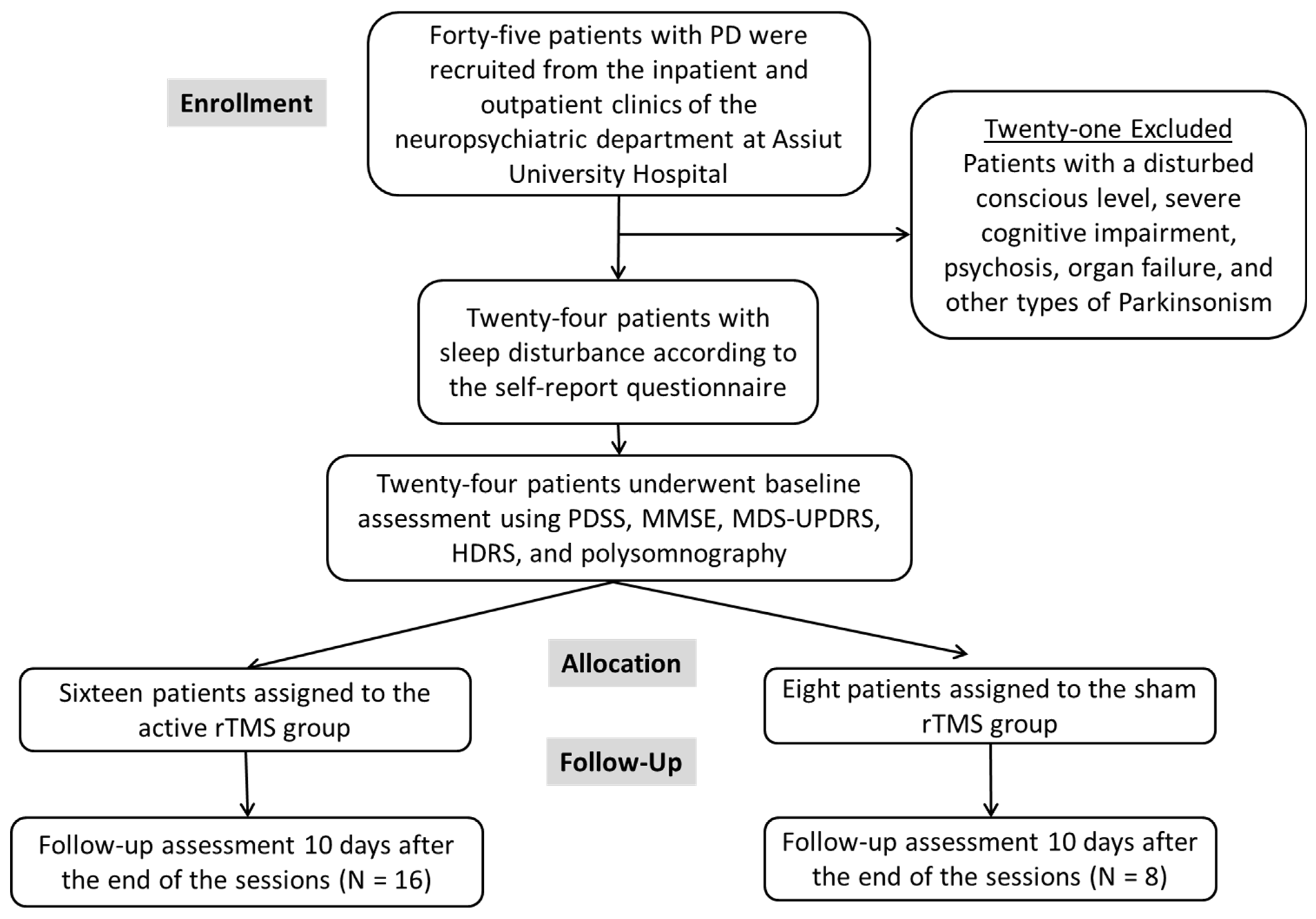
2. Materials and Methods
2.1. Sample Size Calculation
2.2. Methodology
2.2.1. Clinical Assessment
Beck Depression Inventory-II (BDI-II) [30]
2.2.2. Polysomnography
2.3. Randomization (Parallel Design)
2.4. Repetitive Transcranial Magnetic Stimulation Procedure
2.5. Follow Up
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khedr, E.M.; Al Attar, G.S.; Kandil, M.R.; Kamel, N.F.; Abo Elfetoh, N.; Ahmed, M.A. Epidemiological study and clinical profile of Parkinson’s disease in the Assiut Governorate, Egypt: A community-based study. Neuroepidemiology 2012, 38, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Fawi, G.; Abbas, M.A.; Mohammed, T.A.; El-Fetoh, N.A.; Attar, G.A.; Zaki, A.F. Prevalence of Parkinsonism and Parkinson’s disease in Qena governorate/Egypt: A cross-sectional community-based survey. Neurol. Res. 2015, 37, 607–618. [Google Scholar] [CrossRef]
- Martinez-Martin, P. The importance of non-motor disturbances to quality of life in Parkinson’s disease. J. Neurol. Sci. 2011, 310, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; El Fetoh, N.A.; Khalifa, H.; Ahmed, M.A.; El Beh, K.M.A. Prevalence of non motor features in a cohort of Parkinson’s disease patients. Clin. Neurol. Neurosurg. 2013, 115, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, W.G.; de Weerd, A.W. The prevalence of sleep disorders in patients with Parkinson’s disease. A self-reported, community-based survey. Sleep Med. 2002, 3, 147–149. [Google Scholar] [CrossRef]
- Chaudhuri, K.R. Nocturnal symptom complex in PD and its management. Neurology 2003, 61, S17–S23. [Google Scholar] [CrossRef]
- Barone, P.; Amboni, M.; Vitale, C.; Bonavita, V. Treatment of nocturnal disturbances and excessive daytime sleepiness in Parkinson’s disease. Neurology 2004, 63, S35–S38. [Google Scholar] [CrossRef] [PubMed]
- Politis, M.; Piccini, P.; Pavese, N.; Koh, S.B.; Brooks, D.J. Evidence of dopamine dysfunction in the hypothalamus of patients with Parkinson’s disease: An in vivo 11C-raclopride PET study. Exp. Neurol. 2008, 214, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Pascual-Leone, A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003, 2, 145–156. [Google Scholar] [CrossRef]
- Fox, M.D.; Halko, M.A.; Eldaief, M.C.; Pascual-Leone, A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS). Neuroimage 2012, 62, 2232–2243. [Google Scholar] [CrossRef]
- Gratton, C.; Lee, T.G.; Nomura, E.M.; D’Esposito, M. The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Front. Syst. Neurosci. 2013, 7, 124. [Google Scholar] [CrossRef]
