A Narrative Review of the Dorsal Root Ganglia and Spinal Cord Mechanisms of Action of Neuromodulation Therapies in Neuropathic Pain
Abstract
:1. Introduction
2. Mechanisms of Neuropathic Pain
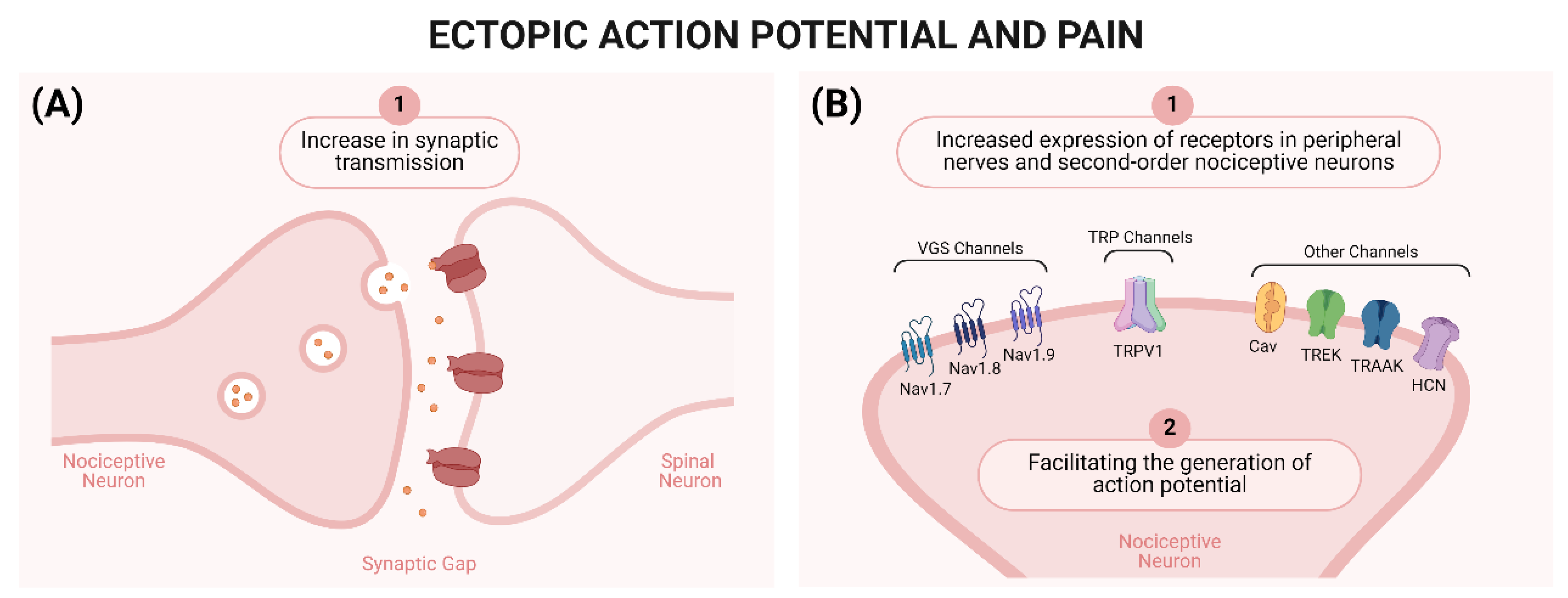
2.1. Peripheral Mechanisms of Neuropathic Pain
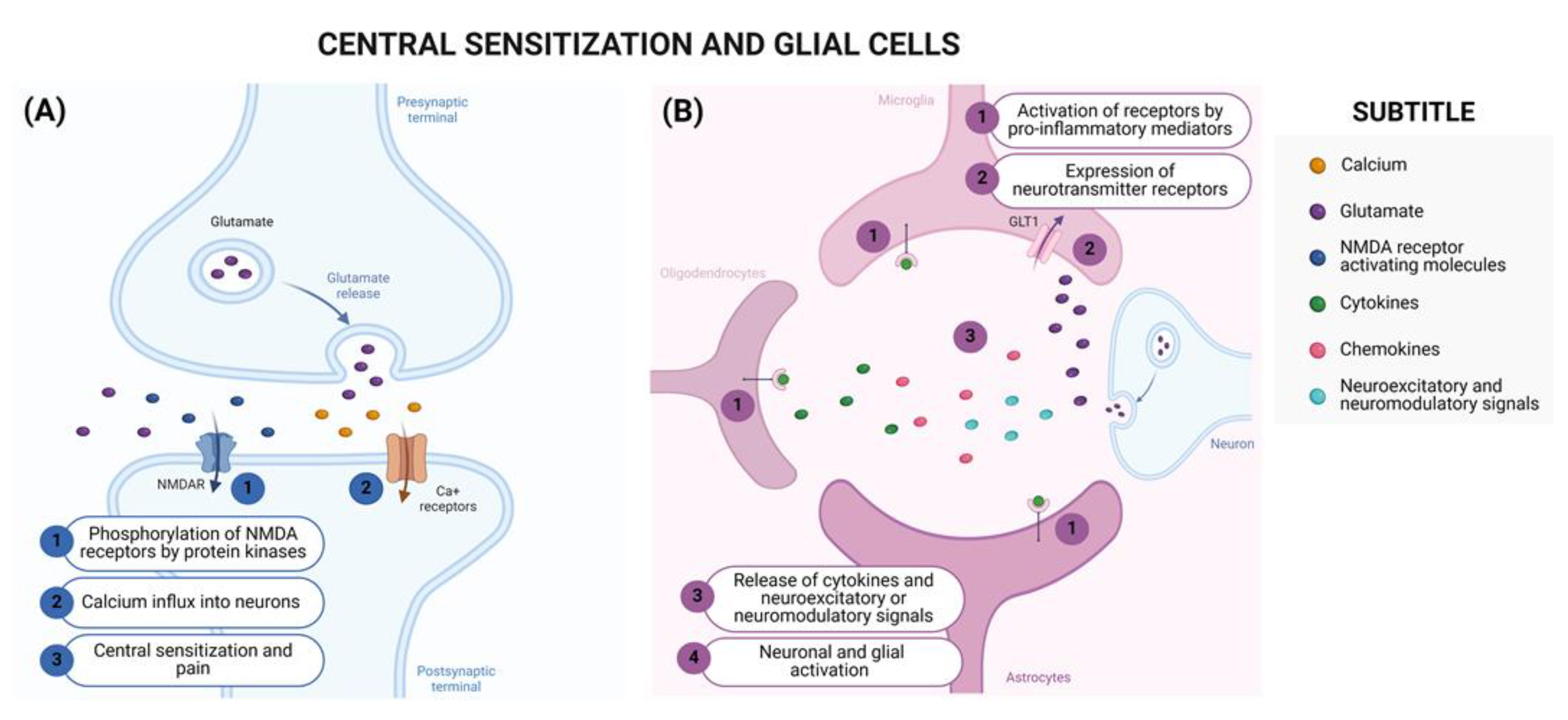
2.2. Central Mechanisms of Neuropathic Pain
3. Current Guidelines for the Treatment of Neuropathic Pain
4. Neuromodulation Therapies
5. Neuromodulation Therapies Targeting DRG and the Spinal Cord in the Management of Neuropathic Pain
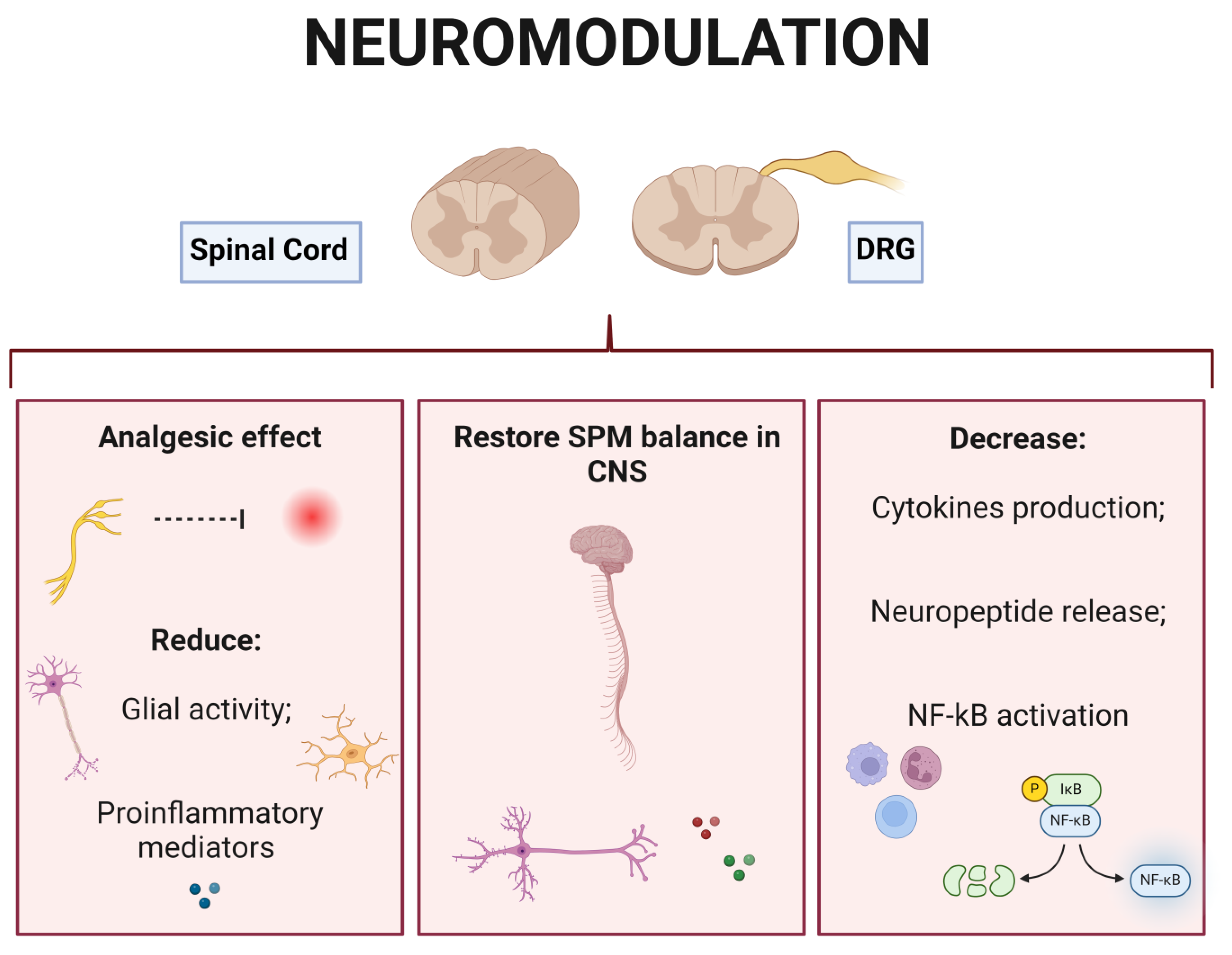
5.1. Neuromodulation Therapies at the DRG Level
5.2. Neuromodulation Therapies Applying SCS
6. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised International Association for the Study of Pain Definition of Pain: Concepts, Challenges, and Compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Amaya, F.; Decosterd, I.; Samad, T.A.; Plumpton, C.; Tate, S.; Mannion, R.J.; Costigan, M.; Woolf, C.J. Diversity of Expression of the Sensory Neuron-Specific TTX-Resistant Voltage-Gated Sodium Ion Channels SNS and SNS2. Mol. Cell. Neurosci. 2000, 15, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.; Gupta, R.; Jordt, S.E.; Chen, Y.; Liedtke, W.B. Regulation of Pain and Itch by TRP Channels. Neurosci. Bull. 2018, 34, 120–142. [Google Scholar] [CrossRef] [PubMed]
- Maximiano, T.K.E.; Carneiro, J.A.; Fattori, V.; Verri, W.A. TRPV1: Receptor Structure, Activation, Modulation and Role in Neuro-Immune Interactions and Pain. Cell Calcium 2024, 119, 102870. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.M.; Verri, W.A.; Vivancos, G.G.; Moreira, I.F.; Reis, S.; Parada, C.A.; Cunha, F.Q.; Ferreira, S.H. An Electronic Pressure-Meter Nociception Paw Test for Mice. Braz. J. Med. Biol. Res. 2004, 37, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.M.; Verri, W.A.; Silva, J.S.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. A Cascade of Cytokines Mediates Mechanical Inflammatory Hypernociception in Mice. Proc. Natl. Acad. Sci. USA 2005, 102, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Verri, W.A.; Cunha, T.M.; Parada, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Hypernociceptive Role of Cytokines and Chemokines: Targets for Analgesic Drug Development? Pharmacol. Ther. 2006, 112, 116–138. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Huh, Y.; Ji, R.R. Roles of Inflammation, Neurogenic Inflammation, and Neuroinflammation in Pain. J. Anesth. 2019, 33, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.D.V.; Piva, M.; Martelossi-Cebinelli, G.; Ribas, M.S.R.; Bianchini, B.H.S.; Heintz, O.K.; Casagrande, R.; Verri, W.A. Stem Cells and Pain. World J. Stem Cells 2023, 15, 1035–1062. [Google Scholar] [CrossRef] [PubMed]
- Meacham, K.; Shepherd, A.; Mohapatra, D.P.; Haroutounian, S. Neuropathic Pain: Central vs. Peripheral Mechanisms. Curr. Pain Headache Rep. 2017, 21, 28. [Google Scholar] [PubMed]
- Shraim, M.A.; Massé-Alarie, H.; Hall, L.M.; Hodges, P.W. Systematic Review and Synthesis of Mechanism-Based Classification Systems for Pain Experienced in the Musculoskeletal System. Clin. J. Pain 2020, 36, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic Pain: An Update on Burden, Best Practices, and New Advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Kosek, E.; Cohen, M.; Baron, R.; Gebhart, G.F.; Mico, J.A.; Rice, A.S.C.; Rief, W.; Sluka, A.K. Do We Need a Third Mechanistic Descriptor for Chronic Pain States? Pain 2016, 157, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Smart, K.M.; Blake, C.; Staines, A.; Doody, C. Clinical Indicators of “Nociceptive”, “peripheral Neuropathic” and “Central” Mechanisms of Musculoskeletal Pain. A Delphi Survey of Expert Clinicians. Man. Ther. 2010, 15, 80–87. [Google Scholar] [CrossRef] [PubMed]
- DiBonaventura, M.D.; Sadosky, A.; Concialdi, K.; Hopps, M.; Kudel, I.; Parsons, B.; Cappelleri, J.C.; Hlavacek, P.; Alexander, A.H.; Stacey, B.R.; et al. The Prevalence of Probable Neuropathic Pain in the US: Results from a Multimodal General-Population Health Survey. J. Pain Res. 2017, 10, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; De Baets, L.; Hodges, P. Phenotyping Nociceptive, Neuropathic, and Nociplastic Pain: Who, How, & Why? Braz. J. Phys. Ther. 2023, 27, 100537. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.A.; Herr, M.J. Physiology, Nociception. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sikandar, S.; Dickenson, A.H. Visceral Pain—The Ins and Outs, the Ups and Downs. Curr. Opin. Support. Palliat. Care 2012, 6, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Malfliet, A.; Nishigami, T. Nociplastic Pain and Central Sensitization in Patients with Chronic Pain Conditions: A Terminology Update for Clinicians. Braz. J. Phys. Ther. 2023, 27, 100518. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic Pain: Towards an Understanding of Prevalent Pain Conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Souza Monteiro de Araujo, D.; Nassini, R.; Geppetti, P.; De Logu, F. TRPA1 as a Therapeutic Target for Nociceptive Pain. Expert Opin. Ther. Targets 2020, 24, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Lantéri-Minet, M.; Attal, N.; Laurent, B.; Touboul, C. Prevalence of Chronic Pain with Neuropathic Characteristics in the General Population. Pain 2008, 136, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Attal, N.; Fermanian, J.; Alchaar, H.; Gautron, M.; Masquelier, E.; Rostaing, S.; Lanteri-Minet, M.; Collin, E.; Grisart, J.; et al. Development and Validation of the Neuropathic Pain Symptom Inventory. Pain 2004, 108, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.D. Challenges of Neuropathic Pain: Focus on Diabetic Neuropathy. J. Neural Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D. Neuropathic Pain: Definition, Assessment and Epidemiology. Rev. Neurol. 2019, 175, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Freynhagen, R.; Parada, H.A.; Calderon-Ospina, C.A.; Chen, J.; Rakhmawati Emril, D.; Fernández-Villacorta, F.J.; Franco, H.; Ho, K.Y.; Lara-Solares, A.; Li, C.C.F.; et al. Current Understanding of the Mixed Pain Concept: A Brief Narrative Review. Curr. Med. Res. Opin. 2019, 35, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Adelmanesh, F.; Jalali, A.; Jazayeri Shooshtari, S.M.; Raissi, G.R.; Ketabchi, S.M.; Shir, Y. Is There an Association Between Lumbosacral Radiculopathy and Painful Gluteal Trigger Points?: A Cross-Sectional Study. Am. J. Phys. Med. Rehabil. 2015, 94, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Nijs, J.; Cagnie, B.; Gerwin, R.D.; Plaza-Manzano, G.; Valera-Calero, J.A.; Arendt-Nielsen, L. Myofascial Pain Syndrome: A Nociceptive Condition Comorbid with Neuropathic or Nociplastic Pain. Life 2023, 13, 694. [Google Scholar] [CrossRef] [PubMed]
- Rowbotham, M.C.; Goli, V.; Kunz, N.R.; Lei, D. Venlafaxine Extended Release in the Treatment of Painful Diabetic Neuropathy: A Double-Blind, Placebo-Controlled Study. Pain 2004, 110, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP Classification of Chronic Pain for ICD-11: Chronic Neuropathic Pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Szok, D.; Tajti, J.; Nyári, A.; Vécsei, L. Therapeutic Approaches for Peripheral and Central Neuropathic Pain. Behav. Neurol. 2019, 2019, 8685954. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Bouhassira, D.; Baron, R. Diagnosis and Assessment of Neuropathic Pain through Questionnaires. Lancet Neurol. 2018, 17, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic Pain in the General Population: A Systematic Review of Epidemiological Studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef] [PubMed]
- De Moulin, D.; Boulanger, A.; Clark, A.J.; Clarke, H.; Dao, T.; Finley, G.A.; Furlan, A.; Gilron, I.; Gordon, A.; Morley-Forster, P.K.; et al. Pharmacological Management of Chronic Neuropathic Pain: Revised Consensus Statement from the Canadian Pain Society. Pain Res. Manag. J. Can. Pain Soc. 2014, 19, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: Systematic Review, Meta-Analysis and Updated NeuPSIG Recommendations. Lancet. Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Estacion, M.; Dib-Hajj, S.D.; Benke, P.J.; Te Morsche, R.H.M.; Eastman, E.M.; Macala, L.J.; Drenth, J.P.H.; Waxman, S.G. NaV1.7 Gain-of-Function Mutations as a Continuum: A1632E Displays Physiological Changes Associated with Erythromelalgia and Paroxysmal Extreme Pain Disorder Mutations and Produces Symptoms of Both Disorders. J. Neurosci. 2008, 28, 11079–11088. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S. Nav1.7 and Nav1.8: Role in the Pathophysiology of Pain. Mol. Pain 2019, 15, 1744806919858801. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.N.; Zheng, Y.L.; Wang, X.Q.; Chen, P.J. Role of Exercise on Inflammation Cytokines of Neuropathic Pain in Animal Models. Mol. Neurobiol. 2024; published online in the Journal Molecular Neurobiology. 1–14. [Google Scholar] [CrossRef]
- Heijnen, S.; Hommel, B.; Kibele, A.; Colzato, L.S. Neuromodulation of Aerobic Exercise—A Review. Front. Psychol. 2016, 6, 1890. [Google Scholar] [CrossRef] [PubMed]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic Pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Cummins, T.R.; Sheets, P.L.; Waxman, S.G. The Roles of Sodium Channels in Nociception: Implications for Mechanisms of Pain. Pain 2007, 131, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Truin, M.; Van Venrooij, P.; Duysens, V.; Deumens, R.; Van Kleef, M.; Joosten, E.A.J. Spinal Cord Stimulation in a Mouse Chronic Neuropathic Pain Model. Neuromodulation 2007, 10, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Salinas, D.L.; Braz, J.M.; Hamel, K.A.; Basbaum, A.I. Pain Relief by Supraspinal Gabapentin Requires Descending Noradrenergic Inhibitory Controls. Pain Rep. 2018, 3, e659. [Google Scholar] [CrossRef] [PubMed]
