Age-Related Aspects of Sex Differences in Event-Related Brain Oscillatory Responses: A Turkish Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Paradigm
2.3. EEG Recording and Data Processing
2.4. Statistical Analysis
3. Results
3.1. Delta Frequency Band Results
3.2. Theta Frequency Band Results
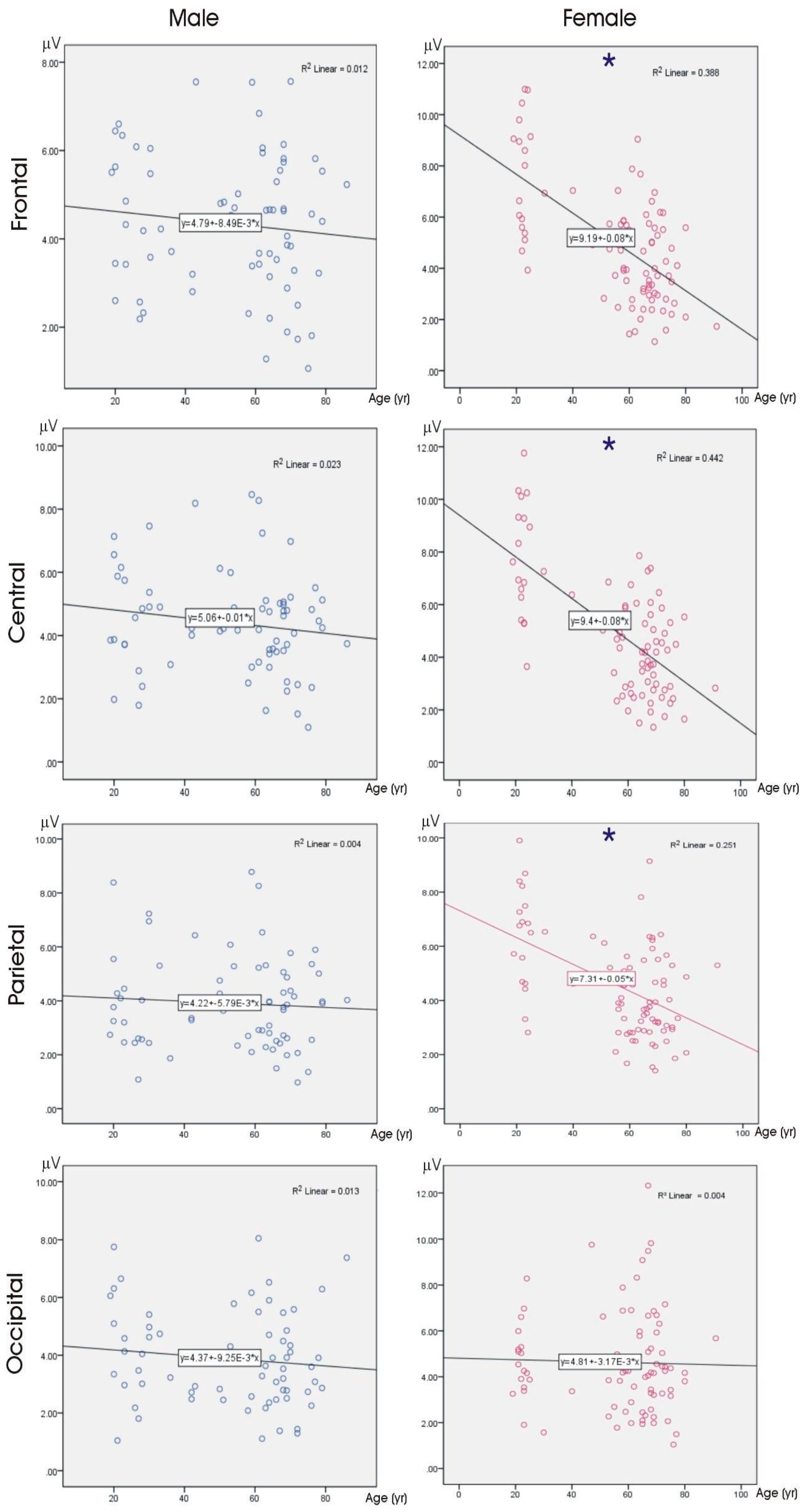
3.3. Correlations
3.3.1. Age
3.3.2. Cognitive Functions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zelco, A.; Wapeesittipan, P.; Joshi, A. Insights into Sex and Gender Differences in Brain and Psychopathologies Using Big Data. Life 2023, 13, 1676. [Google Scholar] [CrossRef] [PubMed]
- Harkin, T.; Snowe, O.J.; Mikulski, B.A.; Waxman, H.A. Drug Safety: Most Drugs Withdrawn in Recent Years Had Greater Health Risks for Women; United States General Accounting Office: Washington, DC, USA, 2001. [Google Scholar]
- Spets, D.S.; Slotnick, S.D. Are there sex differences in brain activity during long-term memory? A systematic review and fMRI activation likelihood estimation meta-analysis. Cogn. Neurosci. 2021, 12, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Malpetti, M.; Ballarini, T.; Presotto, L.; Garibotto, V.; Tettamanti, M.; Perani, D. Alzheimer’s Disease Neuroimaging Initiative (ADNI) Database Network for Efficiency and Standardization of Dementia Diagnosis (NEST-DD) database. Gender differences in healthy aging and Alzheimer’s Dementia: A 18 F-FDG-PET study of brain and cognitive reserve. Hum. Brain Mapp. 2017, 38, 4212–4227. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.S.; Vlassenko, A.G.; Raichle, M.E. Reply to Biskup et al. and Tu et al.: Sex differences in metabolic brain aging. Proc. Natl. Acad. Sci. USA 2019, 116, 10634–10635. [Google Scholar] [CrossRef] [PubMed]
- Perera, G.; Pedersen, L.; Ansel, D.; Alexander, M.; Arrighi, H.M.; Avillach, P.; Foskett, N.; Gini, R.; Gordon, M.F.; Gungabissoon, U.; et al. Dementia prevalence and incidence in a federation of European Electronic Health Record databases: The European Medical Informatics Framework resource. Alzheimers Dement. 2018, 14, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Koran, M.E.I.; Wagener, M.; Hohman, T.J. Alzheimer’s Neuroimaging Initiative. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 2017, 11, 205–213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, R.; Singh, M. Sex differences in cognitive impairment and Alzheimer’s disease. Front. Neuroendocrinol. 2014, 35, 385–403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Babiloni, C.; Triggiani, A.I.; Lizio, R.; Cordone, S.; Tattoli, G.; Bevilacqua, V.; Soricelli, A.; Ferri, R.; Nobili, F.; Gesualdo, L.; et al. Classification of Single Normal and Alzheimer’s Disease Individuals from Cortical Sources of Resting State EEG Rhythms. Front. Neurosci. 2016, 10, 47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maestú, F.; Cuesta, P.; Hasan, O.; Fernandéz, A.; Funke, M.; Schulz, P.E. The Importance of the Validation of M/EEG With Current Biomarkers in Alzheimer’s Disease. Front. Hum. Neurosci. 2019, 13, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Başar-Eroglu, C.; Basar, E.; Demiralp, T.; Schürmann, M. P300-response: Possible psychophysiological correlates in delta and theta frequency channels. A review. Int. J. Psychophysiol. 1992, 13, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Başar, E.; Karakaş, S. Event-Related Oscillations in the Brain. In Brain Function and Oscillations; Springer Series in Synergetics; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar] [CrossRef]