- Fierro, B.; Brighina, F.; D’Amelio, M.; Daniele, O.; Lupo, I.; Ragonese, P.; Palermo, A.; Savettieri, G. Motor intracortical inhibition in PD: L-DOPA modulation of high-frequency rTMS effects. Exp. Brain Res. 2008, 184, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Graf, T.; Engeler, J.; Achermann, P.; Mosimann, U.P.; Noss, R.; Fisch, H.U.; Schlaepfer, T.E. High frequency repetitive transcranial magnetic stimulation (rTMS) of the left dorsolateral cortex: EEG topography during waking and subsequent sleep. Psychiatry Res. 2001, 107, 1–9. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Bianchi, M.T.; Zhan, S.; Jiang, F.; Li, N.; Ding, Y.; Hou, Y.; Wang, L.; Ouyang, Q.; et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: A randomized, double-blind, sham-controlled pilot study. Brain Stimul. 2018, 11, 1103–1109. [Google Scholar] [CrossRef]
- Rosenquist, P.B.; McCall, W.V. Does rTMS treat insomnia? Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 2019, 12, 809. [Google Scholar] [CrossRef]
- Jiang, C.-G.; Zhang, T.; Yue, F.-G.; Yi, M.-L.; Gao, D. Efficacy of Repetitive Transcranial Magnetic Stimulation in the Treatment of Patients with Chronic Primary Insomnia. Cell. Biochem. Biophys. 2013, 67, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Fisicaro, F.; Cantone, M.; Pennisi, M.; Cosentino, F.I.I.; Lanuzza, B.; Tripodi, M.; Bella, R.; Paulus, W.; Ferri, R. Repetitive transcranial magnetic stimulation in primary sleep disorders. Sleep Med. Rev. 2023, 67, 101735. [Google Scholar] [CrossRef]
- van Dijk, K.D.; Møst, E.I.; Van Someren, E.J.; Berendse, H.W.; van der Werf, Y.D. Beneficial effect of transcranial magnetic stimulation on sleep in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2009, 24, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Antczak, J.; Rakowicz, M.; Banach, M.; Derejko, M.; Sienkiewicz, J.; Zalewska, U.; Więcławska, M.; Jernajczyk, W. The Influence of Repetitive Transcranial Magnetic Stimulation on Sleep in Parkinson’s Disease. Biocybern. Biomed. Eng. 2011, 31, 35–46. [Google Scholar] [CrossRef]
- Arias, P.; Vivas, J.; Grieve, K.L.; Cudeiro, J. Double-blind, randomized, placebo controlled trial on the effect of 10 days low-frequency rTMS over the vertex on sleep in Parkinson’s disease. Sleep Med. 2010, 11, 759–765. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, S.; Wu, J.; Wang, L.; Mao, C.; Chen, J.; Liu, C.F. Effects of repetitive transcranial magnetic stimulation over right dorsolateral prefrontal cortex on excessive daytime sleepiness in patients with Parkinson’s disease. Sleep Med. 2022, 100, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, H.A.; Gomaa, M.; Maarouf, M.M.; Daker, L.I. Exploring the effect of transcranial magnetic stimulation on quality of sleep in Parkinson’s disease. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 173. [Google Scholar] [CrossRef]
- Massimini, M.; Ferrarelli, F.; Huber, R.; Esser, S.K.; Singh, H.; Tononi, G. Breakdown of cortical effective connectivity during sleep. Science 2005, 309, 2228–2232. [Google Scholar] [CrossRef] [PubMed]
- Steriade, M.; McCarley, R.W. Brain Control of Wakefulness and Sleep; Springer Science & Business Media: Boston, MA, USA, 2005. [Google Scholar]