- Varshney, V.; Osborn, J.; Chaturvedi, R.; Shah, V.; Chakravarthy, K. Advances in the Interventional Management of Neuropathic Pain. Ann. Transl. Med. 2021, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Uchida, K.; Nakajima, H.; Guerrero, A.R.; Watanabe, S.; Takeura, N.; Sugita, D.; Shimada, S.; Nakatsuka, T.; Baba, H. Early Transcutaneous Electrical Nerve Stimulation Reduces Hyperalgesia and Decreases Activation of Spinal Glial Cells in Mice with Neuropathic Pain. Pain 2014, 155, 1888–1901. [Google Scholar] [CrossRef] [PubMed]
- Liem, L.; Russo, M.; Huygen, F.J.P.M.; Van Buyten, J.P.; Smet, I.; Verrills, P.; Cousins, M.; Brooker, C.; Levy, R.; Deer, T.; et al. One-Year Outcomes of Spinal Cord Stimulation of the Dorsal Root Ganglion in the Treatment of Chronic Neuropathic Pain. Neuromodulation 2015, 18, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, N.; Deer, T.R.; Kramer, J.; Poree, L.; Amirdelfan, K.; Grigsby, E.; Staats, P.; Burton, A.W.; Burgher, A.H.; Scowcroft, J.; et al. Paresthesia-Free Dorsal Root Ganglion Stimulation: An ACCURATE Study Sub-Analysis. Neuromodulation 2020, 23, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Levy, R.M.; Kramer, J.; Poree, L.; Amirdelfan, K.; Grigsby, E.; Staats, P.; Burton, A.W.; Burgher, A.H.; Obray, J.; et al. Dorsal Root Ganglion Stimulation Yielded Higher Treatment Success Rate for Complex Regional Pain Syndrome and Causalgia at 3 and 12 Months: A Randomized Comparative Trial. Pain 2017, 158, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Lindskou, T.A.; Christensen, S.W.; Graven-Nielsen, T. Cuff Algometry for Estimation of Hyperalgesia and Pain Summation. Pain Med. 2017, 18, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Vesper, J.; Slotty, P.; Schu, S.; Poeggel-Kraemer, K.; Littges, H.; Van Looy, P.; Agnesi, F.; Venkatesan, L.; Van Havenbergh, T. Burst SCS Microdosing Is as Efficacious as Standard Burst SCS in Treating Chronic Back and Leg Pain: Results From a Randomized Controlled Trial. Neuromodulation 2019, 22, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.L.; McMillan, J.; Li, L. Benefits of Exercise Intervention in Reducing Neuropathic Pain. Front. Cell. Neurosci. 2014, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Van Laake-Geelen, C.C.M.; Smeets, R.J.E.M.; Quadflieg, S.P.A.B.; Kleijnen, J.; Verbunt, J.A. The Effect of Exercise Therapy Combined with Psychological Therapy on Physical Activity and Quality of Life in Patients with Painful Diabetic Neuropathy: A Systematic Review. Scand. J. Pain 2019, 19, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Krames, E.S.; Hunter Peckham, P.; Rezai, A.; Aboelsaad, F. What Is Neuromodulation? Neuromodulation 2009, 1, 3–8. [Google Scholar] [CrossRef]
- Wall, P.D.; Swert, W.H. Temporary Abolition of Pain in Man. Science 1967, 155, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Sewell, R.D.E. Neuropathic Pain Models and Outcome Measures: A Dual Translational Challenge. Ann. Transl. Med. 2018, 6, S42. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, A.S.; Jain, V.; Singh, N. Animal Models of Neuropathic Pain. Fundam. Clin. Pharmacol. 2011, 25, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elsayed, A.; Vardhan, S.; Aggarwal, A.; Vardhan, M.; Diwan, S.A. Mechanisms of Action of Dorsal Root Ganglion Stimulation. Int. J. Mol. Sci. 2024, 25, 3591. [Google Scholar] [CrossRef] [PubMed]
- Morgalla, M.H.; de Barros Filho, M.F.; Chander, B.S.; Soekadar, S.R.; Tatagiba, M.; Lepski, G. Neurophysiological Effects of Dorsal Root Ganglion Stimulation (DRGS) in Pain Processing at the Cortical Level. Neuromodulation 2019, 22, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Yu, H.; Fischer, G.J.; Kramer, J.M.; Hogan, Q.H. Dorsal Root Ganglionic Field Stimulation Relieves Spontaneous and Induced Neuropathic Pain in Rats. J. Pain 2016, 17, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Datta-Chaudhuri, T.; George, S.J.; Haider, B.; Wong, J.; Hepler, T.D.; Andersson, U.; Brines, M.; Tracey, K.J.; Chavan, S.S. High-Frequency Electrical Stimulation Attenuates Neuronal Release of Inflammatory Mediators and Ameliorates Neuropathic Pain. Bioelectron. Med. 2022, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Shealy, C.N.; Mortimer, J.T.; Reswick, J.B. Electrical Inhibition of Pain by Stimulation of the Dorsal Columns: Preliminary Clinical Report. Anesth. Analg. 