Changes in Spatiotemporal Dynamics of Default Network Oscillations between 19 and 29 Years of Age
Abstract
1. Introduction
1.1. About the Functional Neuroanatomy of the Human Default Mode Network (DMN) and Its Developmental Changes
1.2. Which Brain Areas Shall Be Included in an Ad Hoc Model for the Exploration of Respective Neural Generator Dynamics in the Default Mode Network (DMN)?
1.2.1. Neurophysiological Mapping of the Default Mode Network DMN
1.2.2. Frontal Brain Structures and the Default Mode Network (DMN)
1.2.3. Parietal and Occipital Brain Structures and the Default Mode Network (DMN)
1.2.4. Temporal Brain Structures and the Default Mode Network (DMN)
1.2.5. Subcortical Brain Structures and the Default Mode Network (DMN)
1.2.6. Can Particular Brain Areas Be Excluded from the Default Mode Network (DMN)?
1.3. The Present Work, Exploratory Questions, and Several Working Hypotheses
2. Materials and Methods
2.1. Sample characteristics
2.2. Experimental Procedures
2.3. Multi-Channel-Electroencephalography
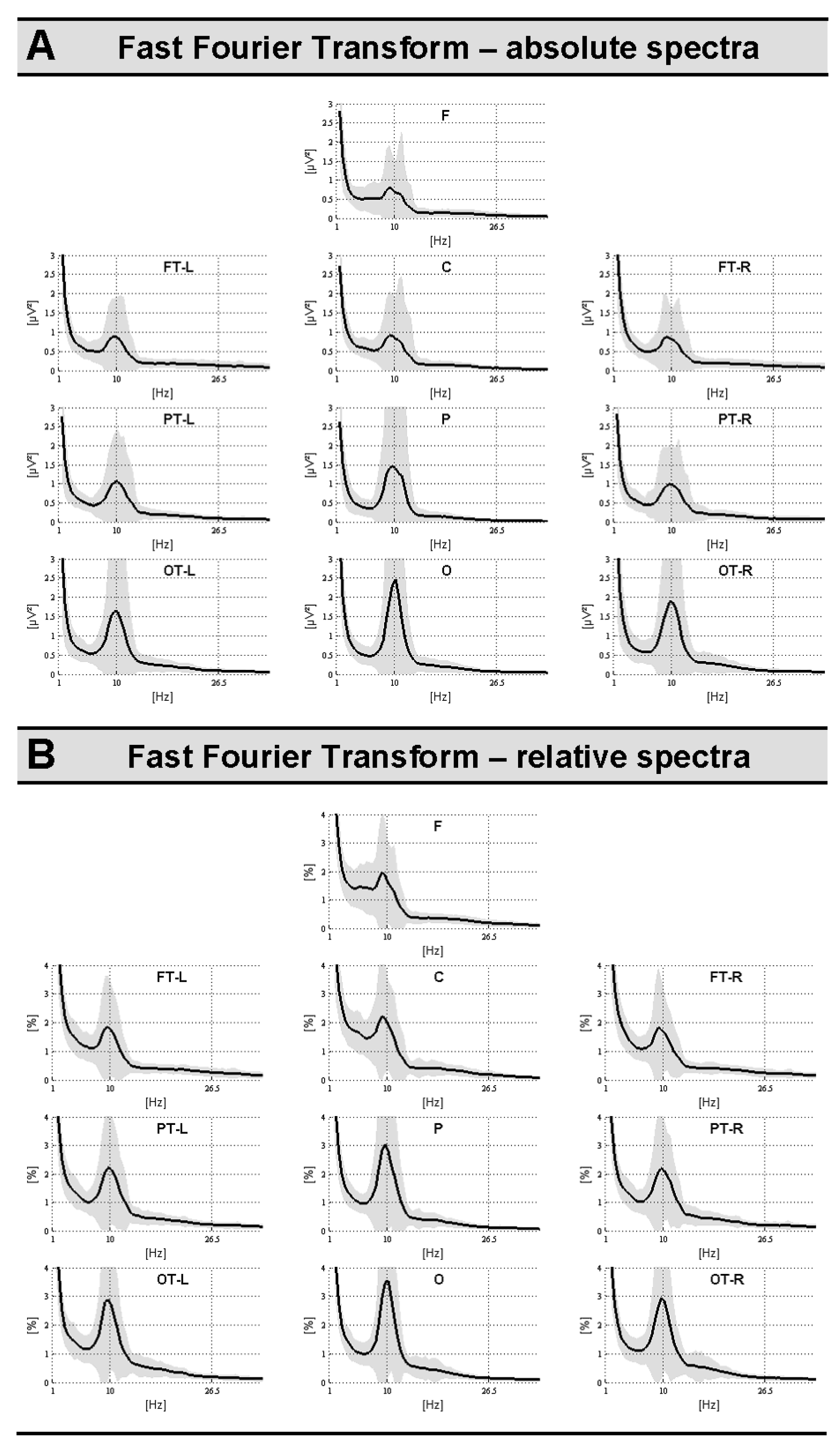
2.3.1. Signal Space: Topographical Fast Fourier Analyses (Absolute and Relative Power Spectra)
2.3.2. Source Space: Default Network Model for Ad Hoc Seeding Procedures
| RS | Anatomical Label | Short Label | TAL-x | TAL-y | TAL-z |
|---|---|---|---|---|---|
| 1 | Ventromedial Prefrontal left | lvmP | −8 | 30 | −24 |
| 2 | Ventromedial Prefrontal right | rvmP | 8 | 30 | −24 |
| 3 | Anterior Ventrolateral Inferior Frontal left | lantVIF | −28 | 25 | −24 |
| 4 | Anterior Ventrolateral Inferior Frontal right | rantVIF | 28 | 25 | −24 |
| 5 | Medial Superior Frontal Gyrus left | lmSFG | −10 | 19 | 52 |
| 6 | Medial Superior Frontal Gyrus right | rmSFG | 10 | 19 | 52 |
| 7 | Middle Frontal Gyrus left | lMFG | −38 | 40 | 20 |
| 8 | Middle Frontal Gyrus right | rMFG | 38 | 40 | 20 |
| 9 | Anterior Cingulate Cortex left | lACC | −10 | 45 | 17 |
| 10 | Anterior Cingulate Cortex right | rACC | 10 | 45 | 17 |
| 11 | Intraparietal Sulcus left | lIPS | −35 | −60 | 50 |
| 12 | Intraparietal Sulcus right | rIPS | 35 | −60 | 50 |
| 13 | Inferior Parietal Lobule left | lIPL | −56 | −36 | 28 |
| 14 | Inferior Parietal Lobule right | rIPL | 56 | −36 | 28 |
| 15 | Precuneus left | lPC | −15 | −58 | 36 |
| 16 | Precuneus right | rPC | 15 | −58 | 36 |