- Chaudhuri, K.R.; Martinez-Martin, P.; Brown, R.G.; Sethi, K.; Stocchi, F.; Odin, P.; Ondo, W.; Abe, K.; Macphee, G.; Macmahon, D.; et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot study. Mov. Disord. 2007, 22, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Moulin, M.; Lewis, E.D.; Crowley, D.C.; Langston, J.; Evans, M. A randomized, double-blind, placebo-controlled, cross-over pilot study to investigate the efficacy of Rest-ZZZ formula in healthy participants with occasional sleeplessness. Sleep Biol. Rhythm. 2023, 21, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Trenkwalder, C.; Kohnen, R.; Högl, B.; Metta, V.; Sixel-Döring, F.; Frauscher, B.; Hülsmann, J.; Martinez-Martin, P.; Chaudhuri, K.R. Parkinson’s disease sleep scale—Validation of the revised version PDSS-2. Mov. Disord. 2011, 26, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Bellaj, T.; Jemaa, S.B.; Romdhane, N.A.; Dhiffallah, M.; Ali, N.B.; Bouaziz, M.; Mrabet, A. Version arabe du Mini Mental State Examination (A-MMSE): Fidélité, validité et données normatives. Tunis Med. 2008, 86, 768–776. [Google Scholar]
- Khalil, H.; Aldaajani, Z.F.; Aldughmi, M.; Al-Sharman, A.; Mohammad, T.; Mehanna, R.; El-Jaafary, S.I.; Dahshan, A.; Ben Djebara, M.; Kamel, W.A.; et al. Validation of the Arabic Version of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale. Mov. Disord. 2022, 37, 826–841. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G.K. BDI-II: Beck Depression Inventory Manual, 2nd ed.; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Al-Musawi, N.M. Psychometric Properties of the Beck Depression Inventory–II With University Students in Bahrain. J. Personal. Assess. 2001, 77, 568–579. [Google Scholar] [CrossRef]
- Wang, Y.P.; Gorenstein, C. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Braz. J. Psychiatry 2013, 35, 416–431. [Google Scholar] [CrossRef]
- Schecklmann, M.; Schmaußer, M.; Klinger, F.; Kreuzer, P.M.; Krenkel, L.; Langguth, B. Resting motor threshold and magnetic field output of the figure-of-8 and the double-cone coil. Sci. Rep. 2020, 10, 1644. [Google Scholar] [CrossRef] [PubMed]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Mariappan, S.; Rashmi, S.; Choeisoongnern, T.; Sittiprapaporn, P.; Chaiyasut, C. Neurological Insights into Sleep Disorders in Parkinson’s Disease. Brain Sci. 2023, 13, 1202. [Google Scholar] [CrossRef] [PubMed]
- Schütz, L.; Sixel-Döring, F.; Hermann, W. Management of Sleep Disturbances in Parkinson’s Disease. J. Park. Dis. 2022, 12, 2029–2058. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; Vaterrodt, T.; Zaunbrecher, L.; Liepelt-Scarfone, I.; Emmert, K.; Roeben, B.; Elshehabi, M.; Hansen, C.; Becker, S.; Nussbaum, S.; et al. Effects of Levodopa on quality of sleep and nocturnal movements in Parkinson’s Disease. J. Neurol. 