1967, 46, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Knotkova, H.; Hamani, C.; Sivanesan, E.; Le Beuffe, M.F.E.; Moon, J.Y.; Cohen, S.P.; Huntoon, M.A. Neuromodulation for Chronic Pain. Lancet 2021, 397, 2111–2124. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.N.; Meyer, R.A. Mechanisms of Neuropathic Pain. Neuron 2006, 52, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Broom, D.C.; Youn, D.H.; Mills, C.D.; Kohno, T.; Suter, M.R.; Moore, K.A.; Decosterd, I.; Coggeshall, R.E.; Woolf, C.J. Blocking Caspase Activity Prevents Transsynaptic Neuronal Apoptosis and the Loss of Inhibition in Lamina II of the Dorsal Horn after Peripheral Nerve Injury. J. Neurosci. 2005, 25, 7317–7323. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanyan, S.; Stemkowski, P.L.; Stebbing, M.J.; Smith, P.A. Sciatic Chronic Constriction Injury Produces Cell-Type-Specific Changes in the Electrophysiological Properties of Rat Substantia Gelatinosa Neurons. J. Neurophysiol. 2006, 96, 579–590. [Google Scholar] [PubMed]
- Hains, B.C.; Waxman, S.G. Sodium Channel Expression and the Molecular Pathophysiology of Pain after SCI. Prog. Brain Res. 2007, 161, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Devor, M. Ectopic Discharge in Aβ Afferents as a Source of Neuropathic Pain. Exp. Brain Res. 2009, 196, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Dib-Hajj, S.D.; Cummins, T.R.; Black, J.A.; Waxman, S.G. Sodium Channels in Normal and Pathological Pain. Annu. Rev. Neurosci. 2010, 33, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.A.; Baker, M.D.; Levato, A.; Ingram, R.; Mallucci, G.; McMahon, S.B.; Wood, J.N. Nerve Injury Induces Robust Allodynia and Ectopic Discharges in Nav1.3 Null Mutant Mice. Mol. Pain 2006, 2, 33. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Wang, F.; Xiang, H.; Bai, X.; Yu, H.; Hogan, Q.H. Inhibition of Neuropathic Hyperalgesia by Intrathecal Bone Marrow Stromal Cells Is Associated with Alteration of Multiple Soluble Factors in Cerebrospinal Fluid. Exp. Brain Res. 2017, 235, 2627–2638. [Google Scholar] [CrossRef] [PubMed]
- Beggs, S.; Salter, M.W. Stereological and Somatotopic Analysis of the Spinal Microglial Response to Peripheral Nerve Injury. Brain Behav. Immun. 2007, 21, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Binder, A.; Wasner, G. Neuropathic Pain: Diagnosis, Pathophysiological Mechanisms, and Treatment. Lancet Neurol. 2010, 9, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Loeser, J.D.; Treede, R.D. The Kyoto Protocol of IASP Basic Pain Terminology. Pain 2008, 137, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Moon, J.Y.; Roh, D.H.; Yoon, S.Y.; Kwon, S.G.; Choi, H.S.; Kang, S.Y.; Han, H.J.; Beitz, A.J.; Lee, J.H. Spinal D-Serine Increases PKC-Dependent GluN1 Phosphorylation Contributing to the Sigma-1 Receptor-Induced Development of Mechanical Allodynia in a Mouse Model of Neuropathic Pain. J. Pain 2017, 18, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.; Kocsis, J.D.; Devor, M. Multiple Interacting Sites of Ectopic Spike Electrogenesis in Primary Sensory Neurons. J. Neurosci. 2005, 25, 2576–2585. [Google Scholar] [CrossRef] [PubMed]
- Bannister, K.; Sachau, J.; Baron, R.; Dickenson, A.H. Neuropathic Pain: Mechanism-Based Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Hayashi, H.; Ishikawa, T.; Shibata, K.; Shigetomi, E.; Shinozaki, Y.; Inada, H.; Roh, S.E.; Kim, S.J.; Lee, G.; et al. Cortical Astrocytes Rewire Somatosensory Cortical Circuits for Peripheral Neuropathic Pain. J. Clin. Investig. 2016, 126, 1983–1997. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.W.; Mehrabani, S.; Liu, S.; Taylor, A.J.; Cahill, C.M. Topography of Microglial Activation in Sensory and Affect Related Regions in Chronic Pain. J. Neurosci. Res. 2017, 95, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.F.; Beggs, S.; Salter, M.W.; De Koninck, Y. Transformation of the Output of Spinal Lamina I Neurons after Nerve Injury and Microglia Stimulation Underlying Neuropathic Pain. Mol. Pain 2007, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.A.M.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from Microglia Causes the Shift in Neuronal Anion Gradient Underlying Neuropathic Pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.; D’Mello, R.; Dickenson, A.H. Peripheral Nerve Injury-Induced Changes in Spinal A2-Adrenoceptor-Mediated Modulation of Mechanically Evoked Dorsal Horn Neuronal Responses. J. Pain 2008, 9, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Scheu, S.; Ali, S.; Ruland, C.; Arolt, V.; Alferink, J. The C-C Chemokines CCL17 and CCL22 and Their Receptor CCR4 in CNS Autoimmunity. Int. J. Mol. Sci. 2017, 18, 2306. [Google Scholar] [CrossRef] [PubMed]