- Başar-Eroglu, C.; Demiralp, T. Event-related theta oscillations: An integrative and comparative approach in the human and animal brain. Int. J. Psychophysiol. 2001, 39, 167–195. [Google Scholar] [CrossRef] [PubMed]
- Yener, G.G.; Fide, E.; Özbek, Y.; Emek-Savaş, D.D.; Aktürk, T.; Çakmur, R.; Güntekin, B. The difference of mild cognitive impairment in Parkinson’s disease from amnestic mild cognitive impairment: Deeper power decrement and no phase-locking in visual event-related responses. Int. J. Psychophysiol. 2019, 139, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Aktürk, T.; de Graaf, T.A.; Erdal, F.; Sack, A.T.; Güntekin, B. Oscillatory delta and theta frequencies differentially support multiple items encoding to optimize memory performance during the digit span task. Neuroimage 2022, 263, 119650. [Google Scholar] [CrossRef] [PubMed]
- Güntekin, B.; Başar, E. Brain oscillations are highly influenced by gender differences. Int. J. Psychophysiol. 2007, 65, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Yerlikaya, D.; Hünerli-Gündüz, D.; Fide, E.; Özbek, Y.; Kıyı, İ.; Öztura, İ.; Yener, G.G. The reliability of P300 and the influence of age; gender and education variables in a 50 years and older normative sample. Int. J. Psychophysiol. 2022, 181, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Blinowska, K.; Bonanni, L.; Cichocki, A.; De Haan, W.; Del Percio, C.; Dubois, B.; Escudero, J.; Fernández, A.; Frisoni, G.; et al. What electrophysiology tells us about Alzheimer’s disease: A window into the synchronization and connectivity of brain neurons. Neurobiol. Aging. 2020, 85, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Lopez, S.; Noce, G.; Ferri, R.; Panerai, S.; Catania, V.; Soricelli, A.; Salvatore, M.; Nobili, F.; Arnaldi, D.; et al. Resting State Alpha Electroencephalographic Rhythms Are Affected by Sex in Cognitively Unimpaired Seniors and Patients with Alzheimer’s Disease and Amnesic Mild Cognitive Impairment: A Retrospective and Exploratory Study. Cereb. Cortex. 2022, 32, 2197–2215. [Google Scholar] [CrossRef] [PubMed]
- Yener, G.G.; Güntekin, B.; Örken, D.N.; Tülay, E.; Forta, H.; Başar, E. Auditory delta event-related oscillatory responses are decreased in Alzheimer’s disease. Behav. Neurol. 2012, 25, 3–11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yener, G.G.; Emek-Savaş, D.D.; Lizio, R.; Çavuşoğlu, B.; Carducci, F.; Ada, E.; Güntekin, B.; Babiloni, C.C.; Başar, E. Frontal delta event-related oscillations relate to frontal volume in mild cognitive impairment and healthy controls. Int. J. Psychophysiol. 2016, 103, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Polich, J.; Kok, A. Cognitive and biological determinants of P300: An integrative review. Biol. Psychol. 1995, 41, 103–146. [Google Scholar] [CrossRef] [PubMed]
- Başar, E.; Başar-Eroğlu, C.; Güntekin, B.; Yener, G.G. Brain’s alpha, beta, gamma, delta, and theta oscillations in neuropsychiatric diseases: Proposal for biomarker strategies. Suppl. Clin. Neurophysiol. 2013, 62, 19–54. [Google Scholar] [CrossRef] [PubMed]