| 17 | Middle Temporal Gyrus left | lMTG | −44 | −66 | 17 |
| 18 | Middle Temporal Gyrus right | rMTG | 44 | −66 | 17 |
| 19 | Medial Temporal Cortex left | lMTC | −55 | 14 | 10 |
| 20 | Medial Temporal Cortex right | rMTC | 55 | 14 | 10 |
| 21 | Hippocampus left | lHIP | −31 | −25 | −8 |
| 22 | Hippocampus right | rHIP | 31 | −25 | −8 |
| 23 | Posterior Cingulate Cortex left | lPCC | −10 | −58 | 11 |
| 24 | Posterior Cingulate right | rPCC | 10 | −58 | 11 |
| 25 | Thalamus left | lTHA | −13 | −17 | 8 |
| 26 | Thalamus right | rTHA | 13 | −17 | 8 |
| 27 | Occipitotemporal junction left | lOTJ | −44 | −71 | 2 |
| 28 | Occipitotemporal junction right | rOTJ | 44 | −71 | 2 |
2.3.3. Source Space: Quantification of Dynamic Follow-Up (FU) Generator Activities
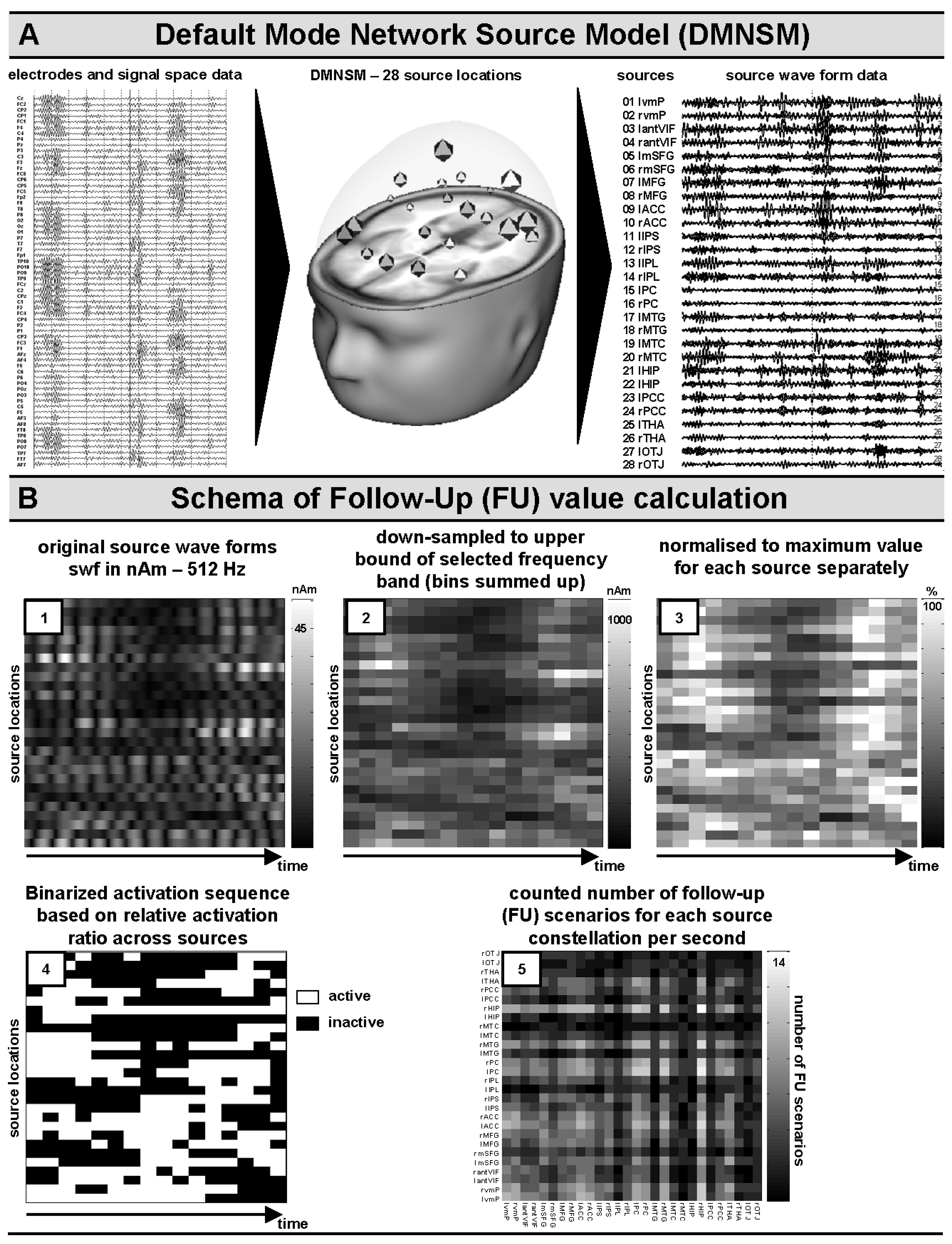
2.3.4. Correlation Analyses
3. Results
3.1. Correlation Analyses
3.1.1. Signal Space Analyses: Regional Absolute and Relative Power Spectra

3.1.2. Source Space Analyses

Correlations between Mean Regional Source (RS) Moments and AGE
Spatiotemporal Dynamics—Follow-Up (FU) Activations and AGE-Related Causal Network Plots (CND)


4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- La, C.; Mossahebi, P.; Nair, V.A.; Bendlin, B.B.; Birn, R.; Meyerand, M.E.; Prabhakaran, V. Age-Related Changes in Inter-Network Connectivity by Component Analysis. Front. Aging Neurosci. 2015, 7, 237. [Google Scholar] [CrossRef]
- Thatcher, R.W. Cyclic cortical reorganization: Origins of human cognitive development. In Human Behavior and the Developing Brain; Dawson, G., Fischer, K.W., Eds.; The Guilford Press: New York, NY, USA, 1994; pp. 232–266. [Google Scholar]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef]
- Sowell, E.R.; Thompson, P.M.; Tessner, K.D.; Toga, A.W. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J. Neurosci. 2001, 21, 8819–8829. [Google Scholar] [CrossRef]
- Basar, E. Brain-Body-Mind in the Nebulous Cartesian System: A Holistic Approach; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Fehr, T. Chancen und Grenzen von Methoden der kognitiven Neurowissenschaften—Funktionelle Magnetresonanztomographie und Biosignalanalyse im Kontext der Entwicklungsneurophysiologie. Z. Gestalt. 2009, 20, 29–43. [Google Scholar]