2021, 268, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.P.; Nombela, C.; Vuono, R.; Jones, P.S.; Fisher, K.; Burn, D.J.; Brooks, D.J.; Reddy, A.B.; Rowe, J.B.; Barker, R.A. Hypothalamic volume loss is associated with reduced melatonin output in Parkinson’s disease. Mov. Disord. 2016, 31, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Krone, L.B.; Fehér, K.D.; Rivero, T.; Omlin, X. Brain stimulation techniques as novel treatment options for insomnia: A systematic review. J. Sleep Res. 2023, 32, e13927. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Farweez, H.M.; Islam, H. Therapeutic effect of repetitive transcranial magnetic stimulation on motor function in Parkinson’s disease patients. Eur. J. Neurol. 2003, 10, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Rothwell, J.C.; Shawky, O.A.; Ahmed, M.A.; Hamdy, A. Effect of daily repetitive transcranial magnetic stimulation on motor performance in Parkinson’s disease. Mov. Disord. 2006, 21, 2201–2205. [Google Scholar] [CrossRef]
- Khedr, E.M.; Al-Fawal, B.; Abdel Wraith, A.; Saber, M.; Hasan, A.M.; Bassiony, A.; Nasr Eldein, A.; Rothwell, J.C. The Effect of 20 Hz versus 1 Hz Repetitive Transcranial Magnetic Stimulation on Motor Dysfunction in Parkinson’s Disease: Which Is More Beneficial? J. Park. Dis. 2019, 9, 379–387. [Google Scholar] [CrossRef]
- Cattaneo, L.; Giampiccolo, D.; Meneghelli, P.; Tramontano, V.; Sala, F. Cortico-cortical connectivity between the superior and inferior parietal lobules and the motor cortex assessed by intraoperative dual cortical stimulation. Brain Stimul. 2020, 13, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Lichstein, K.L.; Stone, K.C.; Donaldson, J.; Nau, S.D.; Soeffing, J.P.; Murray, D.; Lester, K.W.; Aguillard, R.N. Actigraphy validation with insomnia. Sleep 2006, 29, 232–239. [Google Scholar] [PubMed]
- Park, E.J.; Lee, S.J.; Koh, D.Y.; Han, Y.M. Repetitive transcranial magnetic stimulation to treat depression and insomnia with chronic low back pain. Korean J. Pain 2014, 27, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Kay, D.B.; Tanner, J.J.; Bowers, D. Sleep disturbances and depression severity in patients with Parkinson’s disease. Brain Behav. 2018, 8, e00967. [Google Scholar] [CrossRef] [PubMed]
- Michael, P.; Constantinou Juhasz, S.B.; Evagorou, O.; Psalta, L.; Mikellides, G. High-frequency rTMS improves quality of life and depressive symptoms in Parkinson’s disease: A case report. Heliyon 2022, 8, e12196. [Google Scholar] [CrossRef] [PubMed]
- Antczak, J.M.; Poleszczyk, A.; Wichniak, A.; Rakowicz, M.; Parnowski, T.J. The influence of the repetitive transcranial magnetic stimulation on sleep quality in depression. Psychiatr. Pol. 2017, 51, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.; Ludowig, E.; Staresina, B.P.; Wagner, T.; Kranz, T.; Elger, C.E.; Axmacher, N. Medial temporal theta/alpha power enhancement precedes successful memory encoding: Evidence based on intracranial EEG. J. Neurosci. 2011, 31, 5392–5397. [Google Scholar] [CrossRef]