- Güntekin, B.; O’Donnell, B.F. Special Issue: Update on Neural Oscillations in Neuropsychiatric Disorders. Clin. EEG Neurosci. 2023, 54, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Yener, G.G.; Emek-Savaş, D.D.; Güntekin, B.; Başar, E. The visual cognitive network, but not the visual sensory network, is affected in amnestic mild cognitive impairment: A study of brain oscillatory responses. Brain Res. 2014, 1585, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Jakhar, D.; Tucci, F.; Del Percio, C.; Lopez, S.; Soricelli, A.; Salvatore, M.; Ferri, R.; Catania, V.; Massa, F.; et al. Resting state electroencephalographic alpha rhythms are sensitive to Alzheimer’s disease mild cognitive impairment progression at a 6-month follow-up. Neurobiol. Aging. 2024, 137, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Hünerli, D.; Emek-Savaş, D.D.; Çavuşoğlu, B.; Dönmez Çolakoğlu, B.; Ada, E.; Yener, G.G. Mild cognitive impairment in Parkinson’s disease is associated with decreased P300 amplitude and reduced putamen volume. Clin. Neurophysiol. 2019, 130, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Demiralp, T.; Ademoglu, A.; Istefanopulos, Y.; Başar-Eroglu, C.; Başar, E. Wavelet analysis of oddball P300. Int. J. Psychophysiol. 2001, 39, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Yener, G.; Hünerli-Gündüz, D.; Yıldırım, E.; Aktürk, T.; Başar-Eroğlu, C.; Bonanni, L.; Del Percio, C.; Farina, F.; Ferri, R.; Güntekin, B.; et al. Treatment effects on event-related EEG potentials and oscillations in Alzheimer’s disease. Int. J. Psychophysiol. 2022, 177, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, E.; Hanoğlu, L.; Yener, G.; Yerlikaya, D.; Taylor, J.P.; Schumacher, J.; McKeith, I.; Bonanni, L.; Pantano, P.; Piervincenzi, C.; et al. Relationship between default mode network and resting-state electroencephalographic alpha rhythms in cognitively unimpaired seniors and patients with dementia due to Alzheimer’s disease. Cereb. Cortex. 2023, 33, 10514–10527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Georgiev, S.; Minchev, Z.; Christova, C.; Philipova, D. Gender event-related brain oscillatory differences in normal elderly population EEG. Int. J. Bioautomation 2011, 15, 33. [Google Scholar]
- Li, H.; Ruan, J.; Xie, Z.; Wang, H.; Liu, W. Investigation of the critical geometric characteristics of living human skulls utilising medical image analysis techniques. Int. J. Veh. Saf. 2007, 2, 345–367. [Google Scholar] [CrossRef]
- Langrova, J.; Kremláček, J.; Kuba, M.; Kubova, Z.; Szanyi, J. Gender impact on electrophysiological activity of the brain. Physiol. Res. 2012, 61, S119–S127. [Google Scholar] [CrossRef] [PubMed]
- Chorlian, D.B.; Rangaswamy, M.; Manz, N.; Kamarajan, C.; Pandey, A.K.; Edenberg, H.; Kuperman, S.; Porjesz, B. Gender modulates the development of theta event related oscillations in adolescents and young adults. Behav. Brain Res. 2015, 292, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Duffy, F.H.; McAnulty, G.B.; Albert, M.S. The pattern of age-related differences in electrophysiological activity of healthy males and females. Neurobiol. Aging 1993, 14, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Takizawa, Y.; Jiang, Z.Y.; Yamaguchi, N. Gender differences in quantitative EEG at rest and during photic stimulation in normal young adults. Clin. Electroencephalogr. 1994, 25, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Spagna, A.; Wu, T.; Kim, K.; Fan, J. Supramodal executive control of attention: Evidence from unimodal and crossmodal dual conflict effects. Cortex 2020, 133, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, J.; Kolev, V.; Rosso, O.A.; Schürmann, M.; Sakowitz, O.W.; Ozgören, M.; Basar, E. Wavelet entropy analysis of event-related potentials indicates modality-independent theta dominance. J. Neurosci. Methods. 