- Fehr, T. The Neural Architecture of Violence-Related Socialization—Evidence from Functional Neuroimaging. In Handbook of Anger, Aggression, and Violence; Martin, C.R., Preedy, V.R., Patel, V.B., Eds.; Springer: Cham, Swtizerland, 2023. [Google Scholar]
- Lorente de No, R. A Study of Nerve Physiology: Studies from the Rockefeller Institute of Medical Research, 1st ed.; The Rockefeller Institute of Medical Research: New York, NY, USA, 1947. [Google Scholar]
- Basar, E. The theory of the whole-brain-work. Int. J. Psychophysiol. 2006, 60, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Faber, D.S. Field effects in the CNS play functional roles. Front. Neural Circuits 2010, 4, 1541. [Google Scholar] [CrossRef] [PubMed]
- Hindriks, R.; Woolrich, M.; Luckhoo, H.; Joensson, M.; Mohseni, H.; Kringelbach, M.L.; Deco, G. Role of white-matter pathways in coordinating alpha oscillations in resting visual cortex. Neuroimage 2015, 106, 328–339. [Google Scholar] [CrossRef]
- Babiloni, C.; Del Percio, C.; Boccardi, M.; Lizio, R.; Lopez, S.; Carducci, F.; Marzano, N.; Soricelli, A.; Ferri, R.; Triggiani, A.I.; et al. Occipital sources of resting-state alpha rhythms are related to local gray matter density in subjects with amnesic mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2015, 36, 556–570. [Google Scholar] [CrossRef]
- Popov, T.; Tröndle, M.; Baranczuk-Turska, Z.; Pfeiffer, C.; Haufe, S.; Langer, N. Test–retest reliability of resting-state EEG in young and older adults. Psychophysiology 2023, 60, e14268. [Google Scholar] [CrossRef]
- Scherg, M.; Berg, P.; Nakasato, N.; Beniczky, S. Taking the EEG back into the brain: The power of multiple discrete sources. Front. Neurol. 2019, 10, 855. [Google Scholar] [CrossRef]
- Scherg, M. Functional imaging and localization of electromagnetic brain activity. Brain Topogr. 1992, 5, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Scherg, M.; Berg, P. New concepts of brain source imaging and localization. Electroencephalogr. Clin. Neurophysiol. Suppl. 1996, 46, 127–137. [Google Scholar] [PubMed]
- Scherg, M.; Berg, P. Use of prior knowledge in brain electromagnetic source analysis. Brain Topogr. 1991, 4, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Scherg, M. BESA Source Analysis Combining EEG and fMRI. E-Book. 2004. Available online: www.besa.de (accessed on 19 June 2024).