- Bee, L.A.; Dickenson, A.H. Descending Facilitation from the Brainstem Determines Behavioural and Neuronal Hypersensitivity Following Nerve Injury and Efficacy of Pregabalin. Pain 2008, 140, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Takasusuki, T.; Yamaguchi, S.; Hori, Y. NMDA Receptor 2B Subunit-Mediated Synaptic Transmission in the Superficial Dorsal Horn of Peripheral Nerve-Injured Neuropathic Mice. Brain Res. 2007, 1135, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ultenius, C.; Linderoth, B.; Meyerson, B.A.; Wallin, J. Spinal NMDA Receptor Phosphorylation Correlates with the Presence of Neuropathic Signs Following Peripheral Nerve Injury in the Rat. Neurosci. Lett. 2006, 399, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Zarpelon, A.C.; Rodrigues, F.C.; Lopes, A.H.; Souza, G.R.; Carvalho, T.T.; Pinto, L.G.; Xu, D.; Ferreira, S.H.; Alves-Filho, J.C.; McInnes, I.B.; et al. Spinal Cord Oligodendrocyte-Derived Alarmin IL-33 Mediates Neuropathic Pain. FASEB J. 2016, 30, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.L.; Johanek, L.M.; Sanada, L.S.; Sluka, K.A. Spinal Cord Stimulation Reduces Mechanical Hyperalgesia and Glial Cell Activation in Animals with Neuropathic Pain. Anesth. Analg. 2014, 118, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Li, H.; Naser, P.V.; Oswald, M.J.; Kuner, R. Suppression of Neuropathic Pain and Comorbidities by Recurrent Cycles of Repetitive Transcranial Direct Current Motor Cortex Stimulation in Mice. Sci. Rep. 2021, 11, 9735. [Google Scholar] [CrossRef] [PubMed]
- Truin, M.; van Kleef, M.; Verboeket, Y.; Deumens, R.; Honig, W.; Joosten, E.A.J. The Effect of Spinal Cord Stimulation in Mice with Chronic Neuropathic Pain after Partial Ligation of the Sciatic Nerve. Pain 2009, 145, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Tao, X.; Wan, C.; Zhang, X.; Zhao, M.; Xu, M.; Wang, P.; Liu, Y.; Wang, C.; Xi, Q.; et al. Spinal Cord Stimulation Alleviates Neuropathic Pain by Attenuating Microglial Activation via Reducing Colony-Stimulating Factor 1 Levels in the Spinal Cord in a Rat Model of Chronic Constriction Injury. Anesth. Analg. 2022, 135, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.L.; King, E.W.; Johanek, L.M.; Sluka, K.A. Spinal Cord Stimulation Reduces Hypersensitivity through Activation of Opioid Receptors in a Frequency-Dependent Manner. Eur. J. Pain 2013, 17, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, Q.; Yang, F.; Shi, C.; Sivanesan, E.; Liu, S.; Chen, X.; Sarma, S.V.; Vera-Portocarrero, L.P.; Linderoth, B.; et al. The Impact of Electrical Charge Delivery on Inhibition of Mechanical Hypersensitivity in Nerve-Injured Rats by Sub-Sensory Threshold Spinal Cord Stimulation. Neuromodulation 2019, 22, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zaninelli, T.H.; Fattori, V.; Saraiva-Santos, T.; Badaro-Garcia, S.; Staurengo-Ferrari, L.; Andrade, K.C.; Artero, N.A.; Ferraz, C.R.; Bertozzi, M.M.; Rasquel-Oliveira, F.; et al. RvD1 Disrupts Nociceptor Neuron and Macrophage Activation and Neuroimmune Communication, Reducing Pain and Inflammation in Gouty Arthritis in Mice. Br. J. Pharmacol. 2022, 179, 4500–4515. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Jing, X.Y.; Wang, Y.Z.; Yang, B.R.; Lu, Q.; Hu, H.; Kang, L. Exercise, Spinal Microglia and Neuropathic Pain: Potential Molecular Mechanisms. Neurochem. Res. 2024, 49, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Tatikola, S.P.; Natarajan, V.; Desai, V.K.; Asirvatham, A.R.; Rajsekhar, H. Effect of Various Exercise Protocols on Neuropathic Pain in Individuals with Type 2 Diabetes with Peripheral Neuropathy: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. 2022, 16, 102603. [Google Scholar] [CrossRef] [PubMed]
- Koop, M.A.; Sleijser-Koehorst, M.L.S.; Hooijmans, C.R.; Tdlohreg, P.Q.; Lutke Schipholt, I.J.; Scholten-Peeters, G.G.M.; Coppieters, M.W. The Potential Protective Effects of Pre-Injury Exercise on Neuroimmune Responses Following Experimentally-Induced Traumatic Neuropathy: A Systematic Review with Meta-Analysis. Front. Immunol. 2023, 14, 1215566. [Google Scholar] [CrossRef] [PubMed]
- Toloui, A.; Ramawad, H.A.; Gharin, P.; Vaccaro, A.R.; Zarei, H.; Hosseini, M.; Yousefifard, M.; Rahimi-Movaghar, V. The Role of Exercise in the Alleviation of Neuropathic Pain Following Traumatic Spinal Cord Injuries: A Systematic Review and Meta-Analysis. Neurospine 2023, 20, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; Aziz, T.Z.; Garcia-Larrea, L.; Hansson, P.; Jensen, T.S.; Lefaucheur, J.P.; Simpson, B.A.; Taylor, R.S. EFNS Guidelines on Neurostimulation Therapy for Neuropathic Pain. Eur. J. Neurol. 2007, 14, 952–970. [Google Scholar] [CrossRef] [PubMed]