2002, 117, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, M.; Başar-Eroglu, C.; Kolev, V.; Başar, E. Delta responses and cognitive processing: Single-trial evaluations of human visual P300. Int. J. Psychophysiol. 2001, 39, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Yener, G.G.; Güntekin, B.; Oniz, A.; Başar, E. Increased frontal phase-locking of event-related theta oscillations in Alzheimer patients treated with cholinesterase inhibitors. Int. J. Psychophysiol. 2007, 64, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Güntekin, B.; Aktürk, T.; Arakaki, X.; Bonanni, L.; Del Percio, C.; Edelmayer, R.; Farina, F.; Ferri, R.; Hanoğlu, L.; Kumar, S.; et al. Are there consistent abnormalities in event-related EEG oscillations in patients with Alzheimer’s disease compared to other diseases belonging to dementia? Psychophysiology. 2022, 59, e13934. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, Y.; Shiner, T.; Bregman, N.; Fahoum, F.; Giladi, N.; Maidan, I.; Mirelman, A. Event-related oscillations differentiate between cognitive; motor and visual impairments. J. Neurol. 2022, 269, 3529–3540. [Google Scholar] [CrossRef] [PubMed]
- Tülay, E.E.; Güntekin, B.; Yener, G.; Bayram, A.; Başar-Eroğlu, C.; Demiralp, T. Evoked and induced EEG oscillations to visual targets reveal a differential pattern of change along the spectrum of cognitive decline in Alzheimer’s Disease. Int. J. Psychophysiol. 2020, 155, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hünerli-Gündüz, D.; Özbek İşbitiren, Y.; Uzunlar, H.; Çavuşoğlu, B.; Çolakoğlu, B.D.; Ada, E.; Güntekin, B.; Yener, G.G. Reduced power and phase-locking values were accompanied by thalamus; putamen; and hippocampus atrophy in Parkinson’s disease with mild cognitive impairment: An event-related oscillation study. Neurobiol. Aging. 2023, 121, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Missonnier, P.; Gold, G.; Herrmann, F.R.; Fazio-Costa, L.; Michel, J.P.; Deiber, M.P.; Michon, A.; Giannakopoulos, P. Decreased theta event-related synchronization during working memory activation is associated with progressive mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2006, 22, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, Y.; Maidan, I.; Fahoum, F.; Giladi, N.; Bregman, N.; Shiner, T.; Mirelman, A. Differential changes in visual and auditory event-related oscillations in dementia with Lewy bodies. Clin. Neurophysiol. 2020, 131, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Schmiedt, C.; Meistrowitz, A.; Schwendemann, G.; Herrmann, M.; Basar-Eroglu, C. Theta and alpha oscillations reflect differences in memory strategy and visual discrimination performance in patients with Parkinson’s disease. Neurosci. Lett. 2005, 388, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Başar, E.; Güntekin, B.; Yener, G.; Başar-Eroğlu, C. Mindful brain and EEG-neurophysiology. Int. J. Psychophysiol. 2016, 103, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Harmony, T.; Fernández, T.; Silva, J.; Bernal, J.; Díaz-Comas, L.; Reyes, A.; Marosi, E.; Rodríguez, M.; Rodríguez, M. EEG delta activity: An indicator of attention to internal processing during performance of mental tasks. Int. J. Psychophysiol. 1996, 24, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Global Status Report on the Public Health Response to Dementia; World Health Organization: Geneva, Switzerland, 2021. Available online: https://apps.who.int/iris/handle/10665/344701 (accessed on 25 April 2023).