- Somsen, R.J.; van’t Klooster, B.J.; van der Molen, M.W.; van Leeuwen, H.M.; Licht, R. Growth spurts in brain maturation during middle childhood as indexed by EEG power spectra. Biol. Psychol. 1997, 44, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Fehr, T.; Bott, C.; Haeberle, A.; Rockstroh, B. MEG power spectrum and age: Differences between adolescents and adults. In BIOMAG 2002—13th International Conference on Biomagnetism, Jena, Germany, 10–14 August 2002; Nowak, H., Haueisen, J., Gießler, F., Huonker, R., Eds.; VDE Verlag GmbH: Berlin, Germany; Offenbach, Germany, 2002; pp. 320–322. [Google Scholar]
- Finley, A.J.; Angus, D.J.; van Reekum, C.M.; Davidson, R.J.; Schaefer, S.M. Periodic and aperiodic contributions to theta-beta ratios across adulthood. Psychophysiology 2022, 59, e14113. [Google Scholar] [CrossRef] [PubMed]
- Fehr, T. A hybrid model for the neural representation of complex mental processing in the human brain. Cogn. Neurodyn. 2013, 7, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Basar, E. A review of alpha activity in integrative brain function: Fundamental physiology, sensory coding, cognition and pathology. Int. J. Psychophysiol. 2012, 86, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Adrian, E.D.; Matthews, B.H.C. The Berger rhythm: Potential changes from the occipital lobes in man. Brain 1934, 57, 355–385. [Google Scholar] [CrossRef]
- Compston, A. The Berger rhythm: Potential changes from the occipital lobes in man. Brain 2010, 133, 3–6. [Google Scholar] [CrossRef]
- Grandy, T.H.; Werkle-Bergner, M.; Chicherio, C.; Schmiedek, F.; Lovden, M.; Lindenberger, U. Peak individual alpha frequency qualifies as a stable neurophysiological trait marker in healthy younger and older adults. Psychophysiology 2013, 50, 570–582. [Google Scholar] [CrossRef]
- Cragg, L.; Kovacevic, N.; McIntosh, A.R.; Poulsen, C.; Martinu, K.; Leonard, G.; Paus, T. Maturation of EEG power spectra in early adolescence: A longitudinal study. Dev. Sci. 2011, 14, 935–943. [Google Scholar] [CrossRef]
- Miskovic, V.; Ma, X.; Chou, C.A.; Fan, M.; Owens, M.; Sayama, H.; Gibb, B.E. Developmental changes in spontaneous electrocortical activity and network organization from early to late childhood. Neuroimage 2015, 118, 237–247. [Google Scholar] [CrossRef]
- Mason, M.F.; Norton, M.I.; Van Horn, J.D.; Wegner, D.M.; Grafton, S.T.; Macrae, C.N. Wandering minds: The default network and stimulus-independent thought. Science 2007, 315, 393–395. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R. The Brain’s default network and its adaptive role in internal mentation. Neuroscientist 2012, 18, 251–270. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Wen, T.; Mitchell, D.J.; Duncan, J. The functional convergence and heterogeneity of social, episodic, and self-referential thought in the default mode network. Cereb. Cortex 2020, 30, 5915–5929. [Google Scholar] [CrossRef]
- Anticevic, A.; Cole, M.W.; Murray, J.D.; Corlett, P.R.; Wang, X.-J.; Krystal, J.H. The role of default network deactivation in cognition and disease. Trends Cogn. Sci. 2012, 16, 584–592. [Google Scholar] [CrossRef]
- Beckmann, C.F.; Arigita, E.J.S.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Rombouts, S.A.R.B. Reduced resting-state brain activity in the ‘‘default network” in normal aging. Cereb. Cortex 2008, 18, 1856–1864. [Google Scholar] [CrossRef]
- Hafkemeijer, A.; van der Grond, J.; Rombouts, S.A.R.B. Imaging the default mode network in aging and dementia. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 431–441. [Google Scholar] [CrossRef]
- Campbell, K.; Grigg, O.; Saverino, C.; Churchill, N.; Grady, C. Age differences in the intrinsic functional connectivity of default network subsystems. Front. Aging Neurosci. 2013, 5, 73. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Snyder, A.Z.; Vincent, J.L.; Lustig, C.; Head, D.; Raichle, M.E.; Buckner, R.L. Disruption of large-scale brain systems in advanced aging. Neuron 2007, 56, 924–935. [Google Scholar] [CrossRef]
- Persson, J.; Lustig, C.; Nelson, J.K.; Reuter-Lorenz, P.A. Age differences in deactivation: A link to cognitive control? J. Cogn. Neurosci. 2007, 19, 1021–1032. [Google Scholar] [CrossRef]
- Grady, C.L.; Protzner, A.B.; Kovacevic, N.; Strother, S.C.; Afshin-Pour, B.; Wojtowicz, M.; Anderson, J.A.E.; Churchill, N.; McIntosh, A.R. A Multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb. Cortex 2010, 20, 1432–1447. [Google Scholar] [CrossRef]
- Chow, R.; Rabi, R.; Paracha, S.; Hasher, L.; Anderson, N.D.; Alain, C. Default Mode Network and Neural Phase Synchronization in Healthy Aging: A Resting State EEG Study. Neuroscience 2022, 485, 116–128. [Google Scholar] [CrossRef]
- Cabral, J.; Kringelbach, M.L.; Deco, G. Exploring the network dynamics underlying brain activity during rest. Prog. Neurobiol. 2014, 114, 102–131. [Google Scholar] [CrossRef]
- Ghuman, A.S.; McDaniel, J.R.; Martin, A. A wavelet-based method for measuring the oscillatory dynamics of resting-state functional connectivity in MEG. Neuroimage 2011, 56, 69–77. [Google Scholar] [CrossRef]
- Vysata, O.; Kukal, J.; Prochazka, A.; Pazdera, L.; Simko, J.; Valis, M. Age-related changes in EEG coherence. Neurol. Neurochir. Pol. 2014, 48, 35–38. [Google Scholar] [CrossRef]
- Scally, B.; Burke, M.R.; Bunce, D.; Delvenne, J.-F. Resting-state EEG power and connectivity are associated with alpha peak frequency slowing in healthy aging. Neurobiol. Aging 2018, 71, 149–155. [Google Scholar] [CrossRef]
- Smit, D.J.A.; Boersma, M.; Schnack, H.G.; Micheloyannis, S.; Boomsma, D.I.; Hulshoff Pol, H.E.; Stam, C.J.; de Geus, E.J.C.; Valdes-Sosa, P.A. The brain matures with stronger functional connectivity and decreased randomness of its network. PLoS ONE 2012, 7, e36896. [Google Scholar] [CrossRef]
- Moezzi, B.; Pratti, L.M.; Hordacre, B.; Graetz, L.; Berryman, C.; Lavrencic, L.M.; Ridding, M.C.; Keage, H.A.D.; McDonnell, M.D.; Goldsworthy, M.R. Characterization of young and old adult brains: An EEG functional connectivity analysis. Neuroscience 2019, 422, 230–239. [Google Scholar] [CrossRef]
- Kikuchi, M.; Wada, Y.; Koshino, Y.; Nanbu, Y.; Hashimoto, T. Effect of Normal Aging upon Interhemispheric EEG Coherence: Analysis during Rest and Photic Stimulation. Clin. Electroencephalogr. 2000, 31, 170–174. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Bramanti, P.; Rossini, P.M. Human brain networks in physiological aging: A graph theoretical analysis of cortical connectivity from EEG data. J. Alzheimer’s Dis. 2014, 41, 1239–1249. [Google Scholar] [CrossRef]
- Von Bonin, G.V. Essay on the Cerebral Cortex, Charles C. Thomas: Springfield, MA, USA, 1950.