- Strafella, A.P.; Paus, T.; Fraraccio, M.; Dagher, A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain 2003, 126, 2609–2615. [Google Scholar] [CrossRef]


| Variables | Sham Group (n = 8) | Active Group (n = 16) | Z or x2 | p-Value |
|---|---|---|---|---|
| Age (mean ± SD) Median (IQR) | 60.21 ± 1.64 59(3) | 61.82 ± 3.48 62(5) | −1.927 | 0.053 |
| Gender | ||||
| Males, number (%) | 6(75%) | 7(43.8%) | 2.098 | 0.211 |
| Females | 2(25%) | 9(56.3%) | ||
| Duration of illness (mean ± SD) Median (IQR) | 5.87 ± 4.08 4.5(6.5) | 7.12 ± 3.48 9(6.5) | −0.774 | 0.439 |
| BMI (kg/m2) Median (IQR) | 25.69 ± 3.57 26.15(6.18) | 26.95 ± 5.2 26.15(6.74) | - | - |
| MDS-UPDRS (motor section parts II and III) MDS-UPDRS (mean ± SD) | 60.37 ± 33.41 | 66.31 ± 23.41 | −0.532 | 0.601 |
| Beck Depression Rating Scale (HDRS) (mean ± SD) | 15.42 ± 6.57 | 14.87 ± 6.35 | −0.034 | 0.973 |
| Parkinson’s Disease Sleep Scale (PDSS) (mean ± SD) | 14.14 ± 2.19 | 14.12 ± 2.55 | −0.031 | 0.975 |
| Baseline Assessment | Follow-Up 10 Days after Sessions [% of Improvement] | Wilcoxon Measure Analysis | Effect Size | Friedman Measure Analysis Time × Group Interaction | |
|---|---|---|---|---|---|
| Parkinson’s Disease Sleep Scale (PDSS) | |||||
| Sham group (n = 8) Median (IQR) | 14.14 ± 2.19 14(4) | 13.14 ± 3.80 14(4), [1(12.5%)] # | Z = −1.000, p = 0.317 | [0.756] a | p = 0.001 * |
| Real group (n = 16) Median (IQR) | 14.12 ± 2.55 14(3) | 7.62 ± 3.89 6.5(3.75), [13(81.25%)] # | Z = −3.524, p = 0.001 * | [3.724] a | |
| Mann–Whitney at each time of assessment | Z = −0.031, p = 0.975 | Z = −2.818, p = 0.0005 * | |||
| MDS-UPDRS (motor sections II and III) | |||||
| Sham group (n = 8) Median (IQR) | 60.37 ± 33.41 58.5(70) | 56.75 ± 37.64 57(72), [1(12.5%)] # | Z = −1.604, p = 0.109 | [1.377] a | p = 0.001 * |
| Real group (n = 16) Median (IQR) | 66.31 ± 23.41 63.5(42.75) | 45.25 ± 20.86 38.5(27.25), [9(56.25%)] # | Z = −3.521, p = 0.001 * | [3.71] a | |
| Mann–Whitney at each time of assessment | Z = −0.523, p = 0.601 | Z = −0.769, p = 0.442 | |||
| Beck Depression Inventory Scale (BDI) | |||||
| Sham group (n = 8) Median (IQR) | 15.42 ± 6.57 16(9) | 15.28 ± 6.67 16(9), [0(0%)] # | Z = −1.000, p = 0.317 | [0.756] a | p = 0.001 * |
| Real group (n = 16) Median (IQR) | 14.87 ± 6.35 15.5(10.5) | 8.87 ± 5.85 9(9.75), [12(75%)] # | Z = −3.412, p = 0.001 * | [3.269] a | |
| Mann–Whitney at each time of assessment | Z = −0.034, p = 0.973 | Z = −1.848, p = 0.065 | |||
| Baseline Assessment | Follow Up 10 Days after the Sessions [% of Improvement] | Wilcoxon Measure Analysis | Effect Size | Friedman Measure Analysis Time × Group Interaction | |
|---|---|---|---|---|---|
| Sleep Latency (min) [normal sleep latency = normal: <20 min] | |||||
| Sham group (n = 8) Median (IQR) | 29.25 ± 7.92 28.5(17) | 29.12 ± 8.40 28.5(16.5) | Z = −0.106, p = 0.916 | [0.075] a | p = 0.003 * |
| Real group (n = 16) Median (IQR) | 28.87 ± 17.47 25(29.25) | 22.68 ± 15.17 18(21.25) | Z = −3.427, p = 0.001 * | [3.322] a | |
| Mann–Whitney at each time of assessment | Z = −0.400, p = 0.689 | Z = −1.594, p = 0.111 | |||
| Rapid eye movement (REM) latency (min) [REM latency = ≥90 min] | |||||
| Sham group (n = 8) Median (IQR) | 42.39 ± 20.04 45.07(42) | 41.25 ± 21.06 43.5(44) | Z = −1.89, p = 0.059 | [1.796] a | p = 0.007 * |
| Real group (n = 16) | 35.75 ± 21.41 30(40.5) | 51.50 ± 23.76 52(47.75) | Z = 3.519, p = 0.001 * | [3.701] a | |
| Mann–Whitney at each time of assessment | Z = −0.921, p = 0.357 | Z = −1.136, p = 0.256 | |||
| Number of awakenings [normal > five (more than five indicates sleep fragmentation] | |||||
| Sham group (n = 8) Median (IQR) | 5.62 ± 1.40 5.5(2.5) | 6 ± 1.21 5.5(2) | Z = 0.966, p = 0.334 | [0.727] a | p = 0.157 |
| Real group (n = 16) Median (IQR) | 4.93 ± 3.24 4(3.5) | 3.43 ± 2.15 3.5(4) | Z = −2.386, p = 0.017 * | [1.486] a | |
| Mann–Whitney at each time of assessment | Z = −1.399, p = 0.162 | Z = −2.706, p = 0.007 * | |||
| Wake-after-sleep onset index (WASO) [normal WASO = <20–30 min | |||||
| Sham group (n = 8) Median (IQR) | 33.88 ± 8.83 35.5(15) | 36.37 ± 8.26 38(12.75) | Z = −0.677, p = 0.498 | [0.493] a | p = 0.001 * |
| Real group (n = 16) Median (IQR) | 39.5 ± 14.19 40(15) | 22.93 ± 11.79 23(17.25) | Z = −3.442, p = 0.001 * | [3.378] a | |
| Mann–Whitney at each time of assessment | Z = −1.137, p = 0.255 | Z = −0.2.545, p = 0.011 | |||
| Wake % TST [Total Wake Time/Total Sleep Time) × 100 = <10%] | |||||
| Sham group (n = 8) Median (IQR) | 6.85 ± 5.10 6.9(10.4) | 6.53 ± 4.72 6.9(9.4) | Z = −1.46, p = 0.144 | [1.205] a | p = 0.007 * |
| Real group (n = 16) Median (IQR) | 7 ± 4.49 6.7(9.53) | 3.5 ± 3.57 1.5(7.45) | Z = −2.77, p = 0.006 * | [1.92] a | |
| Mann–Whitney at each time of assessment | Z = −0.123, p = 0.902 | Z = −1.84, p = 0.065 | |||
| N1 % TST [(normal stage 1 sleep = 5%] non-REM sleep | |||||
| Sham group (n = 8) Median (IQR) | 1.51 ± 0.72 1.6(0.6) | 1.11 ± 0.75 1.25(1.5) | Z = −2.207, p = 0.027 * | [2.495] a | p = 0.001 * |
| Real group (n = 16) Median (IQR) | 2.56 ± 1.60 2.15(2.2) | 1.51 ± 1.11 1.15(1.4) | Z = −3.18, p = 0.001 * | [2.621] a | |
| Mann–Whitney at each time of assessment | Z = −1.933, p = 0.053 | Z = −0.553, p = 0.58 | |||
| N2%TST [normal stage 2 sleep = 50%] non-REM sleep | |||||
| Sham group (n = 8) Median (IQR) | 67.78 ± 6.89 66.65(11.72) | 66.77 ± 5.63 65(9.35) | Z = −1.524, p = 0.128 * | [1.279] a | p = 0.088 |
| Real group (n = 16) Median (IQR) | 64.08 ± 10.67 65.05(14.72) | 60.10 ± 9.44 62.25(13.75) | Z = −2.13, p = 0.033 * | [1.258] a | |
| Mann–Whitney at each time of assessment | Z = −0.796, p = 0.426 | Z = −1.871, p = 0.061 | |||