- Emek-Savaş, D.D.; Özmüş, G.; Güntekin, B.; Dönmez Çolakoğlu, B.; Çakmur, R.; Başar, E.; Yener, G.G. Decrease of Delta Oscillatory Responses in Cognitively Normal Parkinson’s Disease. Clin. EEG Neurosci. 2017, 48, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, A.; Grothe, M.J.; Ashton, N.J.; Karikari, T.K.; Lantero Rodríguez, J.; Snellman, A.; Suárez-Calvet, M.; Blennow, K.; Zetterberg, H.; Schöll, M.; et al. Longitudinal Associations of Blood Phosphorylated Tau181 and Neurofilament Light Chain with Neurodegeneration in Alzheimer Disease. JAMA Neurol. 2021, 78, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Buckley, R.F.; Mormino, E.C.; Amariglio, R.E.; Properzi, M.J.; Rabin, J.S.; Lim, Y.Y.; Papp, K.V.; Jacobs, H.I.L.; Burnham, S.; Hanseeuw, B.J.; et al. Sex, amyloid, and APOE e4 and risk of cognitive decline in preclinical Alzheimer’s disease: Findings from three well-characterized cohorts. Alzheimers Dement. 2018, 14, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.Z.K.; Zhuang, X.; Leavitt, M.J.; Banks, S.J.; Cummings, J.; Cordes, D.; Papp, K.V.; Jacobs, H.I.L.; Burnham, S.; Hanseeuw, B.J.; et al. Sex moderates amyloid and apolipoprotein e4 effects on default mode network connectivity at rest. Front. Neurol. 2019, 10, 900. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.N.; James, S.A.; Mullen, S.P.; Sutton, B.P.; Wszalek, T.; Mulyana, B.; Mukli, P.; Yabluchanskiy, A. Alzheimer’s Disease Neuroimaging Initiative Consortium; Yang, Y. Sex differences in interacting genetic and functional connectivity biomarkers in Alzheimer’s disease. GeroScience 2024. [Google Scholar] [CrossRef] [PubMed]
- Chino-Vilca, B.; Rodríguez-Rojo, I.C.; Torres-Simón, L.; Cuesta, P.; Vendrell, A.C.; Piñol-Ripoll, G.; Huerto, R.; Tahan, N.; Maestú, F. Sex specific EEG signatures associated with cerebrospinal fluid biomarkers in mild cognitive impairment. Clin. Neurophysiol. 2022, 142, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Arakaki, X.; Azami, H.; Bennys, K.; Blinowska, K.; Bonanni, L.; Bujan, A.; Carrillo, M.C.; Cichocki, A.; de Frutos-Lucas, J.; et al. Measures of resting state EEG rhythms for clinical trials in Alzheimer’s disease: Recommendations of an expert panel. Alzheimers Dement. 2021, 17, 1528–1553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maguire, E.A.; Gadian, D.G.; Johnsrude, I.S.; Good, C.D.; Ashburner, J.; Frackowiak, R.S.; Frith, C.D. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. USA 2000, 97, 4398–4403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirnstein, M.; Hausmann, M. Sex/gender differences in the brain are not trivial—A commentary on Eliot et al. (2021). Neurosci. Biobehav. Rev. 2021, 130, 408–409. [Google Scholar] [CrossRef] [PubMed]