- Fuster, J.M. The cognit: A network model of cortical representation. Int. J. Psychophysiol. 2006, 60, 125–132. [Google Scholar] [CrossRef]
- Fuster, J.M. Cortex and Memory: Emergence of a New Paradigm. J. Cogn. Neurosci. 2009, 21, 2047–2072. [Google Scholar] [CrossRef]
- van Oort, E.S.; Van Walsum, A.V.C.; Norris, D.G. An investigation into the functional and structural connectivity of the Default Mode Network. Neuroimage 2014, 90, 381–389. [Google Scholar] [CrossRef]
- Van Den Heuvel, M.P.; Sporns, O. Rich-club organization of the human connectome. J. Neurosci. 2011, 31, 15775–15786. [Google Scholar] [CrossRef]
- Achard, S.; Salvador, R.; Whitcher, B.; Suckling, J.; Bullmore, E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J. Neurosci. 2006, 26, 63–72. [Google Scholar] [CrossRef]
- Hellyer, P.J.; Shanahan, M.; Scott, G.; Wise, R.J.; Sharp, D.J.; Leech, R. The control of global brain dynamics: Opposing actions of frontoparietal control and default mode networks on attention. J. Neurosci. 2014, 34, 451–461. [Google Scholar] [CrossRef]
- Avelar-Pereira, B.; Bäckman, L.; Wåhlin, A.; Nyberg, L.; Salami, A. Age-related differences in dynamic interactions among default mode, frontoparietal control, and dorsal attention networks during resting-state and interference resolution. Front. Aging Neurosci. 2017, 9, 152. [Google Scholar] [CrossRef]
- Menon, V. 20 years of the default mode network: A review and synthesis. Neuron 2023, 111, 2469–2487. [Google Scholar] [CrossRef]
- Di Plinio, S.; Ferri, F.; Marzet, L.; Romani, G.L.; Northoff, G.; Pizzella, V. Functional connections between activated and deactivated brain regions mediate emotional interference during externally directed cognition. Hum. Brain Mapp. 2018, 39, 3597–3610. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Kelly, A.M.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum. Brain Mapp. 2009, 30, 625–637. [Google Scholar] [CrossRef]
- Konu, D.; Turnbull, A.; Karapanagiotidis, T.; Wang, H.-T.; Brown, L.R.; Jefferies, E.; Smallwood, J. A role for the ventromedial prefrontal cortex in self-generated episodic social cognition. Neuroimage 2020, 218, 116977. [Google Scholar] [CrossRef]
- Alves, P.N.; Foulon, C.; Karolis, V.; Bzdok, D.; Margulies, D.S.; Volle, E.; de Schotten, M.T. An improved neuroanatomical model of the default-mode network reconciles previous neuroimaging and neuropathological findings. Commun. Biol 2019, 2, 370. [Google Scholar] [CrossRef]
- Binder, J.R.; Frost, J.A.; Hammeke, T.A.; Bellgowan, P.; Rao, S.M.; Cox, R.W. Conceptual processing during the conscious resting state: A functional MRI study. J. Cogn. Neurosci. 1999, 11, 80–93. [Google Scholar] [CrossRef]
- Damoiseaux, J.S.; Rombouts, S.A.R.B.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar] [CrossRef]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef]
- Koch, W.; Teipel, S.; Mueller, S.; Buerger, K.; Bokde, A.; Hampel, H.; Coates, U.; Reiser, M.; Meindl, T. Effects of aging on default mode network activity in resting state fMRI: Does the method of analysis matter? Neuroimage 2010, 51, 280–287. [Google Scholar] [CrossRef]
- Schilbach, L.; Eickhoff, S.B.; Rotarska-Jagiela, A.; Fink, G.R.; Vogeley, K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious. Cogn. 2008, 17, 457–467. [Google Scholar] [CrossRef]
- Bluhm, R.L.; Osuch, E.A.; Lanius, R.A.; Boksman, K.; Neufeld, R.W.; Théberge, J.; Williamson, P. Default mode network connectivity: Effects of age, sex, and analytic approach. Neuroreport 2008, 19, 887–891. [Google Scholar] [CrossRef]
- Das, A.; de Los Angeles, C.; Menon, V. Electrophysiological foundations of the human default-mode network revealed by intracranial-EEG recordings during resting state and cognition. Neuroimage 2022, 250, 118927. [Google Scholar] [CrossRef]
- Dixon, M.L.; Moodie, C.A.; Goldin, P.R.; Farb, N.; Heimberg, R.G.; Zhang, J.; Gross, J.J. Frontoparietal and default mode network contributions to self-referential processing in social anxiety disorder. Cogn. Affect. Behav. Neurosci. 2021, 22, 187–198. [Google Scholar] [CrossRef]
- Laird, A.R.; Eickhoff, S.B.; Li, K.; Robin, D.A.; Glahn, D.C.; Fox, P.T. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J. Neurosci. 2009, 29, 14496–14505. [Google Scholar] [CrossRef]
- DeSerisy, M.; Ramphal, B.; Pagliaccio, D.; Raffanello, E.; Tau, G.; Marsh, R.; Posner, J.; Margolis, A.E. Frontoparietal and default mode network connectivity varies with age and intelligence. Dev. Cogn. Neurosci. 2021, 48, 100928. [Google Scholar] [CrossRef]