- De Vos, C.C.; Meier, K.; Zaalberg, P.B.; Nijhuis, H.J.A.; Duyvendak, W.; Vesper, J.; Enggaard, T.P.; Lenders, M.W.P.M. Spinal Cord Stimulation in Patients with Painful Diabetic Neuropathy: A Multicentre Randomized Clinical Trial. Pain 2014, 155, 2426–2431. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Niu, X.; He, B.; Yu, K.; Niu, X.; He, B. Neuromodulation Management of Chronic Neuropathic Pain in the Central Nervous System. Adv. Funct. Mater. 2020, 30, 1908999. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Levy, R.M.; Kramer, J.; Poree, L.; Amirdelfan, K.; Grigsby, E.; Staats, P.; Burgher, A.H.; Scowcroft, J.; Golovac, S.; et al. Comparison of Paresthesia Coverage of Patient’s Pain: Dorsal Root Ganglion vs. Spinal Cord Stimulation. An ACCURATE Study Sub-Analysis. Neuromodulation 2019, 22, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Pawela, C.P.; Kramer, J.M.; Hogan, Q.H. Dorsal Root Ganglion Stimulation Attenuates the BOLD Signal Response to Noxious Sensory Input in Specific Brain Regions: Insights into a Possible Mechanism for Analgesia. Neuroimage 2017, 147, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.E.; Hu, C.Y.; Lee, P.H.; Huang, C.C.; Huang, H.W.; Huang, C.Y.; Lo, H.T.; Liu, W.; Lee, J.S. Sciatic Nerve Stimulation Alleviates Acute Neuropathic Pain via Modulation of Neuroinflammation and Descending Pain Inhibition in a Rodent Model. J. Neuroinflammation 2022, 19, 153. [Google Scholar] [CrossRef] [PubMed]
- Huygen, F.; Liem, L.; Cusack, W.; Kramer, J. Stimulation of the L2-L3 Dorsal Root Ganglia Induces Effective Pain Relief in the Low Back. Pain Pract. 2018, 18, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Piedade, G.S.; Gillner, S.; McPhillips, P.S.; Vesper, J.; Slotty, P.J. Effect of Low-Frequency Dorsal Root Ganglion Stimulation in the Treatment of Chronic Pain. Acta Neurochir. 2023, 165, 947–952. [Google Scholar] [PubMed]
- Stiller, C.O.; Cui, J.G.; O’Connor, W.T.; Brodin, E.; Meyerson, B.A.; Linderoth, B. Release of Gamma-Aminobutyric Acid in the Dorsal Horn and Suppression of Tactile Allodynia by Spinal Cord Stimulation in Mononeuropathic Rats. Neurosurgery 1996, 39, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Linderoth, B.; Stiller, C.O.; Gunasekera, L.; O’Connor, W.T.; Ungerstedt, U.; Brodin, E. Gamma-Aminobutyric Acid Is Released in the Dorsal Horn by Electrical Spinal Cord Stimulation: An in Vivo Microdialysis Study in the Rat. Neurosurgery 1994, 34, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.G.; O’Connor, W.T.; Ungerstedt, U.; Linderoth, B.; Meyerson, B.A. Spinal Cord Stimulation Attenuates Augmented Dorsal Horn Release of Excitatory Amino Acids in Mononeuropathy via a GABAergic Mechanism. Pain 1997, 73, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Gazerani, P. Satellite Glial Cells in Pain Research: A Targeted Viewpoint of Potential and Future Directions. Front. Pain Res. 2021, 2, 646068. [Google Scholar] [CrossRef] [PubMed]
- Leuti, A.; Fava, M.; Pellegrini, N.; Maccarrone, M. Role of Specialized Pro-Resolving Mediators in Neuropathic Pain. Front. Pharmacol. 2021, 12, 717993. [Google Scholar] [CrossRef] [PubMed]
- Rasquel-Oliveira, F.S.; Deroco, M.; Martelossi-cebinelli, G.; Fattori, V.; Casagrande, R.; Verri, W.A. Specialized Pro-Resolving Lipid Mediators: Endogenous Roles and Pharmacological Activities in Infections. Molecules 2023, 28, 5032. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Luo, X.; Zhang, T.; Hershey, B.; Esteller, R.; Ji, R.R. Spinal Cord Stimulation Attenuates Mechanical Allodynia and Increases Central Resolvin D1 Levels in Rats with Spared Nerve Injury. Front. Physiol. 2021, 12, 687046. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.P.; Eldabe, S.; Buchser, E.; Johanek, L.M.; Guan, Y.; Linderoth, B. Parameters of Spinal Cord Stimulation and Their Role in Electrical Charge Delivery: A Review. Neuromodulation 2016, 19, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Joosten, E.A.; Franken, G. Spinal Cord Stimulation in Chronic Neuropathic Pain: Mechanisms of Action, New Locations, New Paradigms. Pain 2020, 161 (Suppl. S1), S104–S113. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaisy, A.; Baranidharan, G.; Palmisani, S.; Pang, D.; Will, O.; Wesley, S.; Crowther, T.; Ward, K.; Castino, P.; Raza, A.; et al. Comparison of Paresthesia Mapping to Anatomical Placement in Burst Spinal Cord Stimulation: Initial Trial Results of the Prospective, Multicenter, Randomized, Double-Blinded, Crossover, CRISP Study. Neuromodulation 2020, 23, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Al-Kaisy, A.; Baranidharan, G.; Sharon, H.; Palmisani, S.; Pang, D.; Will, O.; Wesley, S.; Crowther, T.; Ward, K.; Castino, P.; et al. Comparison of Paresthesia Mapping with Anatomic Placement in Burst Spinal Cord Stimulation: Long-Term Results of the Prospective, Multicenter, Randomized, Double-Blind, Crossover CRISP Study. Neuromodulation 2022, 25, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Thordstein, M.; Svantesson, M.; Rahin, H. Effect of Transspinal Direct Current Stimulation on Afferent Pain Signalling in Humans. J. Clin. Neurosci. 