- Moradifard, S.; Hoseinbeyki, M.; Ganji, S.M.; Minuchehr, Z. Analysis of microRNA and Gene Expression Profiles in Alzheimer’s Disease: A Meta-Analysis Approach. Sci. Rep. 2018, 8, 4767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avila, J.F.; Vonk, J.M.J.; Verney, S.P.; Witkiewitz, K.; Arce Rentería, M.; Schupf, N.; Mayeux, R.; Manly, J.J. Sex/gender differences in cognitive trajectories vary as a function of race/ethnicity. Alzheimer’s Dement. 2019, 15, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, H.; Baez, S.; Medel, V.; Moguilner, S.; Cuadros, J.; Santamaria-Garcia, H.; Tagliazucchi, E.; Valdes-Sosa, P.A.; Lopera, F.; OchoaGómez, J.F.; et al. Brain health in diverse settings: How age, demographics and cognition shape brain function. Neuroimage 2024, 295, 120636. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Umla-Runge, K.; Hofmann, J.; Ferdinand, N.K.; Chan, R.C. Cultural differences in sensitivity to the relationship between objects and contexts: Evidence from P3. Neuroreport 2014, 25, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Sonke, C.J.; Van Boxtel, G.J.; Griesel, D.R.; Poortinga, Y.H. Brain wave concomitants of cross-cultural differences in scores on simple cognitive tasks. J. Cross-Cult. Psychol. 2008, 39, 37–54. [Google Scholar] [CrossRef]
- Lewis, R.S.; Goto, S.G.; Kong, L.L. Culture and context: East Asian American and European American differences in P3 event-related potentials and self-construal. Personal. Soc. Psychol. Bull. 2008, 34, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Kimenai, D.M.; Janssen, E.B.N.J.; Eggers, K.M.; Lindahl, B.; den Ruijter, H.M.; Bekers, O.; Appelman, Y.; Meex, S.J.R. Sex-Specific versus Overall Clinical Decision Limits for Cardiac Troponin I and T for the Diagnosis of Acute Myocardial Infarction: A Systematic Review. Clin. Chem. 2018, 64, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Prado, P.; Birba, A.; Cruzat, J.; Santamaría-García, H.; Parra, M.; Moguilner, S.; Tagliazucchi, E.; Ibáñez, A. Dementia ConnEEGtome: Towards multicentric harmonization of EEG connectivity in neurodegeneration. Int. J. Psychophysiol. 