| N3%TST [stage N3 sleep is considered “deep sleep” or slow wave sleep normal = 20%] non-REM sleep | |||||
| Sham group (n = 8) Median (IQR) | 15.97 ± 6.40 15.9(6.13) | 15.7 ± 6.55 15(7.82) | Z = −0.631, p = 0.528 | [0.458] a | p = 0.827 |
| Real group (n = 16) Median (IQR) | 18.49 ± 10.46 18.7(16.58) | 18.62 ± 9.66 17.5(15.5) | Z = −0.085, p = 0.932 | [0.043] a | |
| Mann–Whitney at each time of assessment | Z = −0.368, p = 0.713 | Z = −0.705, p = 0.481 | |||
| REM %TST [REM %TST = normal 25%] | |||||
| Sham group (n = 8) Median (IQR) | 18.36 ± 8.1 17.5(11.5) | 17.12 ± 8.44 15.5(15.5) | Z = −1.43, p = 0.15 | [1.172] a | p = 0.088 |
| Real group (n = 16) Median (IQR) | 14.66 ± 6.78 16.6(9.78) | 16.68 ± 6.72 18.5(8.25) | Z = −2.72, p = 0.006 * | [1.855] a | |
| Mann–Whitney at each time of assessment | Z = −0.857, p = 0.391 | Z = −0.154, p = 0.878 | |||
| Baseline Assessment | Follow Up 10 Days after Sessions [% of Improvement] | Wilcoxon Measure Analysis | Effect Size | Friedman Measure Analysis Time × Group Interaction | |
|---|---|---|---|---|---|
| Desaturation index [normal ODI < 5 events/hour] | |||||
| Sham group (n = 8) Median (IQR) | 4.94 ± 3.58 5.7(7.3) | 5.4 ± 3.94 5.7(9.4) | Z = −1.34, p = 0.18 | [1.076] a | p = 0.225 |
| Real group (n = 16) Median (IQR) | 6.67 ± 4.84 5.9(9.1) | 4.89 ± 3.12 5.7(5.57) | Z = −1.534, p = 0.125 | [0.83] a | |
| Mann–Whitney at each time of assessment | Z = −0.637, p = 0.524 | Z = −0.062, p = 0.951 | |||
| Average oxygen saturation SpO2 | |||||
| Sham group (n = 8) Median (IQR) | 93.43 ± 1.81 94(3) | 91.85 ± 3.33 92(3) | Z = −1, p = 0.317 | [0.756] a | p = 0.405 |
| Real group (n = 16) Median (IQR) | 92.93 ± 2.58 94(2) | 94.21 ± 2.11 94.5(3.5) | Z = −0.998, p = 0.318 | [0.515] a | |
| Mann–Whitney at each time of assessment | Z = −0.032, p = 0.975 | Z = −1.802, p = 0.071 | |||
| Arousal index (/h) (normal: <five events/hour) | |||||
| Sham group (n = 8) Median (IQR) | 12.74 ± 14.24 5(16.7) | 12.23 ± 12.5 6(13) | Z = −0.105, p = 0.917 | [0.074] a | p = 0.033 * |
| Real group (n = 16) Median (IQR) | 9.95 ± 7.11 9.9(10.63) | 8.11 ± 6.51 6.7(11) | Z = −2.94, p = 0.003 * | [2.168] a | |
| Mann–Whitney at each time of assessment | Z = −0.429, p = 0.668 | Z = −0.460, p = 0.645 | |||
| Periodic leg movement index (PLM index) (/h) [normal = PLM index = <five events/hour] | |||||
| Sham group (n = 8) Median (IQR) | 8.42 ± 8.34 4.38(14.95) | 7.87 ± 8.14 3(16) | Z = −1.214, p = 0.225 | [0.95] a | p = 0.001 * |
| Real group (n = 16) Median (IQR) | 6.75 ± 6.43 4.39(9.5) | 2.08 ± 3.09 1.56(2.26) | Z = −2.97, p = 0.003* | [2.217] a | |
| Mann–Whitney at each time of assessment | Z = −0.553, p = 0.580 | Z = −2.112, p = 0.035 * | |||
| Apnea, hypopnea index per hour AHI/h [normal: AHI < five events/hour] | |||||
| Sham group (n = 8) Median (IQR) | 8.24 ± 4.34 5.13(7.4) | 9.23 ± 5.63 5.13(11.23) | Z = −1.604, p = 0.109 | [1.377] a | p = 0.134 |