- Davey, C.G.; Pujol, J.; Harrison, B.J. Mapping the self in the brain’s default mode network. Neuroimage 2016, 132, 390–397. [Google Scholar] [CrossRef]
- Binkofski, F.C.; Klann, J.; Caspers, S. Chapter 4—On the Neuroanatomy and Functional Role of the Inferior Parietal Lobule and Intraparietal Sulcus. In Neurobiology of Language; Academic Press: Cambridge, MA, USA, 2016; pp. 35–47. [Google Scholar] [CrossRef]
- Mars, R.B.; Jbabdi, S.; Sallet, J.; O’Reilly, J.X.; Croxson, P.L.; Olivier, E.; Noonan, M.P.; Bergmann, C.; Mitchell, A.S.; Baxter, M.G.; et al. Diffusionweighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J. Neurosci. 2011, 31, 4087–4100. [Google Scholar] [CrossRef]
- Thomas Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
- Wu, S.-C.J.; Jenkins, L.M.; Apple, A.C.; Petersen, J.; Xiao, F.; Wang, L.; Yang, F.-P.G. Longitudinal fMRI task reveals neural plasticity in default mode network with disrupted executive-default coupling and selective attention after traumatic brain injury. Brain Imaging Behav. 2020, 14, 1638–1650. [Google Scholar] [CrossRef]
- Smallwood, J.; Bernhardt, B.C.; Leech, R.; Bzdok, D.; Jefferies, E.; Margulies, D.S. The default mode network in cognition: A topographical perspective. Nat. Rev. Neurosci. 2021, 22, 503–513. [Google Scholar] [CrossRef]
- Briggs, R.G.; Tanglay, O.; Dadario, N.B.; Young, I.M.; Fonseka, R.D.; Hormovas, J.; Dhanaraj, V.; Lin, Y.-H.; Kim, S.J.; Bouvette, A.; et al. The unique fiber anatomy of middle temporal gyrus default mode connectivity. Oper. Neurosurg. 2021, 21, E8–E14. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J.; Fan, L.; Li, H.; Zhang, W.; Hu, Q.; Jiang, T. Tractography-based parcellation of the human middle temporal gyrus. Sci. Rep. 2015, 5, 18883. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.I.; Tomasi, D.; Volkow, N.D. Structural and functional connectivity of the precuneus and thalamus to the default mode network. Hum. Brain Mapp. 2017, 38, 938–956. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Xue, S.W. Functional connectivity maps based on hippocampal and thalamic dynamics may account for the default-mode network. Eur. J. Neurosci. 2018, 47, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer, A.; Pontier, B.; Claise, B.; Chassain, C.; Coste, J.; Lemaire, J.-J. Disrupted pallido-thalamo-cortical functional connectivity in chronic disorders of consciousness. Brain Sci. 2021, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Di, X.; Taylor, P.A.; Gohel, S.; Tsai, Y.-H.; Biswal, B.B. Functional topography of the thalamocortical system in human. Brain Struct. Funct. 2016, 221, 1971–1984. [Google Scholar] [CrossRef] [PubMed]
- Trepel, M. Neuroanatomie: Struktur und Funktion, 8th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Fodor, J. The Modularity of Mind; MIT Press: Cambridge, MA, USA, 1983. [Google Scholar]
- Fehr, T.; Milz, P. The individuality index—A measure to quantify the degree of inter-individual variability in intra-cerebral brain electric and metabolic activity. Cogn. Neurodyn. 2019, 13, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1997, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.T., Jr.; McCrae, R.R. Revised NEO Personality Inventory and NEO Five-Factor Inventory; Professional Manual; Psychological Assessment Resources: Odessa, FL, USA, 1992. [Google Scholar]
- Borkenau, P.; Ostendorf, F. NEO-Fünf-Faktoren Inventar (NEO-FFI): Nach Costa und McCrae; Hogrefe: Göttingen, Germany, 1993. [Google Scholar]
- Fehr, T.; Achtziger, A. Contextual modulation of binary decisions in dyadic social interactions. Front. Behav. Neurosci. 2021, 15, 715030. [Google Scholar] [CrossRef] [PubMed]
- Ille, N.; Berg, P.; Scherg, M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. J. Clin. Neurophysiol. 2002, 19, 113–124. [Google Scholar] [CrossRef]
- Talairach, J.; Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain, 1st ed.; Thieme: New York, NY, USA, 1988. [Google Scholar]
- Hopfinger, J.; Khoe, W.; Song, A. Combining Electrophysiology with structural and functional Neuroimaging: ERP’s, PET, MRI, fMRI. In Event-Related Potential: A Methods Handbook; Handy, T.C., Ed.; The MIT Press: Cambridge, MA, USA, 2005; pp. 345–379. [Google Scholar]
- Michel, C.M.; Koenig, T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: A review. NeuroImage 2018, 180, 577–593. [Google Scholar] [CrossRef]