2022, 172, 24–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lopez, K.L.; Monachino, A.D.; Vincent, K.M.; Peck, F.C.; Gabard-Durnam, L.J. Stability; change; and reliable individual differences in electroencephalography measures: A lifespan perspective on progress and opportunities. Neuroimage 2023, 275, 120116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burgess, A.; Gruzelier, J. Individual reliability of amplitude distribution in topographical mapping of EEG. Electroencephalogr. Clin. Neurophysiol. 1993, 86, 219–223. [Google Scholar] [CrossRef] [PubMed]
| Age Groups | ||||||
|---|---|---|---|---|---|---|
| Total Sample | Gender | 19–50 (n = 45) | 51–65 (n = 48) | 66–86 (n = 62) | p Values | |
| Age (yr) * | 55.01 ± 19.41 | Female | 24.80 ± 6.85 | 59.28 ± 3.88 | 71.32 ± 5.00 | 0.197 |
| Male | 29.32 ± 9.46 | 60.16 ± 4.18 | 71.84 ± 5.07 | |||
| Gender ‡ | 155 (86F/69M) | Female | 20 | 29 | 37 | 0.209 |
| Male | 25 | 19 | 25 | |||
| Education (yr) * | 12.75 ± 4.37 | Female | 14.90 ± 1.71 | 12.45 ± 3.98 | 9.65 ± 5.11 | 0.005 |
| Male | 14.79 ± 1.50 | 12.63 ± 3.13 | 14.08 ± 5.26 | |||
| Handedness ‡ | 149 R/4L/2B | Female | 20R | 29R | 36R/1L | 0.126 |
| Male | 24 R/1L | 17R/2B | 23R/2L | |||
| Epoch Number * | 28.75 ± 7.32 | Female | 26.10 ± 8.22 | 27.86 ± 6.88 | 28.14 ± 7.03 | 0.604 |
| Male | 28.16 ± 6.25 | 32.63 ± 7.38 | 30.48 ± 7.60 | |||
| Behavioral Data * | 39.69 ± 2.20 | Female | 40.40 ± 1.50 | 39.48 ± 2.03 | 39.49 ± 2.91 | 0.124 |
| Male | 39.24 ± 2.50 | 39.79 ± 1.58 | 40.04 ± 1.70 | |||
| MMSE * | 29.22 ± 1.04 | Female | 29.75 ± 0.55 | 28.93 ± 1.28 | 28.86 ± 1.12 | 0.592 |
| Male | 29.70 ± 0.70 | 29.26 ± 0.99 | 29.16 ± 0.94 | |||
| Depression ¥ | 4.89 ± 3.69 | Female | 4.89 ± 3.70 | 9.00 ± 6.23 | 7.36 ± 5.00 | NA |
| 5.54 ± 4.51 | Male | 5.58 ± 4.47 | 4.72 ± 4.40 | 4.20 ± 3.60 | ||
| OVMPT Total * | 121.80 ± 12.88 | Female | 128.33 ± 9.24 | 122.38 ± 10.19 | 118.47 ± 12.45 | 0.467 |
| 120.33 ± 14.79 | Male | 130.25 ± 9.31 | 120.64 ± 13.62 | 113.00 ± 14.70 | ||
| OVMPT IR * | 6.23 ± 1.97 | Female | 7.42 ± 1.83 | 6.42 ± 1.58 | 5.25 ± 1.77 | 0.310 |
| 5.99 ± 1.92 | Male | 7.42 ± 1.83 | 6.29 ± 2.02 | 5.25 ± 1.77 | ||
| OVMPT FR * | 13.62 ± 1.20 | Female | 14.00 ± 1.10 | 13.65 ± 1.20 | 13.30 ± 1.29 | 0.456 |
| 13.22 ± 1.43 | Male | 13.75 ± 1.06 | 13.21 ± 1.31 | 12.81 ± 133 | ||
| OVMPT TR * | 14.99 ± 0.11 | Female | 15 | 15 | 15 | 0.995 |
| 15.00 ± 0.00 | Male | 15 | 15 | 15 | ||