| Real group (n = 16) Median (IQR) | 8.09 ± 5.36 8.84(9.8) | 4.01 ± 1.95 4.77(1.8) | Z = −2.553, p = 0.011 * | [1.658] a | |
| Mann–Whitney at each time of assessment | Z = −0.226, p = 0.821 | Z = −2.34, p = 0.019 * | |||
| Variables | PDSS | UPDRS Part III | Beck Depression Rating Scale (HDRS) | |
|---|---|---|---|---|
| Number of awakenings | r | 0.272 | 0.272 | −0.054 |
| p-value | 0.198 | 0.198 | 0.801 | |
| Wake-after-sleep onset index | r | 0.355 | 0.605 | −0.421 |
| p-value | 0.088 | 0.002 * | 0.041 * | |
| Wake % TST | r | 0.371 | 0.406 | −0.148 |
| p-value | 0.074 | 0.049 * | 0.490 | |
| Sleep latency (min) | r | 0.466 * | 0.639 | −0.333 |
| p-value | 0.022 * | 0.001 * | 0.111 | |
| REM latency (min) | r | −0.764 | −0.706 | 0.221 |
| p-value | <0.0001 * | <0.0001 * | 0.299 | |
| N1 % TST (stage 1 non-REM) | r | 0.377 | 0.552 | −0.192 |
| p-value | 0.070 | 0.005 * | 0.368 | |
| N2 % TST (stage 2 non-REM) | r | 0.006 | 0.079 | 0.105 |
| p-value | 0.978 | 0.713 | 0.624 | |
| N3 % TST (stage 3 deep sleep) | r | −0.101 | −0.136 | 0.080 |
| p-value | 0.638 | 0.527 | 0.712 | |
| REM % TST | r | −0.333 | −0.682 | 0.560 |
| p-value | 0.112 | <0.0001 * | 0.004 * | |
| PLMs index (/h) | r | 0.276 | 0.274 | −0.083 |
| p-value | 0.192 | 0.195 | 0.701 | |
| Arousal index (/h) | r | 0.159 | 0.261 | −0.357 |
| p-value | 0.457 | 0.217 | 0.087 | |
| AHI/h | r | 0.261 | 0.488 | −0.207 |
| p-value | 0.230 | 0.018 * | 0.342 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khedr, E.M.; Ahmed, G.K.; Korayem, M.A.; Elamary, S.A.S.H.; El-kholy, M.M.; Haridy, N.A. Short-Term Therapeutic Effect of Repetitive Transcranial Magnetic Stimulations of Sleep Disorders in Parkinson’s Disease: A Randomized Clinical Trial (Pilot Study). Brain Sci. 2024, 14, 556. https://doi.org/10.3390/brainsci14060556
Khedr EM, Ahmed GK, Korayem MA, Elamary SASH, El-kholy MM, Haridy NA. Short-Term Therapeutic Effect of Repetitive Transcranial Magnetic Stimulations of Sleep Disorders in Parkinson’s Disease: A Randomized Clinical Trial (Pilot Study). Brain Sciences. 2024; 14(6):556. https://doi.org/10.3390/brainsci14060556
Chicago/Turabian StyleKhedr, Eman M., Gellan K. Ahmed, Mohammad Ahmad Korayem, Sara Ahmed Salah Hussain Elamary, Maha M. El-kholy, and Nourelhoda A. Haridy. 2024. "Short-Term Therapeutic Effect of Repetitive Transcranial Magnetic Stimulations of Sleep Disorders in Parkinson’s Disease: A Randomized Clinical Trial (Pilot Study)" Brain Sciences 14, no. 6: 556. https://doi.org/10.3390/brainsci14060556
APA StyleKhedr, E. M., Ahmed, G. K., Korayem, M. A., Elamary, S. A. S. H., El-kholy, M. M., & Haridy, N. A. (2024). Short-Term Therapeutic Effect of Repetitive Transcranial Magnetic Stimulations of Sleep Disorders in Parkinson’s Disease: A Randomized Clinical Trial (Pilot Study). Brain Sciences, 14(6), 556. https://doi.org/10.3390/brainsci14060556