- Bledowski, C.; Prvulovic, D.; Hoechstetter, K.; Scherg, M.; Wibral, M.; Goebel, R.; Linden, D.E. Localizing P300 generators in visual target and distractor processing: A combined event-related potential and functional magnetic resonance imaging study. J. Neurosci. 2004, 24, 9353–9360. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Di Nicola, L.M. The brain’s default network: Updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 2019, 20, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Palm, G. Neural associative memories and sparse coding. Neural Netw. 2013, 37, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Fehr, T.; Achtziger, A.; Hinrichs, H.; Herrmann, M. Interindividual differences in oscillatory brain activity in higher cognitive functions—Methodological approaches in analyzing continuous MEG data. In The Cognitive Neuroscience of Individual Differences; Reinvang, I., Greenlee, M.W., Herrmann, M., Eds.; T. Bis Publishers: Oldenburg, Germany, 2003; pp. 101–120. [Google Scholar]
- Achtziger, A.; Fehr, T.; Oettingen, G.; Gollwitzer, P.; Rockstroh, B. Strategies of Intention Formation are Reflected in Continuous MEG Activity. Soc. Neurosci. 2008, 4, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Fehr, T. Lokalisation Langsamer Hirnaktivität bei Schizophrenen Patienten Mittels Magnetenzephalografischer Untersuchungen und Exploration von Zusammenhängen Zwischen Langsamwelliger Hirnaktivität und Symptomatik; Shaker: Aachen, Germany, 2002. [Google Scholar]
- Hampson, S.E.; Goldberg, L.R. Personality Stability and Change over Time. In The Wiley Encyclopedia of Personality and Individual Differences: Models and Theories, 1st ed.; Carducci, B.J., Nave, C.S., Eds.; John Wiley & Sons Ltd.: Portland, OR, USA, 2021; Volume 1, pp. 317–321. [Google Scholar]
- Zhi, S.; Zhao, W.; Wang, R.; Li, Y.; Wang, X.; Liu, S.; Li, J.; Xu, Y. Stability of specific personality network features corresponding to openness trait across different adult age periods: A machine learning analysis. Biochem. Biophys. Res. Commun. 2023, 672, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Koenig, T.; Prichep, L.; Lehmann, D.; Sosa, P.V.; Braeker, E.; Kleinlogel, H.; Isenhart, R.; John, E.R. Millisecond by millisecond, year by year: Normative EEG microstates and developmental stages. NeuroImage 2002, 16, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.M.; Murray, M.M.; Lantz, G.; Gonzalez, S.; Spinelli, L.; de Peralta, R.G. EEG source imaging. Clin. Neurophysiol. 2004, 115, 2195–2222. [Google Scholar] [CrossRef] [PubMed]
- Milz, P.; Faber, P.L.; Lehmann, D.; Koenig, T.; Kochi, K.; Pascual-Marqui, R.D. The functional significance of EEG microstates--associations with modalities of thinking. NeuroImage 2016, 125, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, T.; Vaque-Alcazar, L.; Bartres-Faz, D.; Pero-Cebollero, M.; Canete-Masse, C.; Guardia-Olmos, J. Reduced default mode network effective connectivity in healthy aging is modulated by years of education. NeuroImage 2024, 288, 120532. [Google Scholar] [CrossRef]
- Giannopoulos, A.E.; Zioga, I.; Papageorgiou, P.; Pervanidou, P.; Makris, G.; Chrousos, G.P.; Stachtea, X.; Capsalis, C.; Papageorgiou, C. Evaluating the Modulation of the Acoustic Startle Reflex in Children and Adolescents via Vertical EOG and EEG: Sex, Age, and Behavioral Effects. Front. Psychol. 2022, 16, 798667. [Google Scholar] [CrossRef]
- Giustiniani, A.; Danesin, L.; Bozzetto, B.; Macina, A.; Benavides-Varela, S.; Burgio, F. Functional changes in brain oscillations in dementia: A review. Rev. Neurosci. 2023, 34, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Moreno-De-Luca, A.; Myers, S.M.; Challman, T.D.; Moreno-De-Luca, D.; Evans, D.W.; Ledbetter, D.H. Developmental brain dysfunction: Revival and expansion of old concepts based on new genetic evidence. Lancet Neurol. 2013, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fehr, T.; Mehrens, S.; Haag, M.-C.; Amelung, A.; Gloy, K. Changes in Spatiotemporal Dynamics of Default Network Oscillations between 19 and 29 Years of Age. Brain Sci. 2024, 14, 671. https://doi.org/10.3390/brainsci14070671
Fehr T, Mehrens S, Haag M-C, Amelung A, Gloy K. Changes in Spatiotemporal Dynamics of Default Network Oscillations between 19 and 29 Years of Age. Brain Sciences. 2024; 14(7):671. https://doi.org/10.3390/brainsci14070671
Chicago/Turabian StyleFehr, Thorsten, Sophia Mehrens, Marie-Christine Haag, Anneke Amelung, and Kilian Gloy. 2024. "Changes in Spatiotemporal Dynamics of Default Network Oscillations between 19 and 29 Years of Age" Brain Sciences 14, no. 7: 671. https://doi.org/10.3390/brainsci14070671
APA StyleFehr, T., Mehrens, S., Haag, M.-C., Amelung, A., & Gloy, K. (2024). Changes in Spatiotemporal Dynamics of Default Network Oscillations between 19 and 29 Years of Age. Brain Sciences, 14(7), 671. https://doi.org/10.3390/brainsci14070671