2020, 77, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.M.; Mekhail, N.; Kramer, J.; Poree, L.; Amirdelfan, K.; Grigsby, E.; Staats, P.; Burton, A.W.; Burgher, A.H.; Scowcroft, J.; et al. Therapy Habituation at 12 Months: Spinal Cord Stimulation Versus Dorsal Root Ganglion Stimulation for Complex Regional Pain Syndrome Type I and II. J. Pain 2020, 21, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Mullins, C.F.; Palumbo, G.J.; Harris, S.; Al-Kaisy, O.; Wesley, S.; Yearwood, T.; Al-Kaisy, A. Effectiveness of Combined Dorsal Root Ganglion and Spinal Cord Stimulation: A Retrospective, Single-Centre Case Series for Chronic Focal Neuropathic Pain. Pain Med. 2024, 25, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Lepski, G.; Vahedi, P.; Tatagiba, M.S.; Morgalla, M. Combined Spinal Cord and Peripheral Nerve Field Stimulation for Persistent Post-Herniorrhaphy Pain. Neuromodulation 2013, 16, 84–89. [Google Scholar] [CrossRef] [PubMed]
- North, J.; Loudermilk, E.; Lee, A.; Sachdeva, H.; Kaiafas, D.; Washabaugh, E.; Sheth, S.; Scowcroft, J.; Mekhail, N.; Lampert, B.; et al. Outcomes of a Multicenter, Prospective, Crossover, Randomized Controlled Trial Evaluating Subperception Spinal Cord Stimulation at ≤1.2 KHz in Previously Implanted Subjects. Neuromodulation 2020, 23, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kriek, N.; de Vos, C.C.; Groeneweg, J.G.; Baart, S.J.; Huygen, F.J.P.M. Allodynia, Hyperalgesia, (Quantitative) Sensory Testing and Conditioned Pain Modulation in Patients with Complex Regional Pain Syndrome before and after Spinal Cord Stimulation Therapy. Neuromodulation 2023, 26, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Poply, K.; Haroon, A.; Ganeshan, B.; Nikolic, S.; Sharma, S.; Ahmad, A.; Ellamushi, H.; Parsai, A.; Mehta, V. Dynamic Brain Imaging Response to Spinal Cord Stimulation Differential Frequencies DiFY SCS-PET Clinical Trial. Neuromodulation 2023, 26, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Kissoon, N.R.; Lemahieu, A.M.; Stoltenberg, A.D.; Bendel, M.A.; Lamer, T.J.; Watson, J.C.; Sletten, D.M.; Singer, W. Quantitative Assessment of Painful Diabetic Peripheral Neuropathy after High-Frequency Spinal Cord Stimulation: A Pilot Study. Pain Med. 2023, 24, S41–S47. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.A.; Volschenk, W.; Bailey, D.; Santarelli, D.M.; Holliday, E.; Barker, D.; Dizon, J.; Graham, B. A Novel, Paresthesia-Free Spinal Cord Stimulation Waveform for Chronic Neuropathic Low Back Pain: Six-Month Results of a Prospective, Single-Arm, Dose-Response Study. Neuromodulation 2023, 26, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Liu, Z.; Zhou, W.; Li, X.; Wang, X.; Gong, Q. Short-Term Spinal Cord Stimulation or Pulsed Radiofrequency for Elderly Patients with Postherpetic Neuralgia: A Prospective Randomized Controlled Trial. Neural Plast. 2022, 2022, 7055697. [Google Scholar] [CrossRef] [PubMed]


| Etiology | Pain Syndrome |
|---|---|
| Mechanics (trauma) | Post-surgical neuropathy |
| Cervical and lumbar radiculopathy | |
| Cancer | |
| Traumatic nerve injury | |
| Spinal cord injury | |
| Amputation | |
| Metabolic Disorders | Diabetic neuropathy (insulin-dependent and non-insulin-dependent) |
| Vitamin B12 and folate deficiency | |
| Hypothyroidism | |
| Inflammatory Disorders and Viral Infections | Postherpetic neuralgia |
| HIV neuropathy | |
| Trigeminal neuralgia | |
| Chronic inflammatory demyelinating polyneuropathy | |
| Leprosy | |
| Toxicity | Chemotherapy-induced peripheral neuropathyAlcoholic neuropathy |
| Radiation | Post-radiation neuropathy |
| Heredity | Charcot–Marie–Tooth disease |
| Fabry disease | |
| Autoimmune Diseases | Guillain–Barré Syndrome |
| Multiple sclerosis | |
| Unknown | Idiopathic neuropathy |
| Origin | Pain Syndrome |
|---|---|
| Peripheral | Trigeminal neuralgia |
| Chronic neuropathic pain after peripheral nerve injury | |
| Painful polyneuropathy | |
| Postherpetic neuralgia | |
| Painful radiculopathy | |
| Central | Chronic central neuropathic pain associated with spinal cord injury |
| Chronic central neuropathic pain associated with brain injury | |
| Chronic central pain after stroke | |
| Chronic central neuropathic pain associated with multiple sclerosis |
| Pharmacological Approaches Investigated in Studies | |||
| Pharmacological Class (Drug) | Recommendation | Mechanism | References |
| First-line drugs | |||
| TCA (amitriptyline) | All types of NP | Modulate the activity of descending control systems (inhibit serotonin and noradrenaline reuptake from presynaptic terminals and inhibit cholinergic, adrenergic, and histaminergic receptors) | [42,51] |