| Stroop * | 48.09 ± 19.84 | Female | 31.17 ± 5.85 | 45.46 ± 14.80 | 53.00 ± 20.49 | 0.326 |
| 43.12 ± 18.65 | Male | 40.50 ± 16.31 | 40.57 ± 12.33 | 49.63 ± 26.46 | ||
| Categorical Fluency * | 22.81 ± 4.95 | Female | 25.00 ± 5.02 | 23.69 ± 5.36 | 22.48 ± 4.67 | 0.419 |
| 24.27 ± 4.71 | Male | 26.42 ± 5.14 | 23.93 ± 4.73 | 24.63 ± 5.18 | ||
| Phonemic Fluency * | 44.46 ± 14.09 | Female | 61.17 ± 9.47 | 40.42 ± 11.38 | 38.30 ± 12.04 | 0.007 |
| 44.80 ± 12.79 | Male | 41.50 ± 11.94 | 48.50 ± 16.18 | 41.00 ± 10.68 | ||
| BNT * | 14.79 ± 0.47 | Female | 15 | 14.96 ± 0.20 | 14.78 ± 0.42 | 0.716 |
| 14.94 ± 0.31 | Male | 15 | 15 | 14.81 ± 0.54 | ||
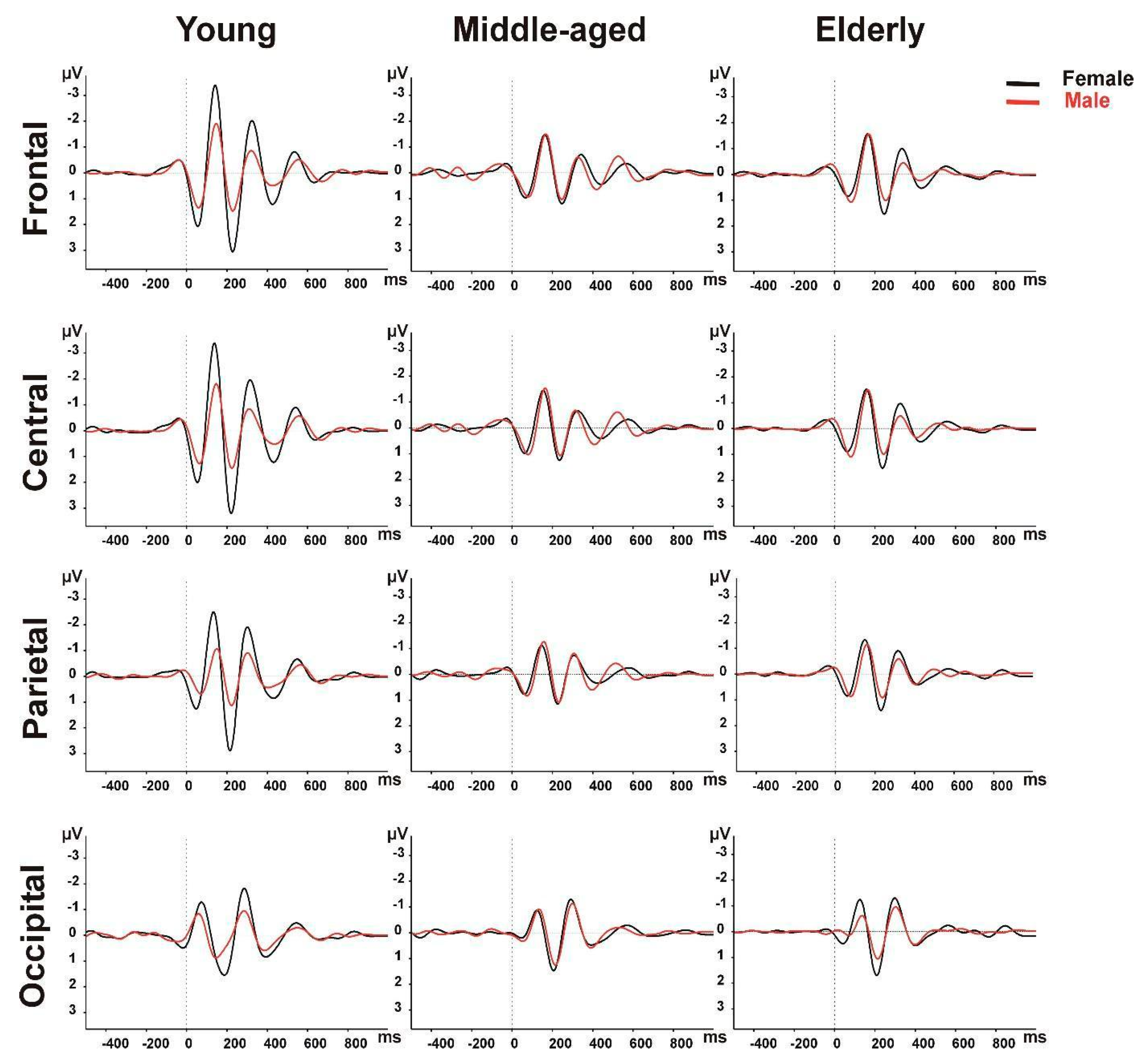
| Female (X− ± SD) | Male (X− ± SD) | p Values | ||
|---|---|---|---|---|
| Young (18–50 yr) | Frontal | 7.41 ± 2.22 | 4.49 ± 1.52 | <0.001 |
| Central | 7.56 ± 2.15 | 4.70 ± 1.69 | <0.001 | |
| Parietal | 6.28 ± 1.83 | 4.03 ± 1.80 | <0.001 | |
| Occipital | 4.80 ± 1.67 | 3.55 ± 1.41 | 0.178 | |
| Middle-Aged (51–65 yr) | Frontal | 4.42 ± 1.95 | 4.34 ± 1.59 | 0.875 |
| Central | 4.35 ± 1.64 | 4.52 ± 1.86 | 0.735 | |
| Parietal | 3.94 ± 1.37 | 4.23 ± 2.04 | 0.552 | |
| Occipital | 4.48 ± 2.02 | 4.10 ± 1.83 | 0.519 | |
| Elderly (>65 yr) | Frontal | 3.94 ± 1.54 | 4.19 ± 1.62 | 0.581 |
| Central | 3.97 ± 1.57 | 4.01 ± 1.37 | 0.940 | |
| Parietal | 4.03 ± 1.67 | 3.55 ± 1.41 | 0.272 | |
| Occipital | 4.65 ± 2.36 | 3.58 ± 1.51 | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yener, G.; Kıyı, İ.; Düzenli-Öztürk, S.; Yerlikaya, D. Age-Related Aspects of Sex Differences in Event-Related Brain Oscillatory Responses: A Turkish Study. Brain Sci. 2024, 14, 567. https://doi.org/10.3390/brainsci14060567
Yener G, Kıyı İ, Düzenli-Öztürk S, Yerlikaya D. Age-Related Aspects of Sex Differences in Event-Related Brain Oscillatory Responses: A Turkish Study. Brain Sciences. 2024; 14(6):567. https://doi.org/10.3390/brainsci14060567
Chicago/Turabian StyleYener, Görsev, İlayda Kıyı, Seren Düzenli-Öztürk, and Deniz Yerlikaya. 2024. "Age-Related Aspects of Sex Differences in Event-Related Brain Oscillatory Responses: A Turkish Study" Brain Sciences 14, no. 6: 567. https://doi.org/10.3390/brainsci14060567
APA StyleYener, G., Kıyı, İ., Düzenli-Öztürk, S., & Yerlikaya, D. (2024). Age-Related Aspects of Sex Differences in Event-Related Brain Oscillatory Responses: A Turkish Study. Brain Sciences, 14(6), 567. https://doi.org/10.3390/brainsci14060567