| Gabapentinoids (gabapentin, pregabalin) | All types of NP | Attenuate central sensitization (brainstem and higher brain centers) by acting on Ca2+ channels (reduce Ca2+ influx) | [37,42,45] |
| SNRI (duloxetine, venlafaxine) | All types of NP data | Affect the reuptake of norepinephrine and serotonin by modulating the activity of the downward control systems | [37,42] |
| Sodium channel blocker anticonvulsants (Carbamazepine, oxcarbazepine) | Trigeminal neuralgia | Act on the peripheral mechanisms that generate pain | [37,42] |
| Second-line drugs | |||
| Weak opioid agonists (tramadol) | All types of NP data | Activate inhibitory pre- and postsynaptic MORs receptors | [37,42] |
| Topical agents (capsaicin, lidocaine) | Peripheral NP and postherpetic neuralgia | TRPV1 channel agonist | [37,42] |
| Third-line drugs | |||
| Strong opioid agonists (morphine, oxycodone) | All types of NP data | Activate inhibitory MORs located in the spinal cord, brain, and periphery (complexity of follow-up and adverse side effects of drugs of abuse) | [37,42] |
| Botulinum toxin A (BoNTA) | SCI and peripheral NP | Dose-dependent toxic action involving chemodenervation, inhibiting acetylcholine release and leading to functional denervation | [33,46] |
| Infusion (IV) therapies (ketamine, lidocaine, bisphosphonates) | CRPS, fibromyalgia, and traumatic spinal cord injury | Antagonism of NMDA receptor, exerting its effect through the modulation of the transmission of ascending nociceptive information and descending inhibitory pathways | [33,46] |
| Non-pharmacological approaches investigated in studies | |||
| Type | Recommendation | Mechanisms | References |
| Interventional therapies | |||
| Sympathetic nerve/ganglion treatment | Intractable NP | Blockage, neurolysis, or ablation | [33,42] |
| Peripheral nerve stimulation | Intractable lumbar NP | Peripheral and central actions, via modulation of inflammation, autonomic nervous system, and endogenous pain inhibition pathways | [33,101] |
| TENS | Intractable NP | Activation of large-diameter non-noxious afferents resulting in the release of inhibitory neurotransmitters which reduce activity in second-order nociceptive transmission cells | [74,102] |
| DRG stimulation | Refractory CRPS and lower limb causalgia | Stimulation of the post-synaptic activation of pain-gating circuitry in the DRG and modulation of the intrinsic excitability of DRG neurons. | [74,102] |
| SCS | Diabetic neuropathy, refractory ND, and fibromyalgia | Administration of electrical impulses capable of suppressing central neuronal hyperexcitability | [74,102] |
| Epidural motor cortex stimulation | Intractable NP | Endogenous opioid and cannabinoid systems via descending inhibitory pathways | [74,102] |
| rTMS | Intractable NP and PLP | Production of electrical currents in the cortex through a transient magnetic field | [74,102] |
| tDCS | Intractable NP, diabetic neuropathy, traumatic spinal cord injury, and fibromyalgia | Modulates axonal membrane potential | [74,102] |
| Physical therapies | |||
| Physical exercises | Peripheral NP | Inducing opioid and cannabinoid pathways, increasing the levels of anti-inflammatory cytokines and neurotransmitters (e.g., serotonin and GABA), and decreasing growth factors such as NGF and BDNF, which results in the inhibition of glial cells in the spinal cord | [33,53] |
| Psychological therapies | |||
| CBT | Chronic NP, diabetic neuropathy, cancer- associated ND, or HIV infection | Improvement in mood and diagnostic catastrophizing | [33,42] |
| Associated interventions with exercise and psychological therapy (mediation, CBT) | Diabetic neuropathy | Summed effects presented in the two descriptions above | [33,54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.D.V.; Martelossi-Cebinelli, G.; Yaekashi, K.M.; Carvalho, T.T.; Borghi, S.M.; Casagrande, R.; Verri, W.A. A Narrative Review of the Dorsal Root Ganglia and Spinal Cord Mechanisms of Action of Neuromodulation Therapies in Neuropathic Pain. Brain Sci. 2024, 14, 589. https://doi.org/10.3390/brainsci14060589
da Silva MDV, Martelossi-Cebinelli G, Yaekashi KM, Carvalho TT, Borghi SM, Casagrande R, Verri WA. A Narrative Review of the Dorsal Root Ganglia and Spinal Cord Mechanisms of Action of Neuromodulation Therapies in Neuropathic Pain. Brain Sciences. 2024; 14(6):589. https://doi.org/10.3390/brainsci14060589
Chicago/Turabian Styleda Silva, Matheus Deroco Veloso, Geovana Martelossi-Cebinelli, Kelly Megumi Yaekashi, Thacyana T. Carvalho, Sergio M. Borghi, Rubia Casagrande, and Waldiceu A. Verri. 2024. "A Narrative Review of the Dorsal Root Ganglia and Spinal Cord Mechanisms of Action of Neuromodulation Therapies in Neuropathic Pain" Brain Sciences 14, no. 6: 589. https://doi.org/10.3390/brainsci